Abstract
The potential protective role of priming wheat seeds with maize green extract (MGE) against the stress effects of drought was studied. Pretreatment using MGE, MGE enriched with polyamines (MGEPA), and drought treatments (irrigation deficit of 30% (severe drought) or 60% (moderate drought) versus 90% relative water content of soil as a control) were applied in a factorial completely randomized design. Under moderate drought, pretreatment with MGEPA outperformed MGE and control, while severely stressed plants died even with pretreatments. Both extracts enhanced normal plant growth and yield and mitigated the deleterious effect of moderately stressed plants. Application of both extracts markedly increased photosynthetic efficiency, membrane stability, relative water content, and accumulation of antioxidants, osmoprotectants, trans- and cis-zeatin, polyamines, and their gene expressions, while levels of superoxide (O2•−) and hydrogen peroxide (H2O2), lipid peroxidation, and electrolyte leakage were decreased. Enzymatic antioxidants and glyoxalase system activities were improved in moderately stressed plants and were further improved with pretreatment with both extracts, thus protecting plants from oxidative damage by up-regulation of the ascorbate–glutathione cycle. Glycine betaine, soluble sugars, and proline levels were greatly increased in pretreated plants, thus maintaining membrane stability and photosynthetic efficiency. The interaction between drought and pretreatment using MGEPA was significant in growing wheat plants in dry environments with 60% relative water content of soil.
1. Introduction
Drought, one of the major types of environmental abiotic stresses, restricts plant performance throughout its life cycle. It limits the productivity of agricultural crops, mostly in dry areas (e.g., semi-arid and arid regions), including Egypt and Saudi Arabia, thus causing an increasing problem over time due to the ongoing climate change. Deficit irrigation water (DIw) adversely affects plant growth, development, and production through decreasing water flux, closure of stomata, and reduced CO2 fixation [1,2,3]. DIw strongly affects plant antioxidant system, metabolism, and physio-biochemistry [4,5,6,7,8]. It causes photooxidation damage to cell components by over-generating reactive species of oxygen (ROS), including radicals of O2•− and H2O2 and eventually photo-inhibition [9], which cause significant oxidative damage to proteins, lipids, and DNA, thus discouraging plant growth [10]. To mitigate the oxidative stress damage, complex ROS-scavenging antioxidant system (enzymes (SOD, POD, CAT, etc.) and low-molecular mass compounds (GSH, AsA, carotenoids, phenols, etc.)) are developed/adopted in plants [11]. The enzymatic antioxidants can directly scavenge ROS or produce one or more of the non-enzymatic antioxidants. SOD is specifically implicated in converting O2•− to H2O2 in cell cytoplasm, chloroplasts, mitochondria, and peroxisomes. In addition, POD acts as a scavenger of H2O2 and CAT eliminates the H2O2 in mitochondria and micro-bodies [12], helping mitigate the adverse effects of oxidative stress.
The effective reduction of cell osmotic potential maintains plant cell metabolism and avoids dehydration damage induced by DIw stress via the accumulation of more levels of glycinebetaine, soluble sugars, and proline [13]. These osmotic compounds maintain an osmotic balance, efficacy, and normality of membrane functions, and protect macromolecules and plasma membranes, providing cellular resistance against DIw stress and dehydration [10,14,15].
In most cases, endogenous components of the developed/adopted antioxidant system in plants are not sufficient to defend against environmental stress. Consequently, plant extract and microbial bio-stimulants may be applied exogenously as an adjuvant to plants to increase their tolerance to stress. Plant extracts can induce plant resistance under various stresses, and microbial biostimulants can increase gene regulation in plants related to drought stress and can also produce biofilm, which helps plants survive under drought stress [16,17,18,19,20,21].
At present, extracts containing natural bio-stimulants obtained from maize grains have been applied for seed priming and have been reported to enhance plant performances under different stress conditions; salinity [22,23], nutrient deficiencies [24], cadmium [25], and salinity plus drought [26]. MGE is an important organic bio-stimulant for different stressed plants. It is rich in various phytohormones (e.g., cytokinins, auxins, and gibberellins), antioxidants, and essential nutrients. It has been used to enhance plant morphology, physiology, biochemistry, and induce plant tolerance against stress damages [22,25]. To date, no work has been done using MGE enriched with antioxidants such as polyamines for wheat grown under DIw stress. Thus, this is the first report that used MGE to stimulate the growth of pretreated wheat plants under DIw stress due to its high content of antioxidants, plant hormones, vitamins, polyamines, and nutrients, making it an effective biological stimulant [22,24,25,26,27] (Table 1). These reports stated that MGE confers seed and their emerged seedlings the ability to resist the unfavorable effects of stress through elevating wheat performance (growth and productivity), physio-biochemical parameters, and different antioxidants. Thus, MGE is an innovative means to provide seeds and developing seedlings that are resistant to damages caused by environmental stresses such as DIw.

Table 1.
Antioxidant and hormone contents detected in maize grain extract (MGE).
Wheat (Triticum aestivum L.) grain and straw are major sources of nourishing the diets of humans and animals, respectively in urban and rural communities due to their high protein and calorie content (approximately 82–85%). However, environmental stresses severely affect wheat productivity; if the available soil water reaches 45–50% of field capacity in the root zone, the wheat plants must be watered [2,26]. The duration and severity of stress, growth physio-biochemical processes, and patterns of differential gene expression are some of the factors that can influence the wheat plant’s response to DIw stress [23,26,28,29].
Although MGE has been used in some investigations for some crop plants to boost their antioxidant defense system against the stress impacts of certain abiotic stresses [22,24,25,26,27], there is no information available on seed priming using MGE (as a bio-stimulant) to encourage plant growth and production by mitigating the damage caused by drought stress. However, by analyzing MGE, we found that it is rich in phytohormones, especially zeatins, but was poor in PAs (Table 1), so enriching this extract with PAs could increase its effectiveness. Therefore, this work is based on the hypothesis that seed soaking in MGE enriched in PAs will mitigate drought stress by enhancing the growth, yield, and physio-biochemical systems of wheat plants. Thus, in this study, the effect of wheat seed priming in MGE (without or with enrichment by PAs) on growth and yield, physio-biochemical characteristics, including the up-regulation of AsA–GSH cycle, glyoxalase system, and PAs gene expression of wheat plants was studied under DIw stress conditions.
2. Materials and Methods
2.1. Plant Material and Its Preparation for Sowing
The experiment was carried out using certified Triticum aestivum (L.) seeds (cv. Giza-168; winter wheat). Filtered calcium hypochlorite (1%, v/v) was used to sterilize the seed surface for 1 h. Then, the seeds were washed directly several times with sterile deionized water. The seeds were divided into three groups for different 6-h soaking treatments. The seeds of the first group were soaked in distilled water to serve as a control. The seeds of the second group were soaked in maize grain extract (MGE, 2%) not enriched with polyamines (PAs). The seeds of the third group were soaked in MGE enriched with PAs (MGEPA, 2%). A weight of 500 g of seeds was soaked in 1 L of each soaking solution, and the soaked seeds were re-dried under the shade with forced air according to the procedure of Sundstrom et al. [30]. The concentration 2% was selected for both MGE and MGEPA based on our initial study carried out with 1, 2, and 3% of each of the extracts. Both levels 2 and 3% conferred similar responses that were better than the response obtained from 1% in terms of wheat seedling growth (data not shown).
