Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Development of Nutrient Solution
2.3. Electrical Conductivity Treatments
2.4. Growth Characteristics
2.5. Macro- and Micro-Elements Contents
2.6. Biochemical Parameters
2.6.1. Total Phenolics
2.6.2. Total Anthocyanins
2.6.3. Antioxidant Capacity
2.6.4. Individual Bioactive Compounds
2.7. Statistical Analysis
3. Results
3.1. Growth Characteristics
3.2. Macro- and Micro-Elements
3.3. Biochemical Parameters
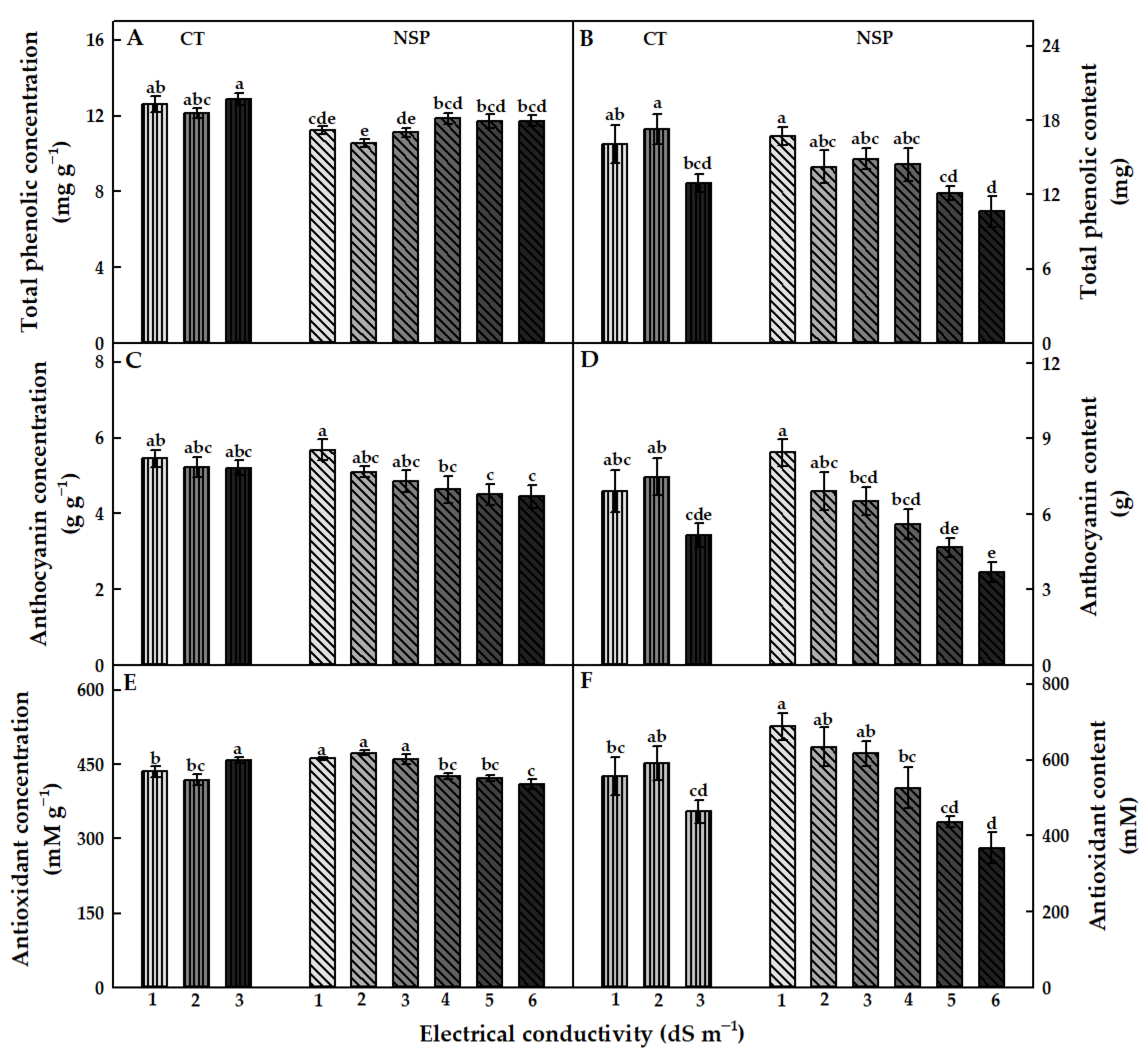
3.3.1. Total Phenolics, Anthocyanins, and Antioxidant Capacity
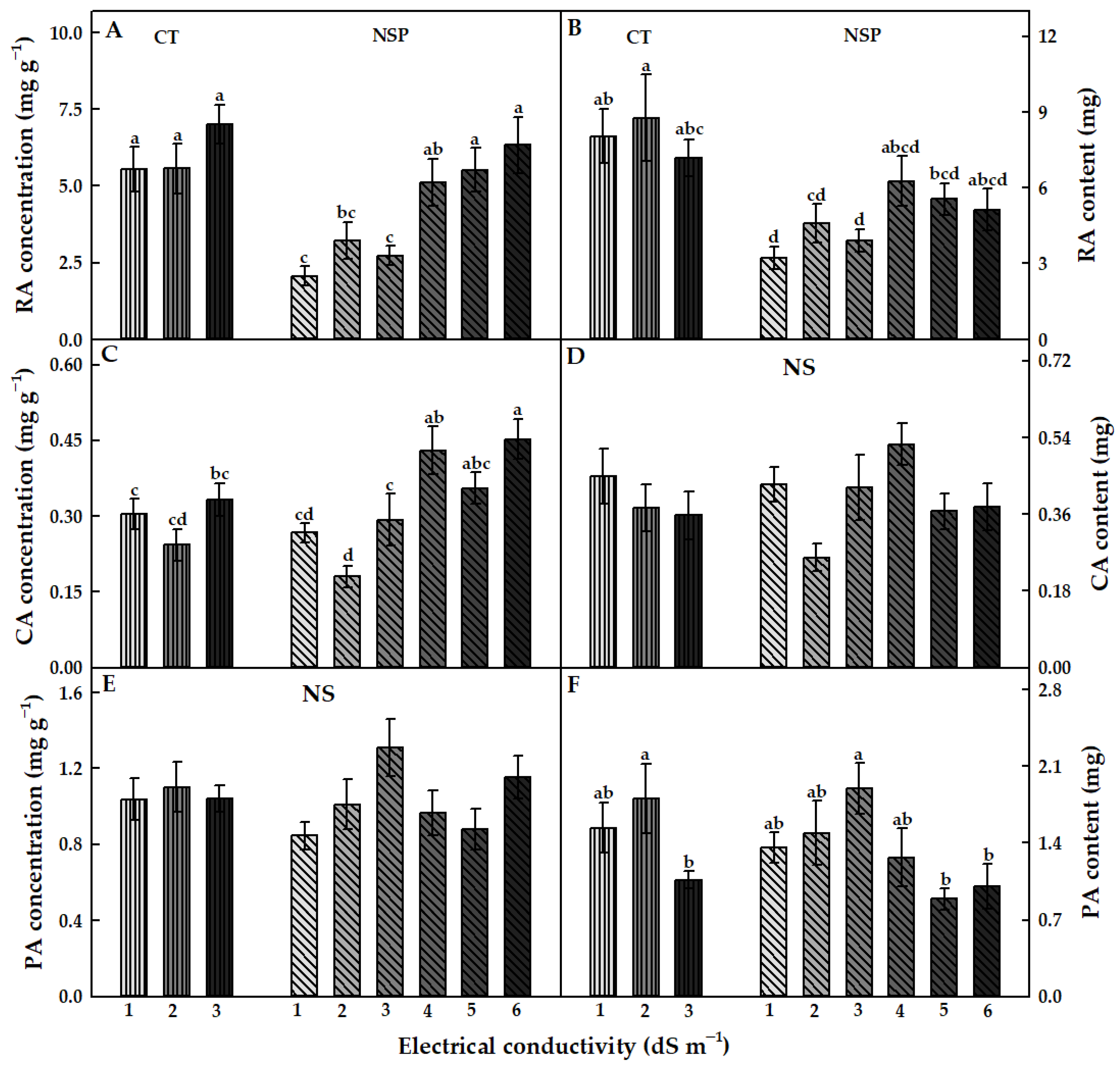
3.3.2. Individual Bioactive Compounds
4. Discussion
4.1. Growth Characteristics
4.2. Macro- and Micro-Elements
4.3. Biochemical Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Kim, N. Genetic diversity and relationships of cultivated and weedy types of Perilla frutescens collected from East Asia revealed by microsatellite markers. Korean J. Breed Sci. 2007, 39, 491–499. [Google Scholar]
- Saklani, S.; Chandra, S.; Gautam, A. Phytochemical investigation and contribution of Perilla frutescence as spices in traditional health care system. Int. J. Pharm. Technol. 2011, 3, 3543–3554. [Google Scholar]
- Shin, T.-Y.; Kim, S.-H.; Kim, S.-H.; Kim, Y.-K.; Park, H.-J.; Chae, B.-S.; Jung, H.-J.; Kim, H.-M. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Perilla frutescens. Immunopharmacol. Immunotoxicol. 2000, 22, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Žekonis, G.; Žekonis, J.; Šadzevičienė, R.; Šimonienė, G.; Kėvelaitis, E. Effect of Perilla frutescens aqueous extract on free radical production by human neutrophil leukocytes. Medicina 2008, 44, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Y.; Huangb, X.; Hanc, J.; Zhenga, W.; Maa, W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 251–257. [Google Scholar] [CrossRef]
