Evaluation of Physiological and Quality Parameters of Green Asparagus Spears Subjected to Three Treatments against the Decline Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Design, Treatments, and Plant Sampling
2.3. Yield
2.4. Determination of Malondialdehyde (MDA) Concentration
2.5. Determination of Photosynthetic Pigment Concentration
2.6. Determination of Reduced Glutathione (GSH) and Total Ascorbate (AsA) Concentrations
2.7. Determination of Total Phenol Concentration
2.8. Determination of Flavonoid Concentration
2.9. Determination of Anthocyanin Concentration
2.10. Determination of Antioxidant Tests: FRAP and TEAC
2.11. Amino Acid Profile Determination
2.12. Determination of Nutrient Concentration
2.13. Statistical Analysis
3. Results
3.1. Asparagus Yield
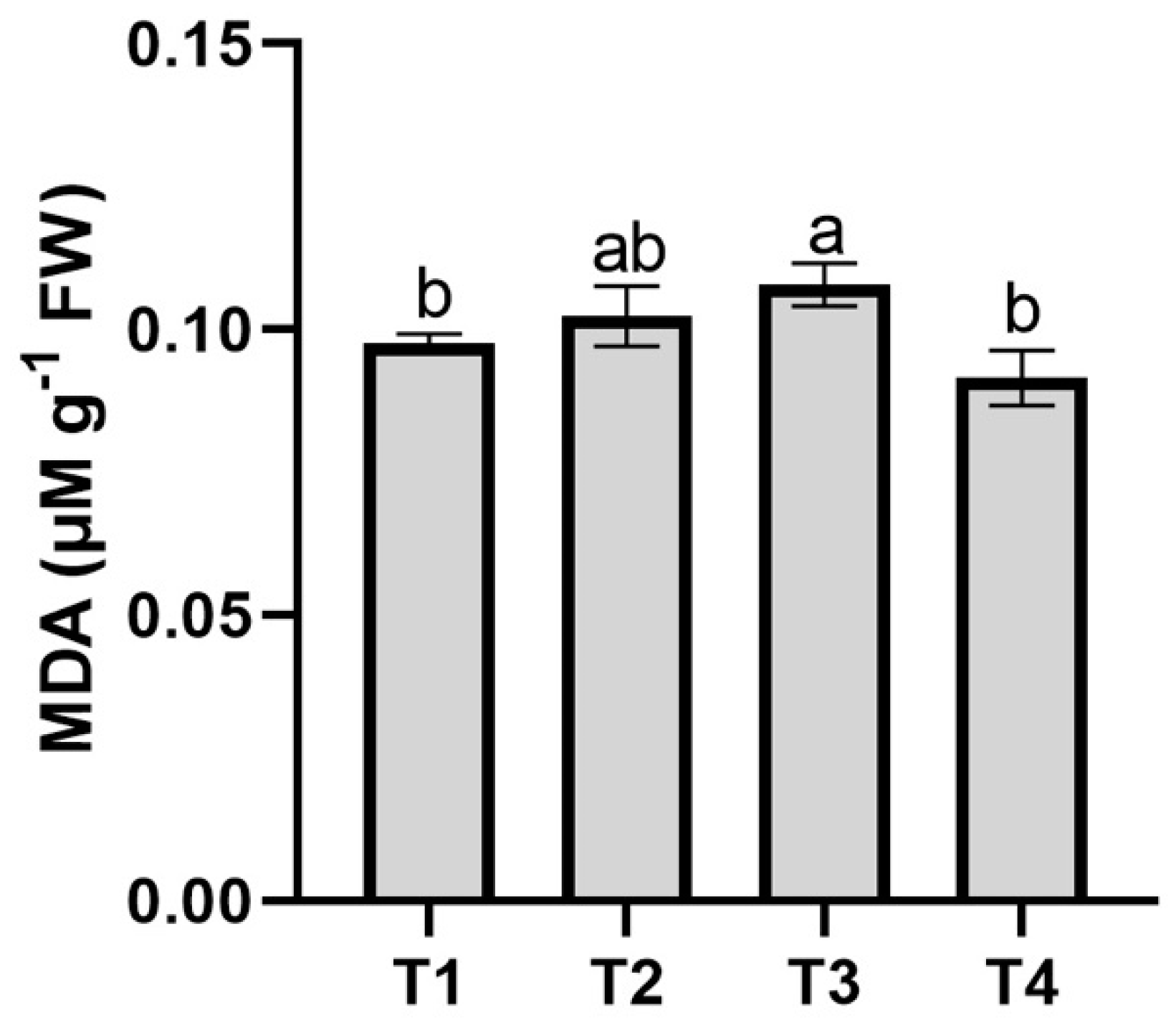
3.2. Oxidative Stress Indicator (MDA)
3.3. Photosynthetic Pigment Concentration
3.4. GSH and AsA Concentrations
3.5. Total Phenol, Flavonoid, and Anthocyanin Concentration
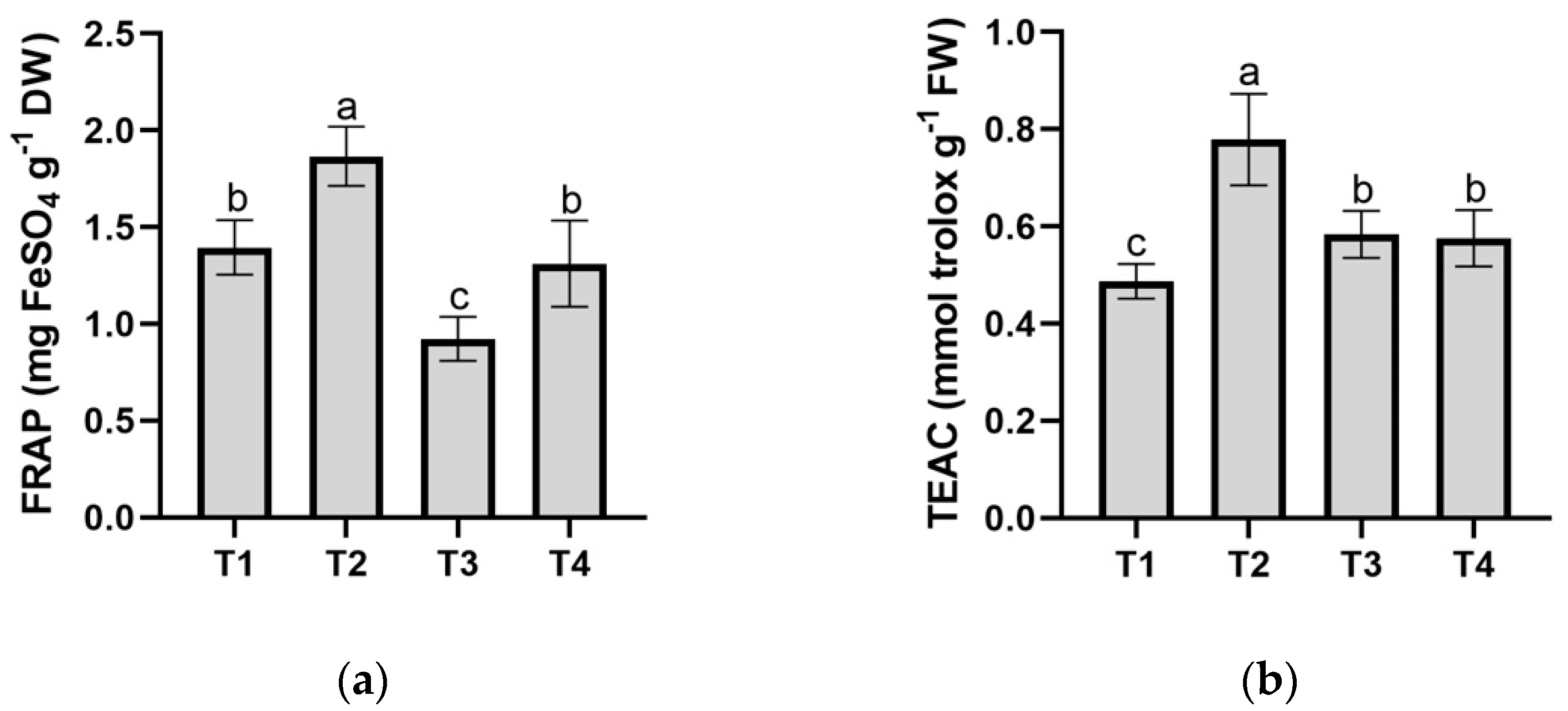
3.6. Antioxidant Test: FRAP and TEAC
3.7. Amino Acids Profile
3.8. Nutrient Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://fenix.fao.org/faostat/internal/es/#data/QC (accessed on 23 March 2021).