2.2. Growing Conditions, Treatments, and Experimental Layout
Black plastic pots (32 cm diameter and 30 cm depth) were used for this study. After sterilization, each pot was filled with 10 kg of pure sand, which was previously cleaned completely of ions with commercial acid and re-cleaned with distilled water. The sand was mixed with compost, vermicompost, and humic acid at a ratio of 90: 6.5: 3: 0.5 (w/w), respectively. Ten uniform and healthy seeds were sown in each pot. The pots were arranged in a greenhouse as follows: the average temperatures were 19 ± 3/10 ± 2 °C for day (13 h)/night (11 h), and the average humidity was 62.0–65.1%. The presence of sunlight inside the greenhouse remained homogeneous. Every three days, all pots were watered with full-strength nutritious solution (pH 5.9) [31], containing Ca(NO3)2×4 H2O, KNO3, KH2PO4, MgSO4×7 H2O, H3BO3, MnCl2×4 H2O, ZnSO4×7 H2O, CuSO4×5 H2O, Na2MoO4×2 H2O, and Fe3+-EDTA+ at the concentrations of 1.25 mM, 1.25 mM, 0.25 mM, 0.50 mM, 11.6 μM, 2.4 μM, 0.24 μM, 0.08 μM, 0.13 μM, and 22.5 μM, respectively. Twenty days after sowing (DAS), water treatments were started with wheat seedlings of the three groups of soaking treatments. Each group of soaking treatments was assigned 45 pots and divided into three subgroups of 15 pots each. Each subgroup (e.g., control, MGE, and MGEPA) was subjected to three irrigation regimes (e.g., 90, 60, or 30% of soil relative water content) to include 9 treatments until harvest. The nutrient solution was applied along with the irrigation treatments, taking into account the equal concentration of nutrients in all pots for all treatments. Based on each water regime, soil moisture level was controlled by weighing each pot and any water loss was supplemented daily. A factorial completely randomized design was the one used to arrange all experimental pots. For the entire duration of the experiment (140 days), all pots were rotated daily to avoid systematic error due to fluctuations in environmental conditions.
2.3. Calculation of Soil Relative Water Content (SRWC) and Preparation of Samples
For the calculation of SRWC, the weighing method was used for the three water treatments (control (90%), 60%, or 30% of SRWC). Daily, the amount of water transpired, and evaporated was compensated by the weight of the pots and watered to the corresponding target SRWC, which was expressed using the following equation:
where Wsoil; current weight of soil, pot, and water, Wpot; empty pot weight, DWsoil; dry soil weight, and WFC; soil weight (soil + pot + water) at field capacity. Samples (the upper fully-expanded leaves) were taken 70 DAS (at the end of the tillering; vegetative growth stage with the first node) to determine the parameters of physiology, biochemistry, and antioxidative defense system components. Five plants (replicates) were functioned for each determination. Grain yield was obtained at the end of experiments (140 DAS) from 100 plants.
SRWC = (Wsoil − Wpot − DWsoil) / (WFC − Wpot − DWsoil) × 100
2.4. Preparation of Maize Grain Extracts (MGE and MGEPA)
The full method outlined in Alzahrani and Rady [25] and Alharby et al. [26] was used to obtain the extract from maize grains using local genotype of Egyptian maize. Wet cotton and clean cloth were used to cover and maintain the grains of maize until they were mushy. The mushy grains were transferred for milling using an appropriate amount of distilled water. Then, the mixture was filtered under vacuum. The filtrate was maintained in black bottles at 4 °C in a refrigerator. The remaining residue was transferred, quantitatively, for another extraction with methyl alcohol (70%) for 72 h, and the filtration process was repeated again. Using a rotary evaporator, the filtrate was completely evaporated to remove alcohol. The alcoholic extract was mixed well with the aqueous extract and, then, the mixture was concentrated to reach the target concentrations, which were used immediately. Otherwise, they were stored in a refrigerator at ‒20 °C until use.
Some major ingredients in MGE were detected. AsA and GSH contents were determined in MGE according to Kampfenkel and Van Montagu [32] and Griffth [33], respectively. Activity of antioxidants was assayed in MGE by using the DPPH-radical scavenging activity [34]. Fresh extract was used for endogenous levels of phytohormones (e.g., auxins, gibberellins, cytokinins, including zeatin-type-cytokinins). The sample was frozen in liquid N for preparing to extract different phytohormones that were then analyzed using the GC/MS system according to Lavrich and Hays [35]. Polyamines (PAs) were extracted and determined as follows: Extraction of PAs was implemented at 4 °C by utilizing 500 mg of top fresh fully (third and fourth)-expanded leaves with 4 mL fresh 5% (v/v) HClO4. The supernatant obtained after centrifugation (15,000× g, 30 min) was utilized to detect the free PAs (e.g., PUT, SPM, and SPD) using HPLC system [36,37,38]. Identification and quantification of PAs were conducted by comparing the retention times with peaks areas using the standards of PAs (observed on 254 nm using a 2487 dual UV-detector; Waters, Milford, MA, USA). Results of all assessments are shown in Table 1. As shown, the contents of PAs detected in MGE were very low (0.5, 0.6, and 0.9 µmol g−1 FW for PUT, SPD, and SPM, respectively), and thus, MGE at 2% level was enriched with PUT, SPD, and SPM by adding them at rates of 0.5, 0.6, and 0.9 mM, respectively. These added concentrations of different PAs tested were based on their contents in MGE.
2.5. Components of Growth and Yield
Seventy DAS (tillering stage), five plants were selected and gently extracted from five randomly selected pots. The plants were gently cleaned from the sand particles by using tap water. After determining the FW of shoots, they were dried at 70 °C up to obtaining a constant DW. The grain yield components [Grain yield (g pot−1) and 1000-grain weight (g)] were assessed 140 DAS (harvest stage) by using 100 plants from each treatment.
2.6. Leaf Pigments, Photosynthetic Efficiency, and Gas Exchange Parameters
A weight of 0.2 g of fresh tissue of leaf was extracted using 80% (v/v) acetone with a clean mortar and pestle to determine chlorophylls and carotenoids [39]. Optical densities of supernatants were measured using spectrophotometer apparatus at wavelengths of 663, 645, and 480 nm. Using top fresh leaves, the fluorometer apparatus (Hansatech Instr. Ltd., Kings Lynn, UK) was utilized to evaluate photosynthetic efficiency components; maximum quantum yield of PSII (Fv/Fm; [40]), photosynthetic performance index of PSII (PIABS) was calculated [41], and hill reaction activity was measured [42]. Using the third upper leaf on five random plants, the infrared gas analyzer apparatus (LCA-4 model, England) was utilized to evaluate each net photosynthesis rate; Pn, CO2 assimilation rate; A, conductance of leafy stomata; gs, and transpiration rate; E. Measurements were assessed 4 times (2 h time interval from 7:00 to 17:00); 40, 50, 60, and 70 DAS.
2.7. Oxidative Stress Biomarkers (Hydrogen Peroxide and Superoxide), and Peroxidation of Lipids
Using the fresh upper leaves free of midribs from 5 randomly selected plants, the methods described in Velikova et al. [43], Kubis [44], and Madhava Rao and Sresty [45] were utilized to determine H2O2, O2•−, and lipid peroxidation (evaluated as malondialdehyde; MDA) contents.
For H2O2, the content (µmol g−1 FW) was evaluated colorimetrically by recording the absorbance readings of samples at 390 nm and the calculations were performed based on a suitable standard curve. For O2•−, the content (µmol g−1 FW) was evaluated using sample fragments (1 × 1 mm, 0.1 g) that flooded using a 10 mM K-phosphate buffer (pH 7.8), which was mixed with each of NBT (0.05%) and NaN3 (10 mM) for 1 h at room temperature. The mixture was heated for 0.25 h on 85 °C. Then, the mixture was cooled rapidly. The absorbance readings were taken at 580 nm. For MDA, the content was evaluated using the extinction coefficient 155 mM−1 cm−1.
2.8. Ion Leakage, Relative Water Content, and Membrane Stability Index
Using fresh top leaves free of midribs from 5 randomly selected plants, the leaf ionic (electrolyte) leakage [46], leaf relative content of water [47], and stability index of cell membranes [46] were estimated.