- Saita, E.; Kishimoto, Y.; Tani, M.; Iizuka, M.; Toyozaki, M.; Sugihara, N.; Kondo, K. Antioxidant activities of Perilla frutescens against low-density lipoprotein oxidation in vitro and in human subjects. J. Oleo Sci. 2012, 61, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of trans-cinnamaldehyde,(−)-perillaldehyde,(−)-citronellal, citral, eugenol and carvacrol on airborne microbes using an airwasher. Biol. Pharm. Bull. 2006, 29, 2292–2294. [Google Scholar] [CrossRef]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Park, J.-S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. HortTechology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Palermo, M.; Paradiso, R.; De Pascale, S.; Fogliano, V. Hydroponic cultivation improves the nutritional quality of soybean and its products. J. Agric. Food Chem. 2012, 60, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Savvas, D.; Adamidis, K. Automated management of nutrient solutions based on target electrical conductivity, pH, and nutrient concentration ratios. J. Plant Nutr. 1999, 22, 1415–1432. [Google Scholar] [CrossRef]
- Nemali, K.S.; van Iersel, M.W. Light intensity and fertilizer concentration: I. Estimating optimal fertilizer concentrations from water-use efficiency of wax begonia. HortScience 2004, 39, 1287–1292. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Optimization of nutrition in soilless systems: A review. Adv. Bot. Res. 2010, 53, 193–245. [Google Scholar]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponics for the Home Grower; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, S.B.; Kim, S.M.; Cho, Y.Y.; Oh, M.M. Evaluating the effects of a newly developed nutrient solution on growth, antioxidants, and chicoric acid contents in Crepidiastrum denticulatum. Hortic. Environ. Biotechnol. 2016, 57, 478–486. [Google Scholar] [CrossRef]
- Lam, V.P.; Lee, M.H.; Park, J.S. Optimization of Indole-3-Acetic Acid Concentration in a Nutrient Solution for Increasing Bioactive Compound Accumulation and Production of Agastache rugosa in a Plant Factory. Agriculture 2020, 10, 343. [Google Scholar] [CrossRef]