- Boletín Mensual de Estadística. Available online: https://www.mapa.gob.es/en/estadistica/temas/publicaciones/boletin-mensual/default.aspx (accessed on 23 March 2021).
- Wang, J.; Liu, Y.; Zhao, J.; Zhang, W.; Pang, X. Saponins Extracted from By-Product of Asparagus Officinalis L. Suppress Tumour Cell Migration and Invasion through Targeting Rho GTPase Signalling Pathway. J. Sci. Food Agric. 2013, 93, 1492–1498. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; López-Moreno, F.J.; Navarro-León, E.; Sánchez, E.; Blasco, B.; Moreno, D.A.; Soriano, T.; Ruiz, J.M. Effects of Asparagus Decline on Nutrients and Phenolic Compounds, Spear Quality, and Allelopathy. Sci. Hortic. 2020, 261, 109029. [Google Scholar] [CrossRef]
- Summerell, B.A.; Leslie, J.F.; Backhouse, D.; Bryden, W.L.; Burgess, L.W. Fusarium: Paul E. Nelson Memorial Symposium. In Fusarium: Paul E. Nelson Memorial Symposium; APS Press: St. Paul, MN, USA, 2001. [Google Scholar]
- Motoki, S.; Ozawa, T.; Komatsu, K.; Tsukada, M.; Hattori, T.; Komura, T.; Oka, J. Allelopathy in Asparagus 1: Reduction of the Allelopathic Effect on Asparagus by the Flowable Agent in Activated Carbon. In X International Asparagus Symposium. International Society for Horticultural Science. Acta Hortic. 2002, 589, 381–386. [Google Scholar] [CrossRef]
- Elmer, W.H. Management of Fusarium Crown and Root Rot of Asparagus. Crop Prot. 2015, 73, 2–6. [Google Scholar] [CrossRef]
- Liu, J.; Matsubara, Y.-I. Interaction between Allelochemicals and Fusarium Root Rot in Asparagus Seedlings Cultured In Vitro. Am. J. Plant Sci. 2018, 9, 543–551. [Google Scholar] [CrossRef]
- Blok, W.J.; Bollen, G.J. The Role of Autotoxins from Root Residues of the Previous Crop in the Replant Disease of Asparagus. Neth. J. Plant Pathol. 1993, 99, 29–40. [Google Scholar] [CrossRef]
- Schreuder, W.; Lamprecht, S.C.; Marasas, W.F.O.; Calitz, F.J. Pathogenicity of Three Fusarium Species Associated with Asparagus Decline in South Africa. Plant Dis. 1995, 79, 177–181. [Google Scholar] [CrossRef]
- Schofield, P.E. Asparagus Decline and Replant Problem in New Zealand. N. Z. J. Crop Hortic. Sci. 1991, 19, 213–220. [Google Scholar] [CrossRef][Green Version]
- Lake, R.J.; Falloon, P.G.; Cook, D.W.M. Replant Problem and Chemical Components of Asparagus Roots. N. Z. J. Crop Hortic. Sci. 1993, 21, 53–58. [Google Scholar] [CrossRef]
- Hartung, A.C.; Stephens, C.T. Effects of Allelopathic Substances Produced by Asparagus on Incidence and Severity of Asparagus Decline Due to Fusarium Crown Rot. J. Chem. Ecol. 1983, 9, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.A.; de Abboud, A.C.S.; do Carmo, M.G.F. Biofumigation with Species of the Brassicaceae Family: A Review. Cienc. Rural 2021, 51, 1–17. [Google Scholar]
- Gamliela, A.; Siti, M.; Arbel, A.; Katan, J. Soil Solarization as a Component of the Integrated Management of Fusarium Crown and Root Rot in Tomato. Acta Hortic. 2009, 808, 321–326. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Zhou, Q.; Gossen, B.D.; McDonald, M.R.; Peng, G.; Turnbull, G.D. Suppression of Clubroot by Dazomet Fumigant. Can. J. Plant Sci. 2017, 98, 172–182. [Google Scholar] [CrossRef]