2.9. Assaying Enzymatic Activities and Ascorbate–Glutathione Cycle Activity
Fresh top leaves free of midribs (0.5 g) from five plants were used to extract antioxidant enzymes for assaying the activities of ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione reductase (GR), and catalase (CAT). The methods of Dhindsa and Matowe [48], Aebi [49], Hasanuzzaman and Fujita [50], Nakano and Asada [51], Foster and Hess [52], Nakano and Asada [51], and Miyake and Asada [53] were employed to assay the activities of SOD (EC: 1.15.1.1), CAT (EC: 1.11.1.6), glutathione S-transferase (GST; EC: 2.5.1.18), APX (EC: 1.11.1.1), GR (EC: 1.6.4.2), DHAR (EC: 1.8.5.1), and MDHAR (EC: 1.6.5.4) in Unit mg−1 protein. Ascorbate (AsA) was determined in the tissue of upper fully expanded fresh leaves according to Huang et al. [54]. Determination of GSH pool was performed by applying the Yu et al. [55] method with a minor modification [56] with using a known concentration of both GSH and GSSG as standard curves.
2.10. Glyoxalase System
To determine the glyoxalase I (Gly I; EC: 4.4.1.5) and glyoxylase II (Gly II; EC: 3.1.2.6), the Hasanuzzaman and Fujita [50] method was applied using fresh top leaves free of midribs from five plants selected randomly. For Gly I, the mixture of assaying consisted of a buffer, pH 7.0 (100 mM K-phosphate), MgSO4 (15 mM), GSH (1.7 mM), and methylglyoxal (3.5 mM) in 900 μL as a final volume. After addition of methylglyoxal, the changes occurred in the absorbance were observed at 240 nm. For Gly II, a reaction mixture (1.5 mL) consisted of a buffer, pH 7.2 (100 mM Tris‒HCl), 200 µM of DTNB, and 1 mM of S-D-lactoylglutathione was applied, and the extinction coefficients 3.37 mM−1 cm−1 and 13.6 mM−1 cm−1 were used to calculate the Gly I and Gly II, respectively.
2.11. Proline, Glycinebetaine (GB), and Soluble Sugars (S. Sugars)
Using fresh top leaves free of midribs from five plants selected randomly and toluene, the extraction of proline was practiced. At 520 nm, the absorbance was recorded [57]. Leaf content (μg proline g−1 FW) of proline was calculated using a suitable standard curve. The Grieve and Grattan [58] method was applied to estimate GB. The formed periodide crystals were monitored colorimetrically at 365 nm after reacting the mixture with cold KI‒I2 (a reagent) under acidic condition. The method of Irigoyen et al. [59] was utilized for extracting (using ethanol, 96%) and determining the content of soluble sugars (S. sugars) in mg g−1 dry leafy mass (DW). The ethanolic extract (100 µL) was reacted with 150 mg of anthrone reagent (freshly prepared in 100 mL H2SO4, 72%), and the mixture was then boiled for 10 min in a water bath. Colorimetrically, the absorbance readings were recorded at 625 nm after cooling.
2.12. Polyamines (PAs) Content Determinations and Relative Expression of Biosynthetic Genes of PAs Relative Expression (Using qRT-PCR)
Chen et al. [36], Guo et al. [37], and Flores and Galston [38] procedures were used with the HPLC system and 0.5 g fresh leaves for extraction of PAs at 4 °C. After a centrifugation process (15,000× g, 30 min), the supernatant was subjected to detect PuT, SpM, and SpD. Retention times were compared with the peak areas (noticed on 254 nm using a 2487 dual UV-detector; Waters, Milford, MA, USA) obtained to identify and quantify the three PAs.
According to the manufacturer’s protocol, RNA isolation (using TRIsure; Bioline, London, UK), cDNA synthesis, and quantitative real-time analysis (qRT-PCR) were implemented with 0.1 g of fresh leaves. DNase I (Thermo Scientific, London, UK) was utilized to digest RNA samples, and then RNA was spectrophotometerically quantified. Using Agarose gel electrophoresis, the determination of overall purity and integrity of RNA was conducted. For the reverse-transcription of RNA to cDNA, 1 µg of total RNA was used with a kit of Sensifast first cDNA synthesis (Bioline, London, UK). Based on the NCBI, the designation of primers and the Genome Database of wheat were as follows: ADC (F: 5′-CAACGACTTTGTTAGCTTTGG-3′, R: 5′-CAGGCTTGGCTTTGGTAA-3′), ODC (F: 5′-GGCCACTTCTTCTAGGTTCA-3′, R: 5′-ACTCGGCGTCTTATATAGCG-3′), SAMDC (F: 5′-CGAGCTTGTGTTGCGTCAG-3′, R: 5′-ATACATTCGCTCACACTGGCA-3′), SPDS (F: 5′-CTGAGAGTATGTGGTTGCAT-3′, R: 5′-CATAGTGGACAGAACCCTTG-3′), SPMS (F: 5′-AGTAGAGAAGATTTTGTACCAGG-3′, R: 5′-GGACATTCCCATAGGTTGAAG-3′), DHS (F: 5′-TCACTCGGAGACATGCTGTT-3′, R: 5′-CAGCCTTATATCTTGTACAATGTCG-3′), and GAPDH (F: 5′-TTGCTCTGAACGACCATTTC-3′, R: 5′-GACACCATCCACATTTATTCTTC-3′).
Using diluted cDNA samples, the qPCR was implemented in a 20 mL reaction mixture (10 mL of SensiFast SYBR Lo-Rox 2X mix (Bioline, London, UK) with 1.2 mL (300 nM) of each primer). The PCR was implemented by using a STRATAGENE MxPro-3000P (Agilent Technologies). Calculation of relative expression was done by using the method of 2-DDCt, where the level of mRNA relative expression was normalized versus the internal standard gene (GAPDH) and was then compared with control.
2.13. Zeatin-Type Cytokinin
Fresh leaf blade was frozen in liquid N, grounded, and extracted for trans-zeatin-type cytokinin; t-Z and cis-zeatin-type cytokinin; c-Z. The analysis was performed according to the Novák et al. [60] and Forcat et al. [61] procedures.
2.14. Experimental Design and Statistical Analysis
The experiment was repeated three times and carried out as a factorial completely randomized design with three soaking treatments (distilled water, 2% MGE, or 2% MGEPA) and three irrigation levels (90% as a control, 60%, or 30% of SRWC) in 15 replications (pots). Data are means (± standard error; SE). Testing for homogeneity of error variances was conducted according to the procedure outlined by Gomez and Gomez [62]. Combined analysis of data of the three experiments was conducted, and statistical analysis of all data was performed by using Statistica (version 9, Tulsa, OK, USA), subjected to two-way ANOVA, and differences among treatment means were affirmed statistically by using the Fisher LSD test at p ≤ 0.05.
3. Results
3.1. Growth, Yield, Leaf Photosynthetic Pigments, and Photosynthetic Efficiency
The treatment Ir60% (moderate drought) or Ir30% (severe drought) markedly decreased wheat plant growth (shoot fresh weight and shoot dry weight), yield (grain yield and 1000-grain weight), leaf pigments (total chlorophylls and total carotenoids), and photosynthetic efficiency (PSII efficiency; Fv/Fm, PSII performance index, and hill reaction activity; HRA) compared to Ir90% (optimal irrigation) (Figure 1 and Figure 2). Severe effect was obtained with Ir30%, which did not give yield because the plants did not survive. However, under Ir90% or Ir60%, seed soaking in 2% MGE or MGEPA significantly increased plant growth, yield, leaf pigments, and photosynthetic efficiency compared to the corresponding controls. MGEPA was more efficient than MGE, and the tested parameters responded better to MGE or MGEPA under Ir60% than Ir90%. On the other hand, under Ir30%, either MGE or MGEPA failed to increase the parameters mentioned above compared to the corresponding control. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all tested attributes (Figure 1 and Figure 2).
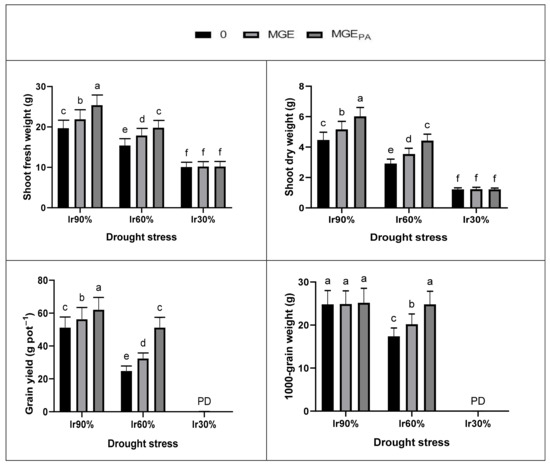
Figure 1.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on growth and yield components of drought-stressed wheat plants.
