Variance Components and Correlations between Doubled Haploid Lines from Two European Flint Landraces and Their Corresponding Testcrosses for Gibberella Ear Rot Resistance, Silking Time, and Plant Height in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Experiments
2.3. Statistical Analysis
3. Results
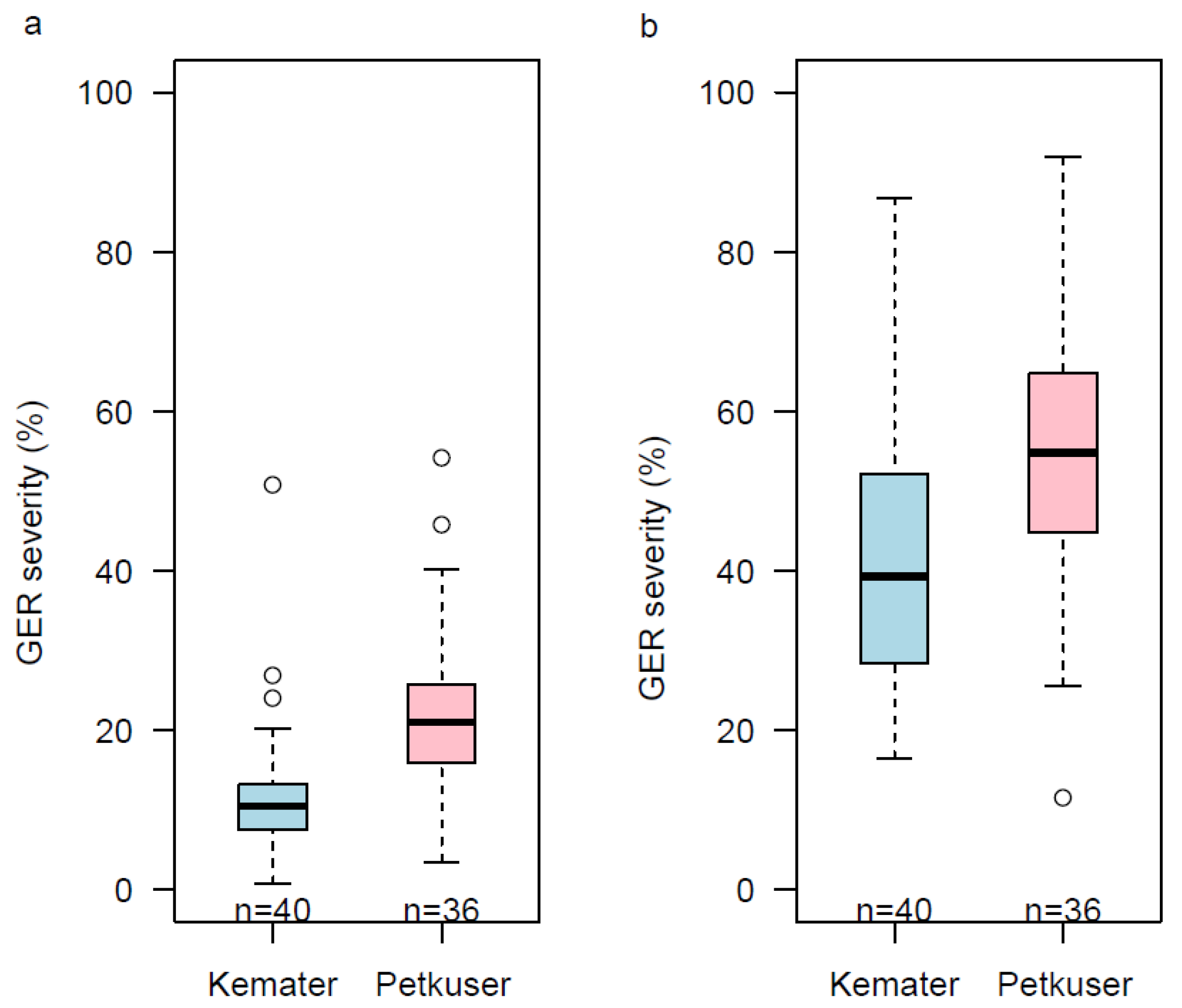
3.1. Testcrosses and DH Line Performances across Environments
3.2. Variance Components and Heritability Estimates in Testcrosses and DH Lines
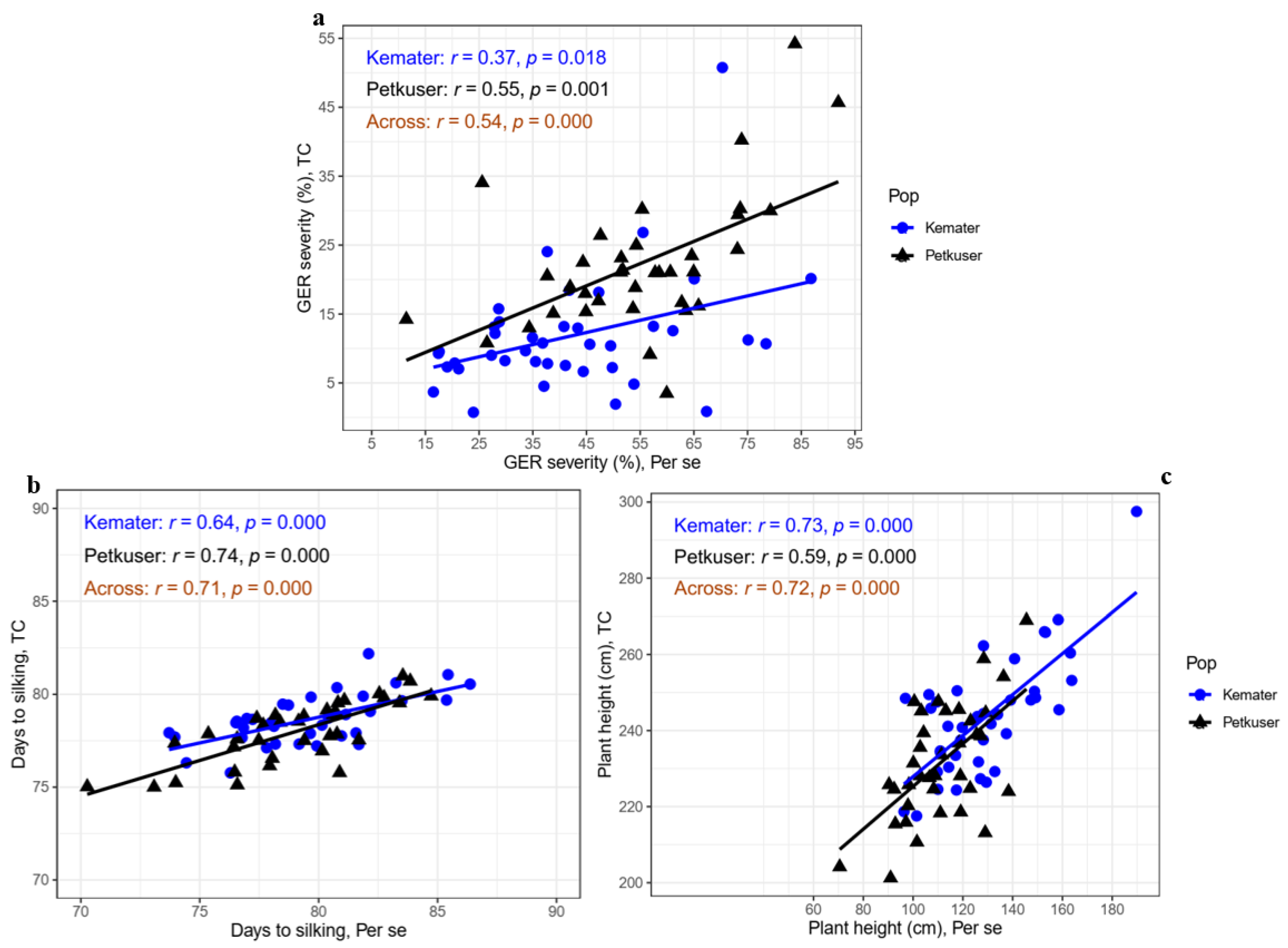
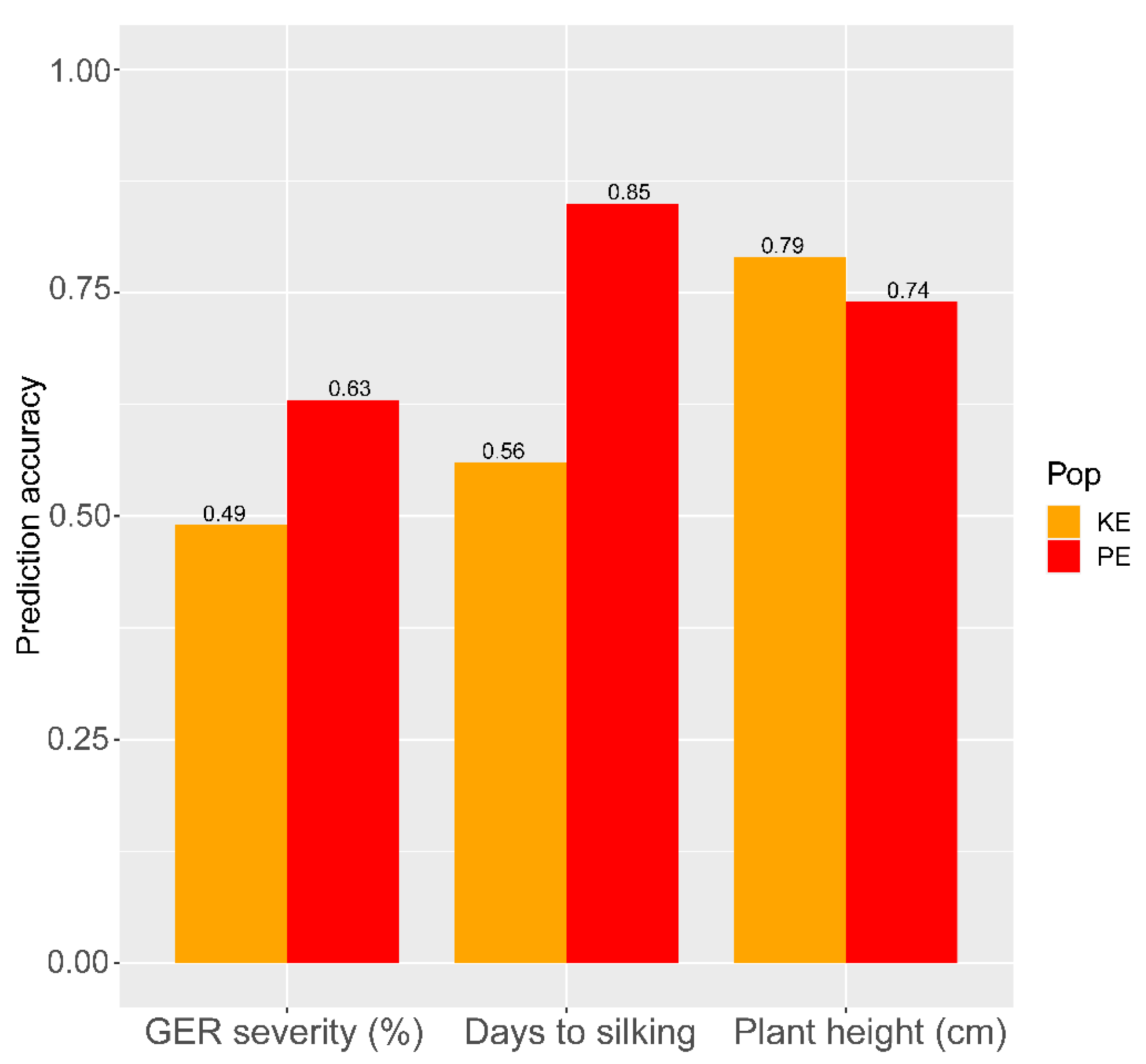
3.3. Correlations and Genomic Prediction Accuracies between Testcrosses and DH Line Performances under Gibberella Ear Rot Infection
4. Discussion
4.1. DH Lines Are Considerably More Susceptible Than Their Testcrosses
4.2. Variance Components Show Large Differences between Lines and Testcrosses
4.3. Moderate Associations and Genomic Prediction Accuracies between Line and Testcross Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dowswell, C.R.; Paliwal, R.L.; Cantrell, R.P. Maize in the Third World; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Verheye, W. Growth and production of maize: Traditional low-input cultivation. In Land Use, Land Cover and Soil Sciences; UNESCO-EOLSS Publishers: Oxford, UK, 2010; pp. 1–23. [Google Scholar]
- Chaudhary, H.K.; Kaila, V.; Rather, S.A. Maize. In Alien Gene Transfer in Crop Plants; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; Volume 2, pp. 27–50. [Google Scholar]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Johnson, J.A.; Tambjerg, L.; Sim, S.; Broeckx-Smith, S.; Reyes, W.; Chaplin-Kramer, R. Closing yield gap is crucial to avoid potential surge in global carbon emissions. Glob. Environ. Chang. 2020, 63, 102100. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to fusarium ear rot in maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Qin, P.; Xu, J.; Jiang, Y.; Hu, L.; Van Der Lee, T.; Waalwijk, C.; Zhang, W.; Xu, X. Survey for toxigenic Fusarium species on maize kernels in China. World Mycotoxin J. 2020, 13, 213–224. [Google Scholar] [CrossRef]
