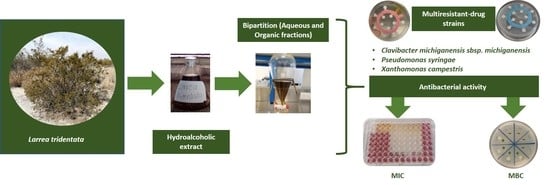
Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Hydroalcoholic Extract
2.3. Fractionation of Larrea Tridentata Hydroalcoholic Extract
2.4. Bacterial Strains and Culture Conditions
2.5. Antibiotic Sensitivity Test
2.6. Antibacterial Activity
2.6.1. Minimal Inhibitory Concentration (MIC)
2.6.2. Minimal Bactericidal Concentration (MBC)
2.7. Statistical Analysis
3. Results
3.1. Test of Antibiotic Sensitivity
3.2. Minimal Inhibitory Concentration (MIC)
3.3. Minimal Bactericidal Concentration (MBC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). México Ante el Desafío del Cambio Climático. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwjN0NWd5MTlAhUCba0KHbGpBbIQFjAAegQIAhAC&url=http%3A%2F%2Fwww.fao.org%2F3%2Fa-i4093s.pdf&usg=AOvVaw07YIaI-PTHin1zf0D3bY-A (accessed on 17 February 2021).
- Food and Agriculture Organization (FAO). Sustainable Small-Scale Livestock Production. Available online: http://www.fao.org/3/ca7660en/CA7660EN.pdf (accessed on 17 February 2021).
- Du, Y.Z.; Wood, L.L.; Saavedra, S.S. Growth behavior and structure of alkyltrichlorosilane monolayers bearing thioacetate and acetate tailgroups. Mater. Sci. Eng. C 1999, 7, 161–169. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, Z.; Yang, W. Breeding for Resistance to Tomato Bacterial Diseases in China: Challenges and Prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Jones, J.; Lacy, G.H.; Bouzar, H.; Minsavage, G.; Stall, R.; Schaad, N.W. Bacterial Spot—Worldwide Distribution, Importance and Review. Acta Hortic. 2004, 695, 27–234. [Google Scholar] [CrossRef]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, J.V.; Grygoryuk, I.P.; Butsenko, L.M. Bacterial diseases of tomato plants in terms of open and covered growing of Ukraine. Ann. Agrar. Sci. 2017, 15, 213–216. [Google Scholar] [CrossRef]
- Soberón, J.R.; Sgariglia, M.A.; Dip Maderuelo, M.R.; Andina, M.L.; Sampietro, D.A.; Vattuone, M.A. Antibacterial activities of Ligaria cuneifolia and Jodina rhombifolia leaf extracts against phytopathogenic and clinical bacteria. J. Biosci. Bioeng. 2014, 118, 599–605. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. J. Crop Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.S.; de Oliveira, M.M.G.; de Melo, J.O.; Blank, A.F.; Corrêa, C.B.; Scher, R. Antimicrobial activity of Lippia gracilis essential oils on the plant pathogen Xanthomonas campestris pv. campestris and their effect on membrane integrity. Pestic. Biochem. Phys. 2019, 160, 40–48. [Google Scholar] [CrossRef]
- Lazarovits, G.; Turnbull, A.; Johnston-Monje, D. Plant Health Management: Biological Control of Plant Pathogens. In Encyclopedia of Agriculture and Food Systems; Van-Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 388–399. [Google Scholar]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 1–10. [Google Scholar] [CrossRef]
- Marutescu, L.; Popa, M.; Saviuc, C.; Lazar, V.; Chifiriuc, M.C. 8—Botanical pesticides with virucidal, bactericidal, and fungicidal activity. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 311–335. [Google Scholar]
- Woodson, R.E.; Schery, R.W.; Porter, D.M. Flora of Panama: Part VI. Family 88. Zygophyllaceae. Ann. Mo. Bot. Gard. 1969, 56, 1–7. [Google Scholar] [CrossRef]
- Ross, I.A. Chemical constituents, traditional and modern medicinal uses. In Medicinal Plants of the World; Springer’s Humana Press: Totowa, NJ, USA, 2007; Volume 3. [Google Scholar]
- Lira-Saldívar, R.H. Estado Actual del Conocimiento sobre las Propiedades Biocidas de la Gobernadora [Larrea tridentata (D.C.) Coville]. Rev. Mex. Fitopatol. 2003, 21, 214–222. [Google Scholar]