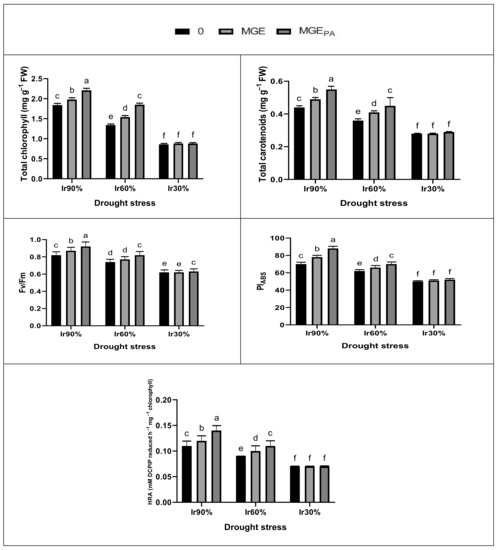
Figure 2.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on leaf photosynthetic pigments, and chlorophyll fluorescence of drought-stressed wheat plants.
3.2. Gas Exchange Parameters and Plant Leaf Tissue Health
Application of moderate or severe drought noticeably decreased gas exchange parameters (Pn, A, gs, and E), MSI, and RWC of wheat plants compared to optimal irrigation (Table 2). Ir30% treatment caused higher reductions than Ir60%. However, under Ir90% or Ir60%, seed soaking with MGE or MGEPA significantly increased gas exchange parameters, while MSI and RWC were only increased under Ir60% compared to the corresponding control. MGEPA was more efficient than MGE, and the tested parameters responded better to MGE or MGEPA under Ir60% than Ir90%. In contrast, either MGE or MGEPA failed to increase the tested parameters under Ir30% compared to the corresponding control. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all tested attributes (Table 2).

Table 2.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on gas exchange parameters of drought-stressed wheat plants.
3.3. Oxidative Stress Levels, Lipid Peroxidation, and Electrolyte Leakage (EL)
Compared to Ir90%, Ir30% treatment noticeably exceeded Ir60% in increasing oxidative stress biomarkers (O2•− and H2O2), lipid peroxidation (assessed as malondialdehyde; MDA accumulation), and EL of wheat plants (Table 3). The tested parameters did not respond to seed soaking in MGE or MGEPA under Ir90% or Ir30% treatment. However, under Ir60%, MGEPA significantly exceeded MGE in reducing O2•−, H2O2, MDA, and EL levels compared to the corresponding control. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all tested parameters (Table 3).

Table 3.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on oxidative stress levels (superoxide; O2•− and hydrogen peroxide; H2O2), malondialdehyde (MDA) accumulation, electrolyte leakage (EL), membrane stability index (MSI), and relative water content (RWC) of drought-stressed wheat plants.
3.4. Activities of Antioxidant Enzymes and Ascorbate–Glutathione (AsA–GSH) Cycle
Ir30% treatment markedly decreased the activities of antioxidant enzymes and AsA–GSH cycle (SOD, CAT, GST, AsA, APX, GR, MDHAR, DHAR, GSH, and GSSG), while Ir60% treatment considerably increased the activities of these antioxidant enzymes and AsA–GSH cycle, except for the activities of AsA, MDHAR, and DHAR activities, which were further decreased in wheat plants compared to Ir90% (Table 4 and Table 5). These tested activities of antioxidant enzymes and AsA–GSH cycle did not respond to seed soaking in MGE or MGEPA under Ir90% or Ir30% treatment. However, under Ir60%, MGEPA significantly exceeded MGE, both of which significantly elevated the activities of antioxidant enzymes and AsA–GSH cycle compared to the corresponding control. The interaction (drought × MGE) was significant (P ≤ 0.05 or 0.01) for all tested parameters (Table 4 and Table 5).

Table 4.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on the activities of antioxidant enzymes of drought-stressed wheat plants.

Table 5.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on the activity of ascorbate–glutathione cycle of drought-stressed wheat plants.
3.5. Glyoxalase System (Gly I and Gly II), Osmoprotectants, and Zeatins
Compared with Ir90%, Ir60% noticeably increased Gly I activity, and osmoprotectants (proline, GB, and S. sugars), t-Z and c-Z contents, except for Gly II activity, which was decreased, while Ir30% markedly decreased all parameters of wheat plants (Table 6). The parameters mentioned above did not respond to seed soaking in MGE or MGEPA under Ir90% or Ir30% treatment, except for trans-zeatin and cis-zeatin contents, which were markedly increased by pretreatment with MGE or MGEPA under Ir90%, with higher efficacy of MGEPA than MGE. However, under Ir60%, MGEPA significantly exceeded MGE in increasing the activities and contents of all parameters mentioned above compared to the corresponding controls. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all tested parameters (Table 6).

Table 6.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on glyoxalase system (Gly I and Gly II), osmoprotectants (e.g., proline, glycine betaine; GB, and soluble sugars; S. sugars), trans- and cis-zeatin accumulation in drought-stressed wheat plants.
3.6. Accumulation of Polyamines (PAs)
The accumulation of PAs (PUT, SPD, and SPM) in wheat plants was markedly raised under Ir60% treatment, while it reduced considerably under Ir30% treatment compared to Ir90% treatment (Table 7). Under optimal irrigation, PAs accumulation was significantly increased with seed soaking in MGE or MGEPA, with higher efficacy of MGEPA than MGE compared to the corresponding control. MGE or MGEPA failed to increase PAs accumulation under Ir30%. However, under Ir60%, MGEPA significantly exceeded MGE in increasing accumulation of all PAs compared to the corresponding control. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all PAs (Table 7).

Table 7.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on polyamines; PAs (putrescine; PuT, spermidine; SpD, and spermine; SpM) contents (nmol g−1 FW) of drought-stressed wheat plants.
3.7. Expression of Biosynthesis Genes of Polyamines (PAs)
Ir60% treatment up-regulated and increased the transcription of all biosynthesis genes of PAs (ADC, SPDS, SPMS, SAMDC, and DHS), but it did not affect ODC gene transcription compared to Ir30% treatment, which did not affect gene transcription (Table 8). Seed soaking in MGE or MGEPA did not affect transcription of all genes mentioned above under Ir30%. However, under either Ir60% or Ir90%, all gene transcripts were up-regulated and increased with MGE or MGEPA compared to the corresponding control. Gene transcriptional responses were more efficient under Ir60% than Ir90%, with higher efficacy for MGEPA than MGE. The interaction (drought × MGE) was significant (p ≤ 0.05 or 0.01) for all tested biosynthesis genes of PAs (Table 8).

Table 8.
Positive seed priming impacts using maize grain extract without (MGE, 2%) or with polyamines enrichment (MGEPA, 2%) on relative expression of PAs biosynthetic genes (by qPCR) of drought-stressed wheat plants.
4. Discussion
No information is available on soaking wheat seeds in maize grain extract enriched with anti-stress stimulants such as polyamines [PAs (MGEPA)] to alleviate the adverse impacts of drought stress. In this study, improvements in wheat plant growth and yield under the effects of drought stress were obtained through improvements in physiological and biochemical attributes due to seed priming in MGE or MGEPA with the advantage of MGEPA. Some articles reported on significant changes in antioxidant defense system including enzymatic and non-enzymatic antioxidants after exposing different plants to drought stress [7,8,16,63] In the current study, this finding corresponds to the measured up-regulation of the ascorbate–glutathione (AsA-GSH) cycle, glyoxalase system, trans- and cis-zeatin, and PAs and their biosynthetic genes under drought stress in response to soaking wheat seeds in MGE or MGEPA. In this regard, the results acquired with MGEPA exceeded those gained with MGE due to enrichment of MGEPA with PAs that increased the efficiency of the extract. Nevertheless, previous studies indicated the importance of MGE (the extract without enrichment with PAs) in mitigating the adverse effects of some stresses (salinity, cadmium, sandy state, and salinity+drought) in some crop plants [22,24,25,26,27], but this study reported a higher benefit of MGEPA than MGE.