- Szabo, B.; Toth, B.; Toldine, E.T.; Varga, M.; Kovacs, N.; Varga, J.; Kocsube, S.; Palagyi, A.; Bagi, F.; Budakov, D.; et al. A new concept to secure food safety standards against fusarium species and aspergillus flavus and their toxins in maize. Toxins 2018, 10, 372. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of fusarium mycotoxins in cereal crops and processed products (Ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef]
- James, A.; Zikankuba, V.L. Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control. 2018, 90, 372–381. [Google Scholar] [CrossRef]
- Gaikpa, D.S.; Miedaner, T. Genomics-assisted breeding for ear rot resistances and reduced mycotoxin contamination in maize: Methods, advances and prospects. Theor. Appl. Genet. 2019, 132, 2721–2739. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Lemmens, M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Martin, M.; Dhillon, B.S.; Miedaner, T.; Melchinger, A.E. Inheritance of resistance to Gibberella ear rot and deoxynivalenol contamination in five flint maize crosses. Plant Breed. 2011, 131, 28–32. [Google Scholar] [CrossRef]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; Von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of fusarium species associated with ear- and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Bolduan, C.; Miedaner, T.; Utz, H.F.; Dhillon, B.S.; Melchinger, A.E. Genetic variation in testcrosses and relationship between line per se and testcross performance for resistance to gibberella ear rot in maize. Crop. Sci. 2010, 50, 1691–1696. [Google Scholar] [CrossRef]
- Löffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Covariation between line and testcross performance for reduced mycotoxin concentrations in European maize after silk channel inoculation of two Fusarium species. Theor. Appl. Genet. 2010, 122, 925–934. [Google Scholar] [CrossRef]