- Arteaga, S.; Andrade-Cetto, A.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Aguilar, C.N.; Teixeira, J.A. Antioxidant capacity and NDGA content of Larrea tridentata (a desert bush) leaves extracted with different solvents. J. Biotechnol. 2010, 150, 500. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, X.-Y.; Liu, J.M.; Song, Y.; Liu, T.T.; Chen, D. NDGA reduces secondary damage after spinal cord injury in rats via anti-inflammatory effects. Brain Res. 2013, 1516, 83–92. [Google Scholar] [CrossRef]
- Rahman, S.; Ansari, R.A.; Rehman, H.; Parvez, S.; Raisuddin, S. Nordihydroguaiaretic Acid from Creosote Bush (Larrea tridentata) Mitigates 12-O-Tetradecanoylphorbol-13-Acetate-Induced Inflammatory and Oxidative Stress Responses of Tumor Promotion Cascade in Mouse Skin. Evid. Based Complement. Altern. Med. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef]
- Skouta, R.; Morán-Santibañez, K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Vasquez, A.H.; Varela-Ramirez, A.; Henderson, V.; Sweeney, J.; Odero-Marah, V. Larrea tridentata Extract Mitigates Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. Antioxidants 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wu, J.; Xing, D.M.; Liu, Y.; Jia, F.H.; Li, D.T. Nordihydroguaiaretic Acid (NDGA) Promotes Functional Recovery after Transient Focal Cerebral Ischemia in Rats. Lat. Am. J. Pharm. 2014, 33, 994–1000. [Google Scholar]
- Siddique, Y.H.; Ali, F. Protective effect of nordihydroguaiaretic acid (NDGA) on the transgenic Drosophila model of Alzheimer’s disease. Chem. Biol. Interact. 2017, 269, 59–66. [Google Scholar] [CrossRef]
- Tovar-Carrillo, K.L.; Saucedo-Acuña, R.A.; Ríos-Arana, J.; Tamayo, G.; Guzmán-Gastellum, D.A.; Díaz-Torres, B.A. Synthesis, Characterization, and In Vitro and In Vivo Evaluations of Cellulose Hydrogels Enriched with Larrea tridentata for Regenerative Applications. Biomed. Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchyo-Tenorio, G.; Rodríguez-Cruz, M.; Andrade-Cetto, A.; Cárdenas-Vázquez, R. Creosote Bush (Larrea tridentata) Improves Insulin Sensitivity and Reduces Plasma and Hepatic Lipids in Hamsters Fed a High Fat and Cholesterol Diet. Front. Pharmacol. 2016, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Bittner, S.; Dong, D.; Cortez, Y.; Dulay, H.; Arshad, S. Creosote bush-derived NDGA attenuates molecular and pathological changes in a novel mouse model of non-alcoholic steatohepatitis (NASH). Mol. Cell. Endocrinol. 2019, 498, 110538. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.W.; Bittner, S.; Bittner, A.; Atwal, S.; Shen, W.J.; Inayathullah, M. Nordihydroguaiaretic Acid, a Lignan from Larrea tridentata (Creosote Bush), Protects Against American Lifestyle-Induced Obesity Syndrome Diet-Induced Metabolic Dysfunction in Mice. J. Pharmacol. Exp. Ther. 2018, 365, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zuntilde, A.; Tapia, E.; Zazueta, C.; Correa, F.; Zatarain-Barron, Z.L.; Hernandez, P.R. Nordihydroguaiaretic acid pretreatment prevents ischemia and reperfusion induced renal injury, oxidant stress and mitochondrial alterations. J. Med. Plant Res. 2012, 6, 2938–2947. [Google Scholar]
- Rojo, A.I.; Medina-Campos, O.N.; Rada, P.; Zúñiga-Toalá, A.; López-Gazcón, A.; Espada, S. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free Radic. Biol. Med. 2012, 52, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.J.; Laster, S.M.; Petty, I.T.D. Inhibition of poxvirus growth by Terameprocol, a methylated derivative of nordihydroguaiaretic acid. Antivir. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.; Hernández, D.; Gallegos, G.; Mendez, M.; Rodríguez, R.; Reyes, A. In vitro antifungal activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind. Crop. Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R.; Aguilar, C.N. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind. Crop. Prod. 2010, 31, 153–157. [Google Scholar] [CrossRef]