In this study, drought stress in terms of deficit irrigation water (DIw) was detrimental to the growth and yield of wheat plants. Damage to wheat growth and yield due to Ir30% (severe drought; 30% of soil relative water content; SRWC) was more severe than that from Ir60% (moderate drought; 60% of SRWC) (Figure 1 and Figure 2, Table 9). The plants did not survive under severe drought even with pretreatment with both extracts (plants died during the flowering stage; Figure 1). This negative finding can be attributed to the drought affecting plant physiology and biochemistry (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). In this study, the decreased growth and yield of drought-stressed wheat plants associated with the overproduction of reactive species of oxygen (ROS), especially H2O2 and O2•− (Table 2, Table 3 and Table 9). This finding is consistent with that obtained by Noctor et al. [64] and Wang et al. [65]. The plant’s first response to drought is closed leaf stomata to reduce loss of water through transpiration (Table 2, Table 3 and Table 9). As a result of continuous photosynthesis in light, the depletion of intercellular CO2 concentration is facilitated by an increased gas diffusion barrier. Therefore, the low CO2 availability catalyzes the oxygenation of ribulose-1,5-bisphosphate, thereby increasing the production of photorespiratory H2O2 in cell peroxisomes [64]. Drought treatments negatively affected leaf photosynthetic pigments, photosynthetic efficiency, gas exchange parameters, cell membranes (increase in lipid peroxidation; MDA and ionic leakage; EL, and decrease in stability index), RWC, zeatins, osmoprotectant and PAs contents, antioxidant enzyme, AsA-GSH cycle and glyoxalase system activities, and transcription of PAs biosynthetic genes (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). Drought-stimulated oxidative stress (increased production of H2O2 and O2•−; Table 2, Table 3 and Table 9) caused these negative findings. Over Ir60%, Ir30% caused exacerbation of MDA and EL and severely affected PSII function efficiency (Figure 2 and Table 9), causing stomatal closure (Table 2 and Table 9), which disrupted the plant antioxidant system (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9), and eventually, the plant did not survive.

Table 9.
Changes (%) in growth, yield, and physio-biochemical parameters relative to the control (Ir90% + 0% MGE) in wheat plants under drought and maize grain extract applications. Three color scale heatmap, yellow as the midpoint of control and parameters with insignificant values compared to control, red for changes below control values, and green for changes over control values.
However, the advantage of seed soaking in both extracts (MGE or MGEPA) was reported under moderate drought (Ir60%). The higher benefit of MGEPA over MGE can be attributed to the additional benefits of PAs granted through pretreatment with MGEPA, where PAs play important roles in mitigating the damage caused by different stresses on crop plants [29,66,67,68]. Yildiztugay et al. [69] reported a relationship between the plant’s biomass (growth) and its water content. Similarly, our results displayed this relationship (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). Despite the benefit of pretreatment with MGE, MGEPA enabled wheat plants to function normally under Ir60% and revealed growth (biomass) and leaf relative water content (RWC), and thus yield similar to that gained by the optimum irrigated plants (Ir90%; control). Wheat plants pretreated with MGEPA and subjected to Ir60% had leaf photosynthetic pigments, photosynthesis efficiency (Fv/Fm, PI, and hill reaction activity; HRA), and gas exchange parameters identical to those obtained with well-watered plants (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). The damage caused by Ir60% was repaired with MGEPA. During the soaking process, the seeds can absorb the bioactive ingredients from the extract such as antioxidants (AsA and GSH) and hormones (salicylic acid and cytokinins (CKs), especially t-Z and c-Z), in addition to the extract PAs (Table 1). These bioactive ingredients enrich the seeds and accelerate metabolism, giving them robust germination and strong seedlings to withstand drought stress.
Under Ir60%, MGEPA preserved leaf photosynthetic pigments and photosynthetic efficiency along with gas exchange parameters, which contributed to the maintenance of wheat yield (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). In this regard, Farooq et al. [16] reported a positive correlation between chlorophyll contents and grain yield in wheat plants. Moreover, the maintenance of PSII function (HRA), antioxidant system, and biosynthesis genes of PAs (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9) along with the beneficial impacts of the bioactive stimulants present in MGEPA (Table 1) contributed well to the plant’s performance under drought stress. Under Ir60%, some antioxidant compounds supported MGEPA-pretreated plants to cause cell membrane stabilization by suppressing H2O2 and O2•− levels, thus reducing MDA and EL levels (Table 3 and Table 9). In this study, as drought stress caused a reduction in chlorophyll content, it caused leaf yellowing and senescence (data not shown). This finding can be attributed to a degradation of chlorophyll and a dysregulation of the photosynthetic apparatus, including decreased HRA, and thus a reduction in wheat yield. Nevertheless, MGEPA enabled wheat plants to stay green by maintaining the chlorophyll content for as long as possible, thus producing better under drought stress (Figure 2, Table 9) due to the bioactive stimulants present in MGEPA. This finding is consistent with that obtained by [22,24,25,26,27] under different abiotic stress conditions. Drought-stressed wheat plant becomes better performer (in terms of growth and yield characteristics) when pretreated with MGE, which has a high ROS-scavenging activity (88.6 ± 1.6%) due to the high contents of different antioxidants, plant hormones, and PAs present in MGE (Table 1). The performance of stressed plant becomes higher with MGEPA due to the PAs added to MGE. Ghassemi et al. [70] reported that PAs improved root growth and development and adjusted cellular water potential after emergence, leading to increased nutrient uptake and translocation in drought-stressed plants. In addition, the enhanced translocation of assimilates, which are photosynthesized in plant leaves, to the edible portion (the grains) is attributed to the longer duration of photosynthesis due to the green leaf survival merit, resulting in a longer period of grain filling under stress [16]. This finding is in line with the finding of this study, which is likely due to the ability of MGEPA-pretreated wheat plants to stimulate the activity of the antioxidant system to withstand DIw stress (Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9) and enable the plants to perform better with regard to the meristematic tissue activities, including the stimulation of cell division and expansion due to maintaining adequate water (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). As a result, it becomes possible to obtain a satisfactory improvement in the growth of drought-stressed plants due to the richness of MGE, especially after its enrichment with PAs, in phytohormones, auxins, CKs including zeatins, gibberellins (GAs), and PAs (Table 1). Therefore, hormonal homeostasis could be induced with MGEPA as a potential mechanism for boosting DIw stress tolerance in plants. This hormonal homeostasis mechanism could function through a complex cross-talk among auxins, CKs and GAs, as well as among plant hormones, PAs, and other biostimulants as an anti-stress network in favor of the plant’s response to DIw stress.
In this study, under low availability of water, MGEPA pretreatment replaced the detrimental effects of DIw to marked increases in wheat growth and yield due to the increase in gas exchange parameters, photosynthetic efficiency including HRA, and the biosynthesis of chlorophyll catalyzed by the biostimulants present in MGEPA, which contributed to elimination of the biomarkers of oxidative stress (H2O2 and O2•−) and preservation of cell membranes and cell water content due to reduced levels of EL and MDA accumulation (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). Different stresses cause a detrimental effect on EL and membrane lipids due to overproduced ROS, which disrupt chlorophyll biosynthesis due to disruption of the pigment protein complex functioning, de novo protein biosynthesis, and chlorophyll-associated components [8,16,71,72]. However, MGEPA bioactive promoters eliminated these harmful effects and mediated improvement in chlorophyll biosynthesis (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). MGEPA pretreatment can stimulate the accumulation of different plant hormones in germinated seeds and thus seedlings, especially CKs, to improve the biosynthesis of chlorophylls as shown in Cortleven et al. [73]. In this study, MGEPA pretreatment improved photosynthesis efficiency and PSII activity (Fv/Fm, PI, and HRA), which could be attributed to suppressing the formation of ROS (1O2, H2O2, and O2•‒), thus protecting the chloroplast structure from DIw-induced oxidative stress damage (Figure 1 and Figure 2, Table 2, Table 3 and Table 9). In this regard, Tikkanen et al. [74] attributed a similar result to the safeguarded light reaction functioning in photosynthesis process, which is functionally synchronized with PSI and PSII enzymes. The functioning of both photosystems is connected to the generation of trans-thylakoid proton, which creates a pH difference for regulating the transfer of electrons to PSI under direct monitoring of the PGR5 protein. As a result of its richness in bioactive stimuli, especially plant hormones, MGEPA can enhance the biosynthesis of polysaccharides and proteins implicated in photosynthesis regulation, in addition to mediating the synthesis of components of redox that may have contributed to photo-protection. In addition, MGEPA can increase carotenoids synthesis that can protect the photosynthesis system from the overproduction of ROS by up-regulating pigment-synthesizing enzyme activities associated with reducing the degradation of enzymes [25,26,75]. This result is consistent with Aldesuquy [76], who reported a positive synergistic impact of plant hormones on pigment and fine chloroplast structure in the flag leaf of stressed wheat plant.