- Amalfitano, C.A.; Del Vacchio, L.D.V.; Somma, S.; Cuciniello, A.C.; Caruso, G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of ‘Friariello’pepper grown in hydroponics. Hortic. Sci. 2017, 44, 91–98. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Hikosaka, Y.; Kanechi, M.; Sato, M.; Uno, Y. Dry-fog aeroponics affects the root growth of leaf lettuce (Lactuca sativa L. cv. Greenspan) by changing the flow rate of spray fertigation. Environ. Control Biol. 2015, 53, 181–187. [Google Scholar] [CrossRef][Green Version]
- Park, Y.G.; Oh, H.J.; Jeong, B.R. Growth and anthocyanin concentration of Perilla frutescens var. acuta Kudo as affected by light source and DIF under controlled environment. Hortic. Environ. Biotechnol. 2013, 54, 103–108. [Google Scholar] [CrossRef]
- Havlin, J.L.; Soltanpour, P. A nitric acid plant tissue digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1980, 11, 969–980. [Google Scholar] [CrossRef]
- Martre, P.; Porter, J.R.; Jamieson, P.D.; Triboï, E. Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiol. 2003, 133, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A.,-B., or-C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Oh, M.M. Physiological and biochemical responses of green and red perilla to LED-based light. J. Sci. Food Agric. 2021, 101, 240–252. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Hydroponics: Its history and use in plant nutrition studies. J. Plant Nutr. 1982, 5, 1003–1030. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Li, Y.L.; Stanghellini, C. Analysis of the effect of EC and potential transpiration on vegetative growth of tomato. Sci. Hortic. 2001, 89, 9–21. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J. Fruit Ornam. Plant Res. 2008, 16, 179–188. [Google Scholar]
- Nyoki, D.; Ndakidemi, P.A. Effects of Bradyrhizobium japonicum and phosphorus supplementation on the productivity of legumes. J. Plant Nutr. Soil Sci. 2014, 3, 894–910. [Google Scholar] [CrossRef]
- Savvas, D.; Passam, H.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- Sadia, A.; Mahasen, M.; Shahrin, S.; Roni, M.; Uddin, A. Phsophorus levels on growth and yield of turnip (Brassica campestris var. rapifera). Bangladesh Res. Publ. J. 2013, 8, 29–33. [Google Scholar]