- Chávez-Solís, A.L.; Pedroza-Sandoval, A.; Nava-Díaz, C.; Cano-Rios, P.; Castro-Franco, R. Control de la cenicilla del melón (Podosphaera xanthii) mediante el uso de extracto de Larrea tridentata (D.C.) Coville (L.). Rev. Chapingo Ser. Zonas Áridas 2014, 13, 103–113. [Google Scholar] [CrossRef][Green Version]
- Valero Galván, J.; González-Díaz, C.A.; González-Fernández, R. Efecto de los extractos acuosos de hojas de plantas de gobernadora (Larreas tridentata), hojasen (Flourensia cernua) y encino (Quercus pungens), sobre el crecimiento micelial in vitro de hongos fitopatógenos. Acta Univ. 2014, 24, 13–19. [Google Scholar]
- Bashyal, B.; Li, L.; Bains, T.; Debnath, A.; LaBarbera, D.V. Larrea tridentata: A novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PLoS Negl. Trop. Dis. 2017, 11, e0005832. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; Camacho-Corona, M.D.R. Potential mechanism of action of 3’-demethoxy-6-O-demethyl-isoguaiacin on methicillin resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef]
- Martins, S.; Amorim, E.L.C.; Sobrinho, T.J.S.P.; Saraiva, A.M.; Pisciottano, M.N.C.; Aguilar, C.N. Antibacterial activity of crude methanolic extract and fractions obtained from Larrea tridentata leaves. Ind. Crop. Prod. 2013, 41, 306–311. [Google Scholar] [CrossRef]
- Snowden, R.; Harrington, H.; Morrill, K.; Jeane, L.; Garrity, J.; Orian, M. A comparison of the anti-Staphylococcus aureus activity of extracts from commonly used medicinal plants. J. Altern. Complement. Med. 2014, 20, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Oakes, E.; Soren, O.; Moussa, C.; Rathor, G.; Liu, Y.; Coates, A. Nordihydroguaiaretic acid enhances the activities of aminoglycosides against methicillin-sensitive and resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2015, 6, 1195. [Google Scholar] [CrossRef]
- Delgadillo-Ruiz, L.; Bañuelos-Valenzuela, R.; Delgadillo-Ruiz, O.; Silva-Vega, M.; Gallegos-Flores, P. Chemical composition and antibacterian effect in vitro of extracts of Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens. Nova Sci. 2017, 9, 273–290. [Google Scholar] [CrossRef]
- Reyes-Melo, K.; García, A.; Romo-Mancillas, A.; Garza-González, E.; Rivas-Galindo, V.M.; Miranda, L.D. Meso-Dihydroguaiaretic acid derivatives with antibacterial and antimycobacterial activity. Bioorg. Med. Chem. 2017, 25, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Gerstel, J.; Turner, T.; Ruiz, G.; Wise, J.; Stein, A.; Jones, G. Identification of botanicals with potential therapeutic use against methicillin-resistant Staphylococcus aureus (MRSA) infections. Phytother. Res. 2018, 32, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Soto, A.F.; Balderas-Rentería, I.; Rivera, G.; Segura-Cabrera, A.; Garza-González, E.; del Rayo Camacho-Corona, M. Potential mechanism of action of meso-dihydroguaiaretic acid on Mycobacterium tuberculosis H37Rv. Molecules 2014, 19, 20170–20182. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Beltrán, S.; Rubio-Badillo, M.Á.; Juárez, E.; Hernández-Sánchez, F.; Torres, M. Nordihydroguaiaretic acid (NDGA) and α-mangostin inhibit the growth of Mycobacterium tuberculosis by inducing autophagy. Int. Immunopharmacol. 2016, 31, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Perez, N.; Hernández-Alvarado, J.L.; Valladares-Carranza, B.; Delgadillo-Ruiz, L.; Ojeda-Ramírez, D.; Sosa-Gutiérrez, C.G.; Morales-Ubaldo, A.L.; Vega-Sánchez, V.; Zaragoza- Bastida, A. Salix babylonica L. as a Natural Anticoccidial Alternative in Growing Rabbits. Evid. Based Complement. Altern. Med. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical Constituents of Salix babylonica L. and their Antibacterial Activity Against Gram-Positive and Gram-Negative Animal Bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Briones-Robles, T.I.; Zaragoza-Bastida, A.; Zamilpa, A.; Ojeda-Ramírez, D.; Mendoza de Gives, P.; Olivarez-Pérez, J.; Rivero-Perez, N. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) Willd against important bacteria in public health. Microb. Pathog. 2019, 136, 103–660. [Google Scholar] [CrossRef] [PubMed]