As previously obtained in some works [22,24,25,26,27], reduced levels of H2O2 and O2•− and thus reduced lipid peroxidation and EL were obtained with the MGEPA-pretreated plant under DIw stress (Table 3 and Table 9). The MGE-mediated enhanced membrane integrity (due to reduced membrane EL and MDA) could be attributed to the bioactive components in present MGEPA (Table 1), which contributed to the maintenance of the components of the antioxidant system, in addition to the low peroxidation levels, which were considerably influenced by DIw stress. MGEPA pretreatment considerably enhanced the activity of antioxidant enzymes and raised the contents of AsA and glutathione forms (e.g., GSH and GSSG), thus protecting the wheat plants from the oxidative stress (H2O2 and O2•−) stimulated by DIw. Similar results are previously gained [22,24,25,26,27] under different stresses. Integrally, after dismutating O2•− to H2O2 by SOD, APX and CAT convert H2O2 to H2O and O2. This mechanism reduces the formation of hydroxyl; OH‒ radicals [77]. Since MGEPA is a rich source of bioactive stimulants, it may stimulate SOD up-regulation to further dismutate O2•− to H2O2. Plants pretreated with MGEPA possessed stimulated AsA and GSH accumulations, which could protect stressed plants from ROS damage stimulated by DIw. All antioxidants (GR, APX, MDHAR, DHAR, GSH, and AsA) are components of the ROS-scavenging pathway (the AsA-GSH cycle), which can be stimulated and up-regulated by MGEPA to elevate tolerance strategies against any potential damage from oxidative stress in the wheat plant (Table 6, Table 7, Table 8 and Table 9). This robust enhancement in the antioxidant system in the DIw-stressed wheat plants pretreated with MGEPA resulted in a marked decrease in ROS accumulation (e.g., H2O2 and O2•−) through the AsA‒GSH cycle and thus increased the protection of pathways of photosynthesis, resulting in better performance (e.g., growth and yield productivity) of wheat plants (Table 6, Table 7, Table 8 and Table 9). The AsA‒GSH cycle includes a sequence of redox reactions, which includes bioactive participation of NADPH, GSH, and AsA [78]. Through this cycle, H2O2 molecules in cell cytosol and chloroplasts are scavenged by APX and CAT, thus preventing H2O2 diffusion to other organelles to avoid any damage. The improved functionality of the ASA–GSH cycle pathway due to pretreatment with MGEPA effectively maintained redox components, including GSH and AsA. These redox components reduced the effects of oxidative stress (H2O2 and O2•−) stimulated by DIw. In this study, the increased antioxidant system activity was accompanied by an enhanced tolerance to DIw stress in the wheat plant. Similar results were gained by [16,25,79]. DHAR and MDHAR activities, which may be up-regulated after pretreatment with MGEPA, improved AsA and GSH to integrate the functions of other enzymes such as GR and APX with non-enzymatic antioxidant components. As a result, a comprehensive impact occurred on H2O2 neutralization and the availability of NADP to effectively protect the electron transport chain [80]. In this study, pretreatment with MGEPA bioactive stimulants protected the electron transport chain implicated in photosynthesis, which may be attributed to the up-regulation of the NADP+/NADPH ratio. This mechanism prevents the flow of electrons into O2 to restrict O2•− production [81]. This study provides a preventive role of MGEPA, given its richness in bioactive stimuli including plant hormones, for the photosynthesis system through the up-regulation of the antioxidant system components.
High levels of GSH can contribute significantly to MGEPA-pretreated wheat plants to maintain the glycoxylase system for methylglyoxal elimination, which may reduce the chances of any dangerous genotoxic influence [82]. Glyoxylase I and II (Gly I and Gly II) constitute the main enzymatic components of the ‘glyoxylase system’. MGEPA bioactive stimuli-stimulated up-regulation in the enzymatic activities of glyoxylase system could be associated with increased levels of GSH. This may have exploited the improved influences of methylglyoxal such as crosstalk with important signalling molecules such as Ca2+, ABA, and ROS [83]. This is the first report conducted with MGEPA for DIw-stressed wheat plants, in which the MGEPA bioactive stimuli can optimize methylglyoxal. Gly I utilizes GSH as a co-factor to convert the methylglyoxal to S-D-lactoylglutathione. In addition, Gly II produces GSH to contribute to redox homeostasis and protection against toxic species [84]. It can be concluded that improvement in endogenous GSH by pretreatment using MGEPA bioactive stimuli and the functioning of methylglyoxal scavenging system could enhance stress tolerance in wheat plants. Ahanger et al. [85] explained up-regulation and the improvement of glyoxylase system activity due to exogenous treatment with kinetin. This result is consistent with our results through the application of CKs-containing MGEPA, which improved activity of glyoxylase system (Table 6 and Table 9). This enhanced glycosylase system activity may protect the system of electron transport by inhibiting any damage to chloroplasts and mitochondrial ultra-structures [82].
In this study, DIw encouraged osmoprotectant accumulations (e.g., GB, S. sugars, and proline), and these accumulations were further enhanced due to pretreatment with MGEPA bioactive stimulants. This accumulation of osmoprotectants may occur due to their absorption from MGEPA and/or their increased biosynthesis catalyzed by MGEPA in soaked seeds then seedlings. These increased osmoprotectants may give the seeds the driving force for strong germination and thus vigorous seedling growth under stress. Improvements of these osmolytes may be effective mechanisms to increase plant tolerance to DIw stress to maintain adequate cell water for healthy metabolism (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). The up-regulation of proline-synthesizing enzymes along with the down-regulation of proline-catabolizing enzymes and/or low incorporation into proteins leads to proline accumulation [86,87]. The improvement in GB, S. sugars, and proline accumulations as a result of MGEPA pretreatment resulted in the maintenance of water balance in DIw-stressed plants to help them withstand DIw stress and avoid increased EL and MDA in plant tissues for healthy metabolic processes, protection of protein turnover, enzymatic activities, and expression of stress protective proteins. These results indicate the protective role of MGEPA for healthy metabolic pathways and effective osmoregulation. Similar results were obtained by Ahanger and Agarwal [85] and Thakur and Sharma [88].
Bypassing MGE, MGEPA attenuated DIw stress and helped the photosynthetic system to perform efficiently (Figure 2, Table 9) in favor of plant metabolism [27], resulting in a lot of savings including hormonal contents (e.g., CKs such as t-Z and c-Z) in DIw-stressed plants (Table 6 and Table 9). Therefore, zeatins play a fundamental role in wheat plant response to DIw stress. Mediating plant response to stress, specifically CKs and their signaling components, predominantly regulate plant defense reactions [89]. T-Z and c-Z have been reported to modulate the wheat plant’s defense response to stress through several mechanisms including defense genes and hormonal regulation [26]. This result confirms the results of this study with t-Z and c-Z-containing MGEPA (Table 6, Table 7, Table 8 and Table 9). This study reported significant increases in trans-zeatin and cis-zeatin contents with MGEPA pretreatment and supported wheat plant’s tolerance to DIw stress (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). Previous reports indicated increased t-Z activity compared to cis-zeatin, which was demonstrated in this study with a higher trans-zeatin content compared to c-Z, which may lead to an increase in the physiological role of t-Z to stimulate a higher increase in PAs contents and their gene expressions under DIw stress due to pretreatment of the plant with MGEPA (Table 6, Table 7, Table 8 and Table 9). This finding may be attributed to the transport, degradation, and conjugation processes of these hormones [90,91].