- Miedaner, T.; Han, S.; Kessel, B.; Ouzunova, M.; Schrag, T.; Utz, F.H.; Melchinger, A.E. Prediction of deoxynivalenol and zearalenone concentrations inFusarium graminearuminoculated backcross populations of maize by symptom rating and near-infrared spectroscopy. Plant Breed. 2015, 134, 529–534. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and pesticides: Their impact on soil health and environment. In Soil Health, 1st ed.; Giri, B., Varma, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Volume 59, pp. 265–285. [Google Scholar]
- Wen, J.; Shen, Y.; Xing, Y.; Wang, Z.; Han, S.; Li, S.; Yang, C.; Hao, D.; Zhang, Y. QTL mapping of resistance to Gibberella ear rot in maize. Mol. Breed. 2020, 40, 94. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Toth, E.T.; Szel, S.; Varga, M.; Toth, B. Resistance of maize hybrids to Fusarium graminearum, F. culmorum, and F. verticillioides ear rots with toothpick and silk channel inoculation, as well as their toxin production. Agronomy 2020, 10, 1283. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Gao, J.; Wu, Y.; Xia, Z.; Zhang, H.; Wu, J. The mechanisms of maize resistance to fusarium verticillioides by comprehensive analysis of RNA-seq data. Front. Plant Sci. 2016, 7, 1654. [Google Scholar] [CrossRef] [PubMed]
- Gaikpa, D.S.; Kessel, B.; Presterl, T.; Ouzunova, M.; Galiano-Carneiro, A.L.; Mayer, M.; Melchinger, A.E.; Schön, C.-C.; Miedaner, T. Exploiting genetic diversity in two European maize landraces for improving Gibberella ear rot resistance using genomic tools. Theor. Appl. Genet. 2021, 134, 793–805. [Google Scholar] [CrossRef]
- Miedaner, T.; Schulthess, A.W.; Gowda, M.; Reif, J.C.; Longin, C.F.H. High accuracy of predicting hybrid performance of Fusarium head blight resistance by mid-parent values in wheat. Theor. Appl. Genet. 2016, 130, 461–470. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Zeleke, H.; Friesen, D.; Blümmel, M.; Twumasi-Afriyie, S. Relationship between the performance of parental inbred lines and hybrids for food-feed traits in maize (Zea mays L.) in Ethiopia. Field Crop. Res. 2013, 153, 86–93. [Google Scholar] [CrossRef]
- Edlich-Muth, C.; Muraya, M.M.; Altmann, T.; Selbig, J. Phenomic prediction of maize hybrids. Biosystems 2016, 146, 102–109. [Google Scholar] [CrossRef]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef]