- Rangel-López, L.; Zaragoza-Bastida, A.; Valladares-Carranza, B.; Peláez-Acero, A.; Sosa-Gutiérrez, C.G.; Hetta, H.F.; Batiha, G.E.S.; Alqahtani, A.; Rivero-Perez, N. In Vitro Antibacterial Potential of Salix babylonica Extract against Bacteria that Affect Oncorhynchus mykiss and Oreochromis spp. Animals 2020, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standards; CLSI Document M7-A5; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Zaragoza-Bastida, A.; Flores-Aguilar, S.C.; Aguilar-Castro, L.M.; Morales-Ubaldo, A.L.; Valladares-Carranza, B.; Rangel-López, L.; Olmedo-Juárez, A.; Rosenfeld-Miranda, C.E.; Rivero-Perez, N. Antibacterial and Hemolytic Activity of Crotalus Triseriatus and Crotalus Ravus Venom. Animals 2020, 10, 281. [Google Scholar] [CrossRef]
- López-Pueyo, M.J.; Barcenilla-Gaite, F.; Amaya-Villar, R.; Garnacho-Montero, J. Multirresistencia antibiótica en unidades de críticos. Med. Intensiv. 2011, 35, 41–53. [Google Scholar] [CrossRef]
- García-Salazar, E.M. El agua residual como generadora del espacio de la actividad agrícola en el Valle del Mezquital, Hidalgo, México. Estud. Soc. REVISTA Aliment. Contemp. Desarro. Reg. 2019, 29, 2–34. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Zongo, C.; McNally, A.; Ricci, V.; Etoa, F.X.; Thiele-Bruhn, S.; Piddock, L.J.V. Wastewater used for urban agriculture in West Africa as a reservoir for antibacterial resistance dissemination. Environ. Res. 2019, 168, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; McNally, A.; Etoa, F.X.; Piddock, L.J.V. Antibiotic resistance genes are abundant and diverse in raw sewage used for urban agriculture in Africa and associated with urban population density. Environ. Pollut. 2019, 251, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Rodríguez, R.; Ruiz, J.; Morales-Adame, D.; Castillo, F.; Hernández-Castillo, F.D.; Aguilar, C.N. Antibacterial activity of plant extracts obtained with alternative organics solvents against food-borne pathogen bacteria. Ind. Crop. Prod. 2012, 37, 445–450. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Yi-Hsu, J. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Cartaya, O.; Reynaldo, I. Flavonoides: Características químicas y aplicaciones. Cult. Trop. 2001, 22, 5–14. [Google Scholar]
- Martínez- Aguilar, Y.; Soto-Rodríguez, F.; Almeida-Saavedra, M.; Hermosilla-Espinosa, R.; Martínez-Yero, O. Metabolitos secundarios y actividad antibacteriana in vitro de extractos de hojas de Anacardium occidentale L. (marañón). Rev. Cuba. Plantas Med. 2012, 17, 320–329. [Google Scholar]
- Gómez-Guiñán, Y.; Hidalgo, J.; Jiménez, M.; Salcedo, J. Obtención de extractos orgánicos con actividad antimicrobiana a partir de Penicillium sp. (Moniliales) aislado de la esponja Ircinia felix (Porifera: Demospongiae). Rev. Biol. Trop. 2003, 51, 141–147. [Google Scholar]
- Rivas-Cáceres, R.R.; Luis Stephano-Hornedo, J.; Lugo, J.; Vaca, R.; Del Aguila, P.; Yañez-Ocampo, G.; Mora-Herrera, M.E.; Camacho-Díaz, L.M.; Cipriano-Salazar, M.; Alaba, P.A. Bactericidal effect of silver nanoparticles against propagation of Clavibacter michiganensis infection in Lycopersicon esculentum Mill. Microb. Pathog. 2018, 115, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos-Valenzuela, R.; Delgadillo-Ruiz, L.; Echavarría-Cháirez, F.; Delgadillo-Ruiz, O.; Meza-López, C. Composición química y FTIR de extractos etanólicos de Larrea tridentata, Origanum vulgare, Artemisa ludoviciana y Ruta graveolens. Agrociencia 2018, 52, 309–321. [Google Scholar]