In this study, the MGEPA-conferred increase in PAs contents and their gene expressions contributed to distinct defense mechanisms, which improved the tolerance of the wheat plant to DIw stress (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). The integrated defense mechanisms of antioxidant and osmoregulation systems along with hormonal content and gene expressions of PAs, regulated by MGEPA not only evaluate efficacy in limiting the effects of hazardous stress but also modulate physiological status to terminate the trade-off of plant integrity related to defense responses (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). Therefore, wheat plants could survive better with MGEPA pretreatment in areas with a shortage of water such as dry ones. MGEPA pretreatment stimulated more accumulation in PAs (PUT, SPD, and SPM) that act as signaling molecules to focus primarily on metabolism in relation to conservation against DIw stress in wheat plants (Table 7 and Table 9). This finding, together with other cumulative zeatins and antioxidants (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9), is likely due to the attenuation of the adverse effects of DIw stress through its powerful antioxidant roles [29,67] and their gene expressions [26]. Cumulative levels of endogenous PAs under DIw stress conditions have been linked to effective up-regulation of SPDS, ADC, DHS, SPMS, and SAMDC gene expression but not to the ODC gene (Table 8 and Table 9). Similar results were reported by Ebeed et al. [67] and Alharby et al. [26]. The results of this study indicate that PAs, PUT, SPD, and SPM were synthesized under DIw stress through the SPDS, ADC, DHS, SPMS, and SAMDC pathways (and not from ODC) in wheat plants. Various enzymes were involved in the pathway from PUT to SPM and SPD, including SAMDC, SPDS, and SPMS gene expression. The endogenous levels of SPD, SPM, and PUT that accumulated by MGEPA in DIw-stressed wheat plants were connected to the up-regulated gene expression levels of the ADC, SPDS, SPMS, SAMDC, and DHS genes, giving wheat plants a robust antioxidant defense to withstand DIw stress (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9).
MGEPA efficiently outperformed MGE in promoting wheat plant growth, grain yield, physio-biochemistry, and antioxidant defense system components (Figure 1 and Figure 2, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9). These promoted results can be attributed to MGEPA’s further enrichment of PAs along with its high antioxidants and phytohormone contents, as well as its higher antioxidant activity (88.6%) (Table 1). This makes MGEPA possess pivotal mechanisms for inhibiting or at least minimizing the dangerous impacts of oxidative stress, cell membrane lipid peroxidation, and ionic leakage [25,26]. Therefore, MGEPA, an eco-friendly strategy, is a powerful natural extract that contains many antioxidants and biostimulants for plant growth under stress.
5. Conclusions
Drought stress led to a severe decrease in wheat plant growth and yield due to stress negative effects such as decreased chlorophyll content, photosynthetic efficiency, water content, and gas exchange, in addition to PAs gene expression, and increased membrane lipid peroxidation and electrolyte leakage due to increased oxidative stress. These negative effects can be attenuated by using a plant extract such as biostimulants-rich MGE enriched with PAs (MGEPA) as a seed soaking strategy. MGEPA (2%) pretreatment promoted wheat plant growth and yield by improving photosynthetic efficiency, antioxidant and osmoregulation systems, and PAs gene expression. This was due to reduced oxidative stress damage by MGEPA through improved enzymatic and non-enzymatic antioxidant activities and up-regulation of the ascorbate–glutathione cycle and the glyoxalase system. All these positive results were reflected in the decrease in memebrane lipid peroxidation and the leakage of electrolytes under drought stress. This study predicts that plant extracts such as biostimulants-rich MGE enriched with PAs possess signaling networks that interfere with many physiological and biochemical pathways to develop DIw-tolerant crops for effective sustainable agriculture.
Author Contributions
H.F.A., K.R.H. and M.M.R. visualized and designed experiments, H.F.A., M.M.R. and Y.M.A. performed experiments, H.S.A.-Z., M.M.R. and H.F.A. analyzed data, H.A. and M.M.R. contributed to reagents/materials/analysis tools, H.F.A., H.S.A.-Z., K.R.H., Y.M.A. and H.A. wrote the manuscript, M.M.R. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (RG-1-130-40). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
MGE; Maize grain extract, MGEPA; Maize grain extract enriched with polyamines, O2•−; Superoxide, H2O2; Hydrogen peroxide, DIw; Deficit irrigation water, CO2; Carbon dioxide, ROS; reactive oxygen species, DNA; deoxyribonucleic acid, RNA; Ribonucleic acid, SOD; Superoxide dismutase, POD; Peroxidase, CAT; Catalase, GR; Glutathione reductase, APX; Ascorbate peroxidase, GST; Glutathione S-transferase, MDHAR; Monodehydroascorbate reductase, DHAR; Dehydroascorbate reductase, GSH; Reduced glutathione, GSSG; Oxidized glutathione, AsA; Ascorbate, OH; Hydroxyl, PAs; Polyamines, mM; Millimole, μM; Micromole, DAS; Days after sowing, SRWC; Soil relative water content, DPPH; 1,1-diphenyl-2-picrylhydrazyl, PUT; Putrescine, SPM, Spermine, SPD, Spermidine, HPLC; High-performance liquid chromatography, GC/MS; Gas chromatography–mass spectrometry, FW; Fresh weight, DW; Dry weight, PSII; Photosystem II, PI; performance index, Fv/Fm; A normalized ratio created by dividing variable fluorescence (Fv) by maximum fluorescence (Fm), Pn; Net photosynthesis rate, A; CO2 assimilation rate, gs; Stomatal conductance, E; Transpiration rate, MDA; Malondialdehyde, PVP; Polyvinyl pyrrolidone, NBT; 4-Nitro blue tetrazolium chloride, EDTA; Ethylenediamine tetraacetic acid, HPO3; Metaphosphoric acid, Gly I; Glyoxalase I, Gly II; Glyoxalase II, GB; Glycinebetaine, S. sugars; Soluble sugars, H2SO4; Sulphuric acid, nm; Nanometer, qRT-PCR; Real-Time Quantitative Reverse Transcription, nM; Nanometer, GAPDH; Glyceraldehyde 3-phosphate dehydrogenase gene, ADC; Arginine decarboxylase gene, ODC; Ornithine decarboxylase gene, SAMDC; S-Adenosylmethionine decarboxylase proenzyme gene, SPDS; Spermidine synthase gene, SPMS, Spermine synthase gene, DHS; Deoxyhypusine synthase gene, GAPDH; Glyceraldehyde-3-phosphate dehydrogenase gene, t-Z; Trans-zeatin-type cytokinin, c-Z; Cis-zeatin-type cytokinin, ANOVA; Analysis of variance, Ir60%; Moderate drought, Ir30%; Severe drought, Ir90%; Optimum irrigation, HRA; Hill reaction activity, MSI; Membrane stability index, RWC; Relative water content, EL; Electrolyte leakage, CKs; Cytokinines, PGR5; Proton gradient regulation-5, AsA-GSH cycle; Ascorbate-glutathione cycle, and ABA; Abscisic acid.