- Bauer, E.; Falque, M.; Walter, H.; Bauland, C.; Camisan, C.; Campo, L.; Meyer, N.; Ranc, N.; Rincent, R.; Schipprack, W.; et al. Intraspecific variation of recombination rate in maize. Genome Biol. 2013, 14, R103. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Mather, D.E.; Hamilton, R.I. Distribution of deoxynivalenol in Fusarium graminearum-infected maize ears. Phyto-pathology 1996, 86, 110–114. [Google Scholar] [CrossRef]
- Reid, L.; Hamilton, R. Effects of inoculation position, timing, macroconidial concentration, and irrigation on resistance of maize toFusarium graminearuminfection through kernels. Can. J. Plant Pathol. 1996, 18, 279–285. [Google Scholar] [CrossRef]
- Butler, D.; Cullis, B.; Gilmour, A.; Gogel, B. Analysis of Mixed Models for S–Language Environments: ASReml–R Reference Manual; Queensland DPI: Brisbane, Australia, 2007; Available online: http://www.vsni.co.uk/resources/doc/asreml-R.pdf (accessed on 25 March 2021).
- Hallauer, A.; Russell, W.A.; Lamkey, K. Corn breeding. In Corn and Corn Improvement, 3rd ed.; Sprague, G.F., Dudley, J.W., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1988; Volume 18, pp. 463–564. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Endelman, J.B. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome 2011, 4, 250–255. [Google Scholar] [CrossRef]
- Endelman, J.B.; Jannink, J.-L. Shrinkage estimation of the realized relationship matrix. G3 Genes Genomes Genet. 2012, 2, 1405–1413. [Google Scholar] [CrossRef]
- Unterseer, S.; Bauer, E.; Haberer, G.; Seidel, M.; Knaak, C.; Ouzunova, M.; Meitinger, T.; Strom, T.M.; Fries, R.; Pausch, H.; et al. A powerful tool for genome analysis in maize: Development and evaluation of the high density 600 k SNP genotyping array. BMC Genom. 2014, 15, 823. [Google Scholar] [CrossRef]
- Matzinger, D.F. Comparison of three types of testers for the evaluation of inbred lines of corn1. Agron. J. 1953, 45, 493–495. [Google Scholar] [CrossRef]