- Abou El-Magd, M.; Zaki, M.; Eldewiny, C.Y. Effect of planting dates and different levels of potassium fertilizers on growth, yield and chemical composition of sweet fennel cultivars under newly reclaimed sandy soil conditions. J. Am. Sci. 2010, 6, 89–105. [Google Scholar]
- Netto, A.T.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yang, J.; Wang, H.; Zou, J.; He, J. Effects of nitrogen application rate and leaf age on the distribution pattern of leaf SPAD readings in the rice canopy. PLoS ONE 2014, 9, e88421. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Belkhodja, R.; Morales, F.; Sanz, M.; Abadía, A.; Abadía, J. Iron deficiency in peach trees: Effects on leaf chlorophyll and nutrient concentrations in flowers and leaves. Plant Soil 1998, 203, 257–268. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Lucini, L.; Rea, E.; Colla, G. Nutrient solution concentration affects growth, mineral composition, phenolic acids, and flavonoids in leaves of artichoke and cardoon. HortScience 2012, 47, 1424–1429. [Google Scholar] [CrossRef]
- Steiner, A.A. The selective capacity of plants for ions and its importance for the composition and treatment of the nutrient solution. Acta Hortic. 1980, 98, 87–98. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant. Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Voogt, W.; Sonneveld, C. Nutrient management in closed growing systems for greenhouse production. In Plant Production in Closed Ecosystems; Springer: Berlin/Heidelberg, Germany, 1997; pp. 83–102. [Google Scholar]
- Winsor, G.; Adams, P.; Fiske, P.; Smith, A. Diagnosis of Mineral Disorders in Plants. v. 1. Principles.-v. 2. Vegetables.-v. 3: Glasshouse Crops; Her Majesty’s Stationery Office: London, UK, 1987.
- Chen, Y.; Guo, Q.; Liu, L.; Liao, L.; Zhu, Z. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J. Med. Plant. Res. 2011, 5, 1749–1755. [Google Scholar]