- Fritsch, H.J.; Huber, R. Basamid granular—A halogen-free soil disinfestant. Acta Hortic. 1995, 76–85. [Google Scholar] [CrossRef]
- Prider, J.; Williams, A. Using Dazomet to Reduce Broomrape Seed Banks in Soils with Low Moisture Content. Crop Prot. 2014, 59, 43–50. [Google Scholar] [CrossRef]
- Kantikowati, E.; Yusdian, Y.; Suryani, C. Chicken Manure and Biofertilizer for Increasing Growth and Yield of Potato (Solanum Tuberosum L.) of Granola Varieties. IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012017. [Google Scholar]
- Borrego-Benjumea, A.; Basallote-Ureba, M.J.; Melero-Vara, J.M. Eficacia de Enmiendas Orgánicas, Temperatura de Suelo y Cultivares En El Control de La Podredumbre de Raíces y Cuello de Espárrago. In Proceedings of the Resúmenes del XV Congreso de la Sociedad Española de Fitopatología, Vitoria-Gasteiz, Spain, 27 September–1 October 2010; pp. 383–undefined. [Google Scholar]
- Vida, C.; Vicente, A.; Cazorla, F.M. The Role of Organic Amendments to Soil for Crop Protection: Induction of Suppression of Soilborne Pathogens. Ann. Appl. Biol. 2020, 176, 1–15. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and Ascorbic Acid in Spinach (Spinacia Oleracea) Chloroplasts. The Effect of Hydrogen Peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Gronwald, J.W.; Fuerst, E.P.; Eberlein, C.V.; Egli, M.A. Effect of Herbicide Antidotes on Glutathione Content and Glutathione S-Transferase Activity of Sorghum Shoots. Pestic. Biochem. Physiol. 1987, 29, 66–76. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Wolf, B. A Comprehensive System of Leaf Analyses and Its Use for Diagnosing Crop Nutrient Status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Krom, M.D. Spectrophotometric Determination of Ammonia: A Study of a Modified Berthelot Reaction Using Salicylate and Dichloroisocyanurate. Anal. 1980, 105, 305–316. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid Peroxidation—DNA Damage by Malondialdehyde. Mutat. Res. - Fundam. Mol. Mech. Mutagenesis 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Tenorio, M.; Villanueva, M.; Sagardoy, M. Changes in Carotenoids and Chlorophylls in Fresh Green Asparagus (Asparagus Officinalis L) Stored under Modified Atmosphere Packaging. J. Sci. Food Agric. 2004, 84, 357–365. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Zhang, Z.; Cao, Y.; Li, J.; Luo, L. Allyl Isothiocyanate (AITC) Triggered Toxicity and FsYvc1 (a STRPC Family Member) Responded Sense in Fusarium Solani. Front. Microbiol. 2020, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Papoulias, E.; Siomos, A.; Koukounaras, A.; Gerasopoulos, D.; Kazakis, E. Effects of Genetic, Pre- and Post-Harvest Factors on Phenolic Content and Antioxidant Capacity of White Asparagus Spears. Int. J. Mol. Sci. 2009, 10, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional Comparison of Fresh, Frozen and Canned Fruits and Vegetables. Part 1. Vitamins C and B and Phenolic Compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Rosales, M.A.