- Butrón, A.; Reid, L.M.; Santiago, R.; Cao, A.; Malvar, R.A. Inheritance of maize resistance to gibberella and fusarium ear rots and kernel contamination with deoxynivalenol and fumonisins. Plant Pathol. 2015, 64, 1053–1060. [Google Scholar] [CrossRef]
- Roessler, K.; Muyle, A.; Diez, C.M.; Gaut, G.R.J.; Bousios, A.; Stitzer, M.C.; Seymour, D.K.; Doebley, J.F.; Liu, Q.; Gaut, B.S. The genome-wide dynamics of purging during selfing in maize. Nat. Plants 2019, 5, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Schipprack, W.; Utz, H.F.; Melchinger, A.E. Tapping the genetic diversity of landraces in allogamous crops with doubled haploid lines: A case study from European flint maize. Theor. Appl. Genet. 2017, 130, 861–873. [Google Scholar] [CrossRef]
- Strigens, A.; Schipprack, W.; Reif, J.C.; Melchinger, A.E. Unlocking the genetic diversity of maize landraces with doubled haploids opens new avenues for breeding. PLoS ONE 2013, 8, e57234. [Google Scholar] [CrossRef] [PubMed]
- Wricke, G.; Weber, E. Quantitative Genetics and Selection in Plant Breeding; Walter de Gruyter: Berlin, Germany, 2010. [Google Scholar]
- Wang, N.; Wang, H.; Zhang, A.; Liu, Y.; Yu, D.; Hao, Z.; Ilut, D.; Glaubitz, J.C.; Gao, Y.; Jones, E.; et al. Genomic prediction across years in a maize doubled haploid breeding program to accelerate early-stage testcross testing. Theor. Appl. Genet. 2020, 133, 2869–2879. [Google Scholar] [CrossRef]
| Parameter | DH Lines–4 Env | DH Lines–2 Env | TC–2 Env | |||
|---|---|---|---|---|---|---|
| KE | PE | KE | PE | KE | PE | |
| GER (%) | ||||||
| Minimum | 16.49 | 11.44 | 25.11 | 33.09 | 0.74 | 3.48 |
| Maximum | 86.82 | 91.90 | 93.60 | 94.54 | 50.76 | 54.20 |
| Mean | 42.13 | 55.04 | 54.98 | 63.92 | 11.82 | 22.33 |
| DS (days) | ||||||
| Minimum | 73.71 | 70.27 | 75.76 | 74.27 | 75.77 | 75.00 |
| Maximum | 86.37 | 84.73 | 92.21 | 86.99 | 82.19 | 80.99 |
| Mean | 79.54 | 78.98 | 82.57 | 81.07 | 78.53 | 77.92 |
| PHT (cm) | ||||||
| Minimum | 96.32 | 70.51 | 96.53 | 72.66 | 217.56 | 201.23 |
| Maximum | 189.79 | 145.55 | 203.80 | 149.69 | 297.52 | 268.95 |
| Mean | 129.30 | 110.98 | 133.84 | 114.24 | 243.70 | 231.49 |