- Martins, S.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Sep. Purif. Technol. 2012, 88, 163–167. [Google Scholar] [CrossRef]
- Ruiz, J.; Ascacio-Valdés, J.; Rodriguez, R.; Morales, D.; Aguilar, C. Phytochemical screening of extracts from some Mexican plants used in traditional medicine. J. Med. Plants 2011, 5, 2791–2797. [Google Scholar]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Rodríguez-Herrera, R.; Aguilar-González, C. Análisis de ácido elágico en algunas plantas del semidesierto Mexicano. Rev. Mex. Cienc. Farm. 2013, 44, 36–40. [Google Scholar]
- Gnabre, J.; Bates, R.; Huang, R.C. Creosote bush lignans for human disease treatment and prevention: Perspectives on combination therapy. J. Tradit. Complement. Med. 2015, 5, 119–126. [Google Scholar] [CrossRef]
| Antimicrobial | Bacteria | ||
|---|---|---|---|
| C. michiganensis subsp. Michiganensis | P. syringae | X. campestris | |
| Amikacin | 10 (R) | 20 (S) | 15 (I) |
| Ampicillin | 7 (R) | 15 (S) | 7 (R) |
| Carbenicillin | 7 (R) | 16 (I) | 22(S) |
| Cephalothin | 7 (R) | 11 (R) | 7 (R) |
| Cefotaxime | 7 (R) | 7 (R) | 7 (R) |
| Ciprofloxacin | 30 (S) | 30 (S) | 7 (R) |
| Clindamycin | 15 (I) | 7 (R) | 34 (S) |
| Chloramphenicol | 8 (R) | 31 (S) | 30 (S) |
| Dicloxacillin | 12 (I) | 7 (R) | 7 (R) |
| Erythromycin | 7 (R) | 7 (R) | 23 (S) |
| Gentamicin | 7 (R) | 21 (S) | 12 (R) |
| Netilmicin | 8 (R) | 20 (S) | 10 (R) |
| Nitrofurantoin | 9 (R) | 22 (S) | 8 (R) |
| Norfloxacin | 15 (I) | 20 (S) | 7 (R) |
| Penicillin | 7 (R) | 7 (R) | 22 (S) |
| Sulfamethoxazole/trimethoprim | 7 (R) | 7 (R) | 17 (S) |
| Tetracycline | 20 (S) | 20 (S) | 20 (S) |
| Vancomycin | 7 (R) | 7 (R) | 21 (S) |
| Evaluated Treatment | Bacteria | |||
|---|---|---|---|---|
| C. michiganensis sbsp. Michiganensis | P. syringae | X. campestris | p-Value | |
| LTHE | 6.25 cB | 6.25 cB | 0.39 bA | 0.0001 |
| LTAq-F | 6.25 cA | 6.25 cA | NA | 0.0001 |
| LTEtOAc-F | 3.12 bB | 3.12 bB | 0.39 bA | 0.0001 |
| Kanamycin | 0.25 a* | 0.25 a* | 8 a* | |
| p-value | 0.0001 | 0.0001 | 0.0001 | |
| Evaluated Treatment | Bacteria | |||
|---|---|---|---|---|
| C. nichiganensis sbsp. Michiganensis | P. syringae | X. campestris | p-Value | |
| LTHE | 12.5 cB | 12.5 cB | 0.78 bA | 0.0001 |
| LTAq-F | NA | NA | NA | |
| LTEtOAc-F | 6.25 bB | 6.25 bB | 0.78 bA | 0.0001 |
| Kanamycin | 0.5 a* | 0.5 a* | 16 a* | |
| p-value | 0.0001 | 0.0001 | 0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ubaldo, A.L.; Rivero-Perez, N.; Avila-Ramos, F.; Aquino-Torres, E.; Prieto-Méndez, J.; Hetta, H.F.; El-Saber Batiha, G.; Zaragoza-Bastida, A. Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria. Agronomy 2021, 11, 957. https://doi.org/10.3390/agronomy11050957
Morales-Ubaldo AL, Rivero-Perez N, Avila-Ramos F, Aquino-Torres E, Prieto-Méndez J, Hetta HF, El-Saber Batiha G, Zaragoza-Bastida A. Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria. Agronomy. 2021; 11(5):957. https://doi.org/10.3390/agronomy11050957
Chicago/Turabian StyleMorales-Ubaldo, Ana Lizet, Nallely Rivero-Perez, Fidel Avila-Ramos, Eliazar Aquino-Torres, Judith Prieto-Méndez, Helal F. Hetta, Gaber El-Saber Batiha, and Adrian Zaragoza-Bastida. 2021. "Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria" Agronomy 11, no. 5: 957. https://doi.org/10.3390/agronomy11050957
APA StyleMorales-Ubaldo, A. L., Rivero-Perez, N., Avila-Ramos, F., Aquino-Torres, E., Prieto-Méndez, J., Hetta, H. F., El-Saber Batiha, G., & Zaragoza-Bastida, A. (2021). Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria. Agronomy, 11(5), 957. https://doi.org/10.3390/agronomy11050957