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.d.M.; Blasco, B.; Ríos, J.J.; Soriano, T.; Castilla, N.; Romero, L.; Ruiz, J.M. The Effect of Environmental Conditions on Nutritional Quality of Cherry Tomato Fruits: Evaluation of Two Experimental Mediterranean Greenhouses. J. Sci. Food Agric. 2011, 91, 152–162. [Google Scholar] [CrossRef]
- Farooq, M.; Rizwan, M.; Nawaz, A.; Rehman, A.; Ahmad, R. Application of Natural Plant Extracts Improves the Tolerance against Combined Terminal Heat and Drought Stresses in Bread Wheat. J. Agron. Crop Sci. 2017, 203, 528–538. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The Role of Amino Acid Metabolism during Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Holländer-Czytko, H.; Grabowski, J.; Sandorf, I.; Weckermann, K.; Weiler, E.W. Tocopherol Content and Activities of Tyrosine Aminotransferase and Cystine Lyase in Arabidopsis under Stress Conditions. J. Plant Physiol. 2005, 162, 767–770. [Google Scholar]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, T.; Lidon, F.J.C. Zinc in Plants—An Overview. Emir. J. Food Agric. 2012, 24, 322–334. [Google Scholar]

| Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Carotenoids (mg g−1 FW) | |
|---|---|---|---|
| T1 | 0.053 ± 0.006 a | 0.041 ± 0.006 a | 0.034 ± 0.005 a |
| T2 | 0.048 ± 0.007 ab | 0.040 ± 0.006 ab | 0.032 ± 0.005 ab |
| T3 | 0.035 ± 0.002 c | 0.032 ± 0.001 c | 0.023 ± 0.001 c |
| T4 | 0.045 ± 0.005 b | 0.036 ± 0.002 bc | 0.029 ± 0.003 b |
| p-value | *** | ** | *** |
| LSD0.05 | 0.005 | 0.004 | 0.003 |
| GSH (µg g−1 FW) | AsA (µg g−1 FW) | |
|---|---|---|
| T1 | 0.53 ± 0.06 ab | 0.28 ± 0.02 c |
| T2 | 0.54 ± 0.09 a | 0.28 ± 0.02 c |
| T3 | 0.46 ± 0.05 bc | 0.33 ± 0.01 b |
| T4 | 0.40 ± 0.09 c | 0.36 ± 0.05 a |
| p-value | ** | *** |
| LSD0.05 | 0.07 | 0.03 |
| Total Phenols (mg g−1 FW) | Flavonoids (mg g−1 FW) | Anthocyanins (mg g−1 FW) | |
|---|---|---|---|
| T1 | 4.32 ± 0.53 a | 4.57 ± 0.55 b | 8.95 ± 0.42 b |
| T2 | 4.59 ± 0.78 a | 5.22 ± 0.61 a | 10.73 ± 0.97 a |
| T3 | 2.95 ± 0.73 b | 2.89 ± 0.28 c | 7.67 ± 0.35 c |
| T4 | 3.27 ± 0.48 b | 4.25 ± 0.36 b | 5.88 ± 2.56 d |
| p-value | *** | *** | *** |
| LSD0.05 | 0.61 | 0.45 | 0.50 |
| Arg | His | Glu | Asp | Pro | Ser | Gly | Thr | Ala | Tyr | Val | Ile | Leu | Phe | Met | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 20.56 ± 0.82 b | 15.57 ± 0.19 b | 3.43 ± 0.14 a | 1.91 ± 0.08 a | 16.99 ± 0.68 b | 4.67 ± 0.19 c | 2.08 ± 0.08 b | 1.27 ± 0.05 c | 5.20 ± 0.21 c | 9.31 ± 0.37 c | 1.35 ± 0.05 c | 0.07 ± 0.00 d | 0.26 ± 0.01 c | 0.22 ± 0.01 b | nd |
| T2 | 24.91 ± 1.00 a | 17.10 ± 0.21 a | 3.52 ± 0.14 a | 1.32 ± 0.05 b | 10.10 ± 0.40 c | 5.35 ± 0.21 b | 2.16 ± 0.09 b | 1.21 ± 0.05 b | 6.60 ± 0.26 a | 11.89 ± 0.48 b | 1.41 ± 0.06 b | 0.08 ± 0.00 c | 0.28 ± 0.01 c | 0.15 ± 0.01 c | 0.26 ± 0.01 a |