| Population | Parameter | GER (%) | DS (days) | PHT (cm) |
|---|---|---|---|---|
| DH lines | ||||
| KE | 259.81 | 8.87 | 416.66 | |
| 78.82 | 3.28 | 60.04 | ||
| 308.70 | 3.58 | 86.26 | ||
| 0.82 | 0.88 | 0.94 | ||
| PE | 211.48 | 11.16 | 207.73 | |
| 114.31 | 2.40 | 55.56 | ||
| 308.70 | 3.58 | 86.26 | ||
| 0.76 | 0.91 | 0.89 | ||
| TC | ||||
| KE | 39.48 | 1.36 | 207.33 | |
| 31.49 | 0.26 | 28.65 | ||
| 63.13 | 1.02 | 68.72 | ||
| 0.56 | 0.78 | 0.87 | ||
| PE | 67.26 | 2.35 | 191.90 | |
| 28.14 | 0.16 | 26.79 | ||
| 63.13 | 1.02 | 68.72 | ||
| 0.69 | 0.88 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akohoue, F.; Gaikpa, D.S.; Kessel, B.; Presterl, T.; Miedaner, T. Variance Components and Correlations between Doubled Haploid Lines from Two European Flint Landraces and Their Corresponding Testcrosses for Gibberella Ear Rot Resistance, Silking Time, and Plant Height in Maize. Agronomy 2021, 11, 1039. https://doi.org/10.3390/agronomy11061039
Akohoue F, Gaikpa DS, Kessel B, Presterl T, Miedaner T. Variance Components and Correlations between Doubled Haploid Lines from Two European Flint Landraces and Their Corresponding Testcrosses for Gibberella Ear Rot Resistance, Silking Time, and Plant Height in Maize. Agronomy. 2021; 11(6):1039. https://doi.org/10.3390/agronomy11061039
Chicago/Turabian StyleAkohoue, Félicien, David Sewordor Gaikpa, Bettina Kessel, Thomas Presterl, and Thomas Miedaner. 2021. "Variance Components and Correlations between Doubled Haploid Lines from Two European Flint Landraces and Their Corresponding Testcrosses for Gibberella Ear Rot Resistance, Silking Time, and Plant Height in Maize" Agronomy 11, no. 6: 1039. https://doi.org/10.3390/agronomy11061039
APA StyleAkohoue, F., Gaikpa, D. S., Kessel, B., Presterl, T., & Miedaner, T. (2021). Variance Components and Correlations between Doubled Haploid Lines from Two European Flint Landraces and Their Corresponding Testcrosses for Gibberella Ear Rot Resistance, Silking Time, and Plant Height in Maize. Agronomy, 11(6), 1039. https://doi.org/10.3390/agronomy11061039