References
- Kiliç, H.; Yağbasanlar, T. The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) cultivars. Not. Bot. Hort Agrobot. Cluj-Napoca 2010, 38, 164–170. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Taha, R.S.; Rady, M.M. Effect of summer-fall deficit irrigation on morpho-physiological, anatomical responses, fruit yield and water use efficiency of cucumber under salt affected soil. Sci. Hortic. 2018, 237, 148–155. [Google Scholar] [CrossRef]
- Merwad, A.M.A.; Desoky, E.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Desoky, E.M.; Mansour, E.; Yasin, M.A.T.; El-Sobky, E.E.A.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Spanish J. Agric. Res. 2020, 18, e0802. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous application improves drought tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Sanoubar, R.; Cellini, A.; Gianfranco, G.; Spinelli, F. Osmoprotectants and antioxidative enzymes as screening tools for salinity tolerance in radish (Raphanus sativus). Hortic. Plant J. 2020, 6, 14–24. [Google Scholar] [CrossRef]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Farooq, M.; Rizwan, M.; Nawaz, A.; Rehman, A.; Ahmad, R. Application of natural plant extracts improves the tolerance against combined terminal heat and drought stresses in bread wheat. J. Agron. Crop Sci. 2017, 203, 528–538. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.A.; Rady, M.M. Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Commun. Soil Sci. Plant Anal. 2018, 49, 967–983. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops—A review. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Silva, E.O.; Martins, S.J.; Alves, E. Essential oils for the control of bacterial speck in tomato crop. Afr. J. Agric. Res. 2014, 9, 2624–2629. [Google Scholar] [CrossRef]
- Martins, S.J.; Medeiros, F.H.V.; Lakshmanan, V.; Bais, H.P. Impact of seed exudates on growth and biofilm formation of Bacillus amyloliquefaciens ALB629 in common bean. Front. Microbiol. 2018, 8, 2631. [Google Scholar] [CrossRef]
- Martins, S.J.; Rocha, G.A.; Georg, R.C.; Ulhôa, C.J.; Cunha, M.G.; Rocha, M.R.; Araújo, L.G.; Vaz, K.S.; Dianese, E.C.; Oshiquiri, L.H.; et al. Plant-associated bacteria mitigate drought stress in soybean. Environ. Sci. Pollut. Res. 2018, 25, 13676–13686. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Presoaking application of propolis and maize grain extracts alleviates salinity stress in common bean (Phaseolus vulgaris L.). Sci. Hortic. 2014, 168, 210–217. [Google Scholar] [CrossRef]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Alharby, H.F.; Alzahrani, Y.M.; Rady, M.M. Seeds pretreatment with zeatins or maize grain-derived organic biostimulant improved hormonal contents, polyamine gene expression, and salinity and drought tolerance of wheat. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar]
- Rady, M.M.; Talaat, N.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Denby, K.; Gehring, C. Engineering drought and salinity tolerance in plants: Lessons from genome-wide expression profiling in Arabidopsis. Trends Biotechnol. 2005, 23, 547–552. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Sundstrom, F.J.; Reader, R.B.; Edwards, R.L. Effect of seed treatment and planting method on Tabasco pepper. J. Amer. Soc. Hortic. Sci. 1987, 112, 641–644. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil; College of Agriculture, Agricultural Experiment Station, University of California: Baltimore, MA, USA, 1950. [Google Scholar]
- Kampfenkel, K.; Van Montagu, M. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, J.H.; Jeong, S.M.; Kim, D.R.; Ha, J.U.; Nam, K.C. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agric. Food Chem. 2003, 51, 4400–4403. [Google Scholar] [CrossRef]
- Lavrich, R.J.; Hays, M.D. Validation studies of thermal extraction-GC/MS applied to source emissions aerosols. 1. Semivolatile analyte-nonvolatile matrix interactions. Anal. Chem. 2007, 79, 3635–3645. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Fang, J.; Lu, S. Physiological responses of a centipedegrass mutant to chilling stress. Agron. J. 2013, 105, 1814–1820. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [CrossRef]
- Flores, E.; Galston, A.W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982, 69, 701–706. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Bregman, A. Laboratory Investigations in Cell and Molecular Biology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Kubis, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Effect of humic acid as an additive to growing media to enhance the production of eggplant and tomato transplants. J. Hortic. Sci. Biotechnol. 2014, 89, 237–244. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.; Berardino, R.; de Pinto, M.C.; di Toppi, L.S.; Storelli, M.M.; Tommasi, F.; De Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Novák, O.; Hauserová, E.; Amakorová, P.; Doležal, K.; Strnad, M. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 2008, 69, 2214–2224. [Google Scholar] [CrossRef]
- Forcat, S.; Bennett, M.H.; Mansfield, J.W.; Grant, M.R. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 2008, 4, 16. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: Singapore, 1984; p. 680. [Google Scholar]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Zhang, L.-H.; Ma, J.; Li, X.-Y.; Li, Y.; Zhang, R.-P.; Wang, R.-Q. Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage. Agric. Sci. China 2010, 9, 633–641. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Taie, H.A.A.; El-Yazal, M.A.S.; Ahmed, S.M.A.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef]
- Yildiztugay, A.; Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Biochar triggers systemic tolerance against cobalt stress in wheat leaves through regulation of water status and antioxidant metabolism. J. Soil Sci. Plant Nutr. 2019, 19, 935–947. [Google Scholar] [CrossRef]
- Ghassemi, S.; Farhangi-Abriz, S.; Faegi-Analou, R.; Ghorbanpour, M.; Lajayer, B.A. Monitoring cell energy, physiological functions and grain yield in field-grown mung bean exposed to exogenously applied polyamines under drought stress. J. Soil Sci. Plant Nutr. 2018, 18, 1108–1125. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Zaki, S.S.; Rady, M.M. Moringa oleifera leaf extract improves growth, physio-chemical attributes, antioxidant defence system and yields of salt-stressed Phaseolus vulgaris L. plants. Int. J. Chem. Tech. Res. 2015, 8, 120–134. [Google Scholar]
- Cortleven, A.; Marg, I.; Yamburenko, M.V.; Schlicke, H.; Hill, K.; Grimm, B.; Schaller, G.E.; Schmülling, T. Cytokinin regulates the etioplast-chloroplast transition through the two-component signaling system and activation of chloroplast-related genes. Plant Physiol. 2016, 172, 464–478. [Google Scholar] [CrossRef]
- Tikkanen, M.; Rantala, S.; Aro, E.-M. Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 2015, 6, 521. [Google Scholar] [CrossRef]
- Behera, R.K.; Mishra, P.C.; Choudhury, N.K. High irradiance and water stress induce alterations in pigment composition and chloroplast activities of primary wheat leaves. J. Plant Physiol. 2002, 159, 967–973. [Google Scholar] [CrossRef]
- Aldesuquy, H. Synergistic effect of phytohormones on pigment and fine structure of chloroplasts in flag leaf of wheat plants irrigated by seawater. Egypt. J. Basic Appl. Sci. 2015, 2, 310–317. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. Growth, photosynthesis and oxidative responses of Solanum melongena L. seedlings to cadmium stress: Mechanism of toxicity amelioration by kinetin. Sci. Hortic. 2014, 176, 1–10. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Z.; Li, X.; Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 2012, 34, 2105–2114. [Google Scholar] [CrossRef]
- Gupta, B.K.; Sahoo, K.K.; Ghosh, A.; Tripathi, A.K.; Anwar, K.; Das, P.; Singh, A.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Environ. 2017, 41, 1186–1200. [Google Scholar] [CrossRef]
- Li, Z.G. Methylglyoxal and glyoxalase system in plants: Old players, new concepts. Bot. Rev. 2016, 82, 183–203. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Reddy, M.; Sopory, S.K. Methylglyoxal detoxification by glyoxalase system: A survival strategy during environmental stresses. Physiol. Mol. Biol. Plant 2005, 11, 1. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Ahmad, P. Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 2010, 56, 575–588. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.D. Salt-stress-induced proline accumulation in germinating embryos: Evidence suggesting a role of proline in seed germination. J. Arid Environ. 2005, 62, 517–523. [Google Scholar] [CrossRef]
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and Regulation of Cytokinins in Plant Response to Drought Stress—A review. Plants 2020, 9, 422. [Google Scholar] [CrossRef]
- Gajdosová, S.; Spichal, L.; Kaminek, M.; Hoyerová, K.; Novák, O.; Dobrev, P.I.; Galuszka, P.; Klıma, P.; Gaudinova, A.; Zizkova, E.; et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 2011, 62, 2827–2840. [Google Scholar] [CrossRef]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-Zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).