- Chishaki, N.; Horiguchi, T. Responses of secondary metabolism in plants to nutrient deficiency. In Plant Nutrition for Sustainable Food Production and Environment; Springer: Berlin/Heidelberg, Germany, 1997; pp. 341–345. [Google Scholar]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.; Li, B. Anthocyanin and flavonoid production from Perilla frutescens: Pilot plant scale processing including cross-flow microfiltration and reverse osmosis. J. Agric. Food Chem. 2006, 54, 4297–4303. [Google Scholar] [CrossRef]
- Hilbert, G.; Soyer, J.; Molot, C.; Giraudon, J.; Milin, S. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. Vitis 2003, 42, 69–76. [Google Scholar]
- Horiguchi, T. Effects of nitrogen, phosphorus, and manganese deficiencies on the formation of anthocyanin and other phenolic compounds in plants. Jpn. J. Soil Sci. Plant. Nutr. 1989, 60, 226–232. [Google Scholar]
- Ito, M.; Toyoda, M.; Honda, G. Chemical composition of the essential oil of Perilla frutescens. Nat. Med. 1999, 53, 32–36. [Google Scholar]
- Seo, M.W.; Yang, D.S.; Kays, S.J.; Kim, J.-H.; Woo, J.H.; Park, K.W. Effects of nutrient solution electrical conductivity and sulfur, magnesium, and phosphorus concentration on sesquiterpene lactones in hydroponically grown lettuce (Lactuca sativa L.). Sci. Hortic. 2009, 122, 369–374. [Google Scholar] [CrossRef]
- David, E.; Mischan, M.; Marques, M.; Boaro, C. Physiological indexese macro-and micronutrients in plant tissue and essential oil of Mentha piperita L. grown in nutrient solution with variation in N, P, K and Mg levels. Rev. Bras. Plantas Med. 2014, 16, 97–106. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Petropoulos, S.A.; Fernandes, Â.; Barros, L.; Tzortzakis, N.; Ferreira, I.C. Effect of phosphorus application rate on Mentha spicata L. grown in deep flow technique (DFT). Food Chem. 2019, 276, 84–92. [Google Scholar] [CrossRef]
- Yoshida, T.; Morisada, S.; Kameoka, K. On the distribution of oil gland and the accumulation of essential oil in Perilla species. Jpn. J. Crop Sci. 1969, 38, 333–337. [Google Scholar] [CrossRef][Green Version]
- Ogawa, E.; Hikosaka, S.; Goto, E. Effects of nutrient solution temperature on the concentration of major bioactive compounds in red perilla. J. Agric. Meteorol. 2018, 74, 71–78. [Google Scholar] [CrossRef]
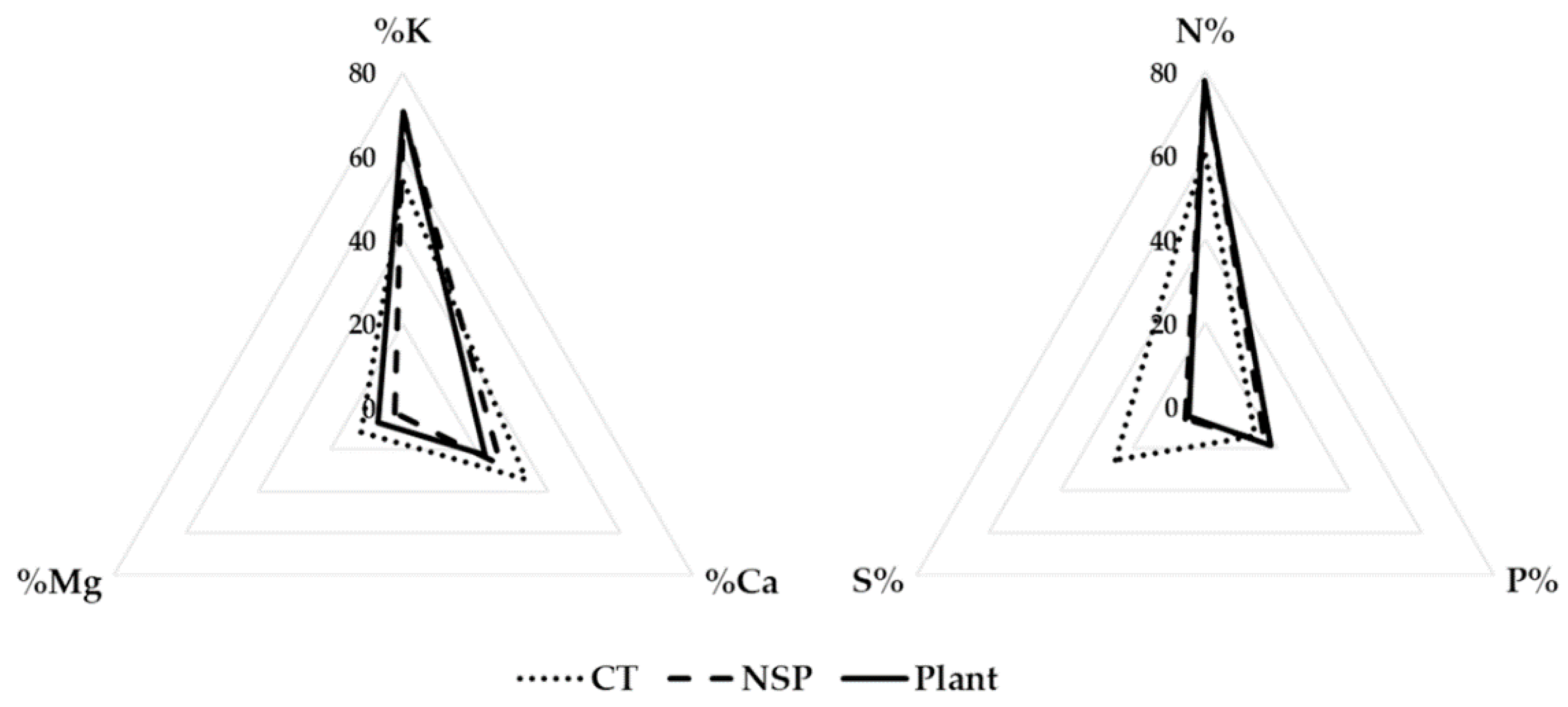