| T3 | 14.31 ± 0.57 c | 11.94 ± 0.16 c | 1.97 ± 0.08 b | 1.16 ± 0.05 c | 5.72 ± 0.23 d | 4.02 ± 0.16 d | 2.21 ± 0.09 b | 1.06 ± 0.04 b | 4.09 ± 0.16 d | 2.81 ± 0.11 d | 1.10 ± 0.04 b | 0.13 ± 0.01 b | 0.33 ± 0.01 b | nd | 0.15 ± 0.01 b |
| T4 | 26.18 ± 1.05 a | 10.89 ± 0.25 c | 2.94 ± 0.12 c | 1.96 ± 0.08 a | 25.45 ± 1.02 a | 6.28 ± 0.25 a | 2.75 ± 0.11 a | 1.95 ± 0.08 a | 6.08 ± 0.24 b | 16.21 ± 0.65 a | 2.44 ± 0.10 a | 0.22 ± 0.01 a | 0.74 ± 0.03 a | 0.57 ± 0.02 a | nd |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| LSD0.05 | 1.66 | 1.06 | 0.23 | 0.12 | 1.23 | 0.39 | 0.17 | 0.11 | 0.42 | 0.84 | 0.12 | 0.01 | 0.03 | 0.02 | 0.01 |
| N | K | P | S | Ca | Mg | |
|---|---|---|---|---|---|---|
| T1 | 84.54 ± 7.97 a | 18.00 ± 0.36 c | 4.69 ± 0.09 b | 2.83 ± 0.06 d | 2.22 ± 0.04 c | 0.74 ± 0.01 c |
| T2 | 76.31 ± 13.15 ab | 19.52 ± 0.39 b | 4.85 ± 0.10 b | 4.26 ± 0.09 b | 3.50 ± 0.07 b | 0.88 ± 0.02 b |
| T3 | 67.03 ± 13.48 bc | 22.91 ± 0.46 a | 6.19 ± 0.12 a | 5.00 ± 0.10 a | 3.39 ± 0.07 b | 1.12 ±0.02 a |
| T4 | 59.72 ± 6.42 c | 17.65 ± 0.35 c | 4.25 ± 0.09 c | 3.41 ± 0.07 c | 3.92 ± 0.08 a | 0.79 ± 0.02 d |
| p-value | *** | *** | *** | *** | *** | *** |
| LSD0.05 | 10.29 | 0.74 | 0.19 | 0.15 | 0.12 | 0.03 |
| B | Fe | Mn | Zn | Cu | |
|---|---|---|---|---|---|
| T1 | 15.86 ± 0.32 a | 135.47 ± 2.71 d | 16.19 ± 0.32 d | 31.66 ± 0.63 d | 5.33 ± 0.11 d |
| T2 | 10.29 ± 0.21 b | 232.35 ± 4.65 b | 19.42 ± 0.39 b | 40.01 ± 0.80 b | 7.07 ± 0.14 b |
| T3 | 9.13 ± 0.18 c | 177.82 ± 3.56 c | 21.70 ± 0.43 a | 56.36 ± 1.13 a | 7.11 ± 0.14 a |
| T4 | 9.10 ± 0.18 c | 255.43 ± 5.11 a | 18.25 ± 0.36 c | 33.96 ± 0.68 c | 6.83 ± 0.14 c |
| p-value | *** | *** | *** | *** | *** |
| LSD0.05 | 0.20 | 7.74 | 0.72 | 1.57 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Moreno, F.J.; Atero-Calvo, S.; Navarro-León, E.; Blasco, B.; Soriano, T.; Ruiz, J.M. Evaluation of Physiological and Quality Parameters of Green Asparagus Spears Subjected to Three Treatments against the Decline Syndrome. Agronomy 2021, 11, 937. https://doi.org/10.3390/agronomy11050937
López-Moreno FJ, Atero-Calvo S, Navarro-León E, Blasco B, Soriano T, Ruiz JM. Evaluation of Physiological and Quality Parameters of Green Asparagus Spears Subjected to Three Treatments against the Decline Syndrome. Agronomy. 2021; 11(5):937. https://doi.org/10.3390/agronomy11050937
Chicago/Turabian StyleLópez-Moreno, Francisco Javier, Santiago Atero-Calvo, Eloy Navarro-León, Begoña Blasco, Teresa Soriano, and Juan Manuel Ruiz. 2021. "Evaluation of Physiological and Quality Parameters of Green Asparagus Spears Subjected to Three Treatments against the Decline Syndrome" Agronomy 11, no. 5: 937. https://doi.org/10.3390/agronomy11050937
APA StyleLópez-Moreno, F. J., Atero-Calvo, S., Navarro-León, E., Blasco, B., Soriano, T., & Ruiz, J. M. (2021). Evaluation of Physiological and Quality Parameters of Green Asparagus Spears Subjected to Three Treatments against the Decline Syndrome. Agronomy, 11(5), 937. https://doi.org/10.3390/agronomy11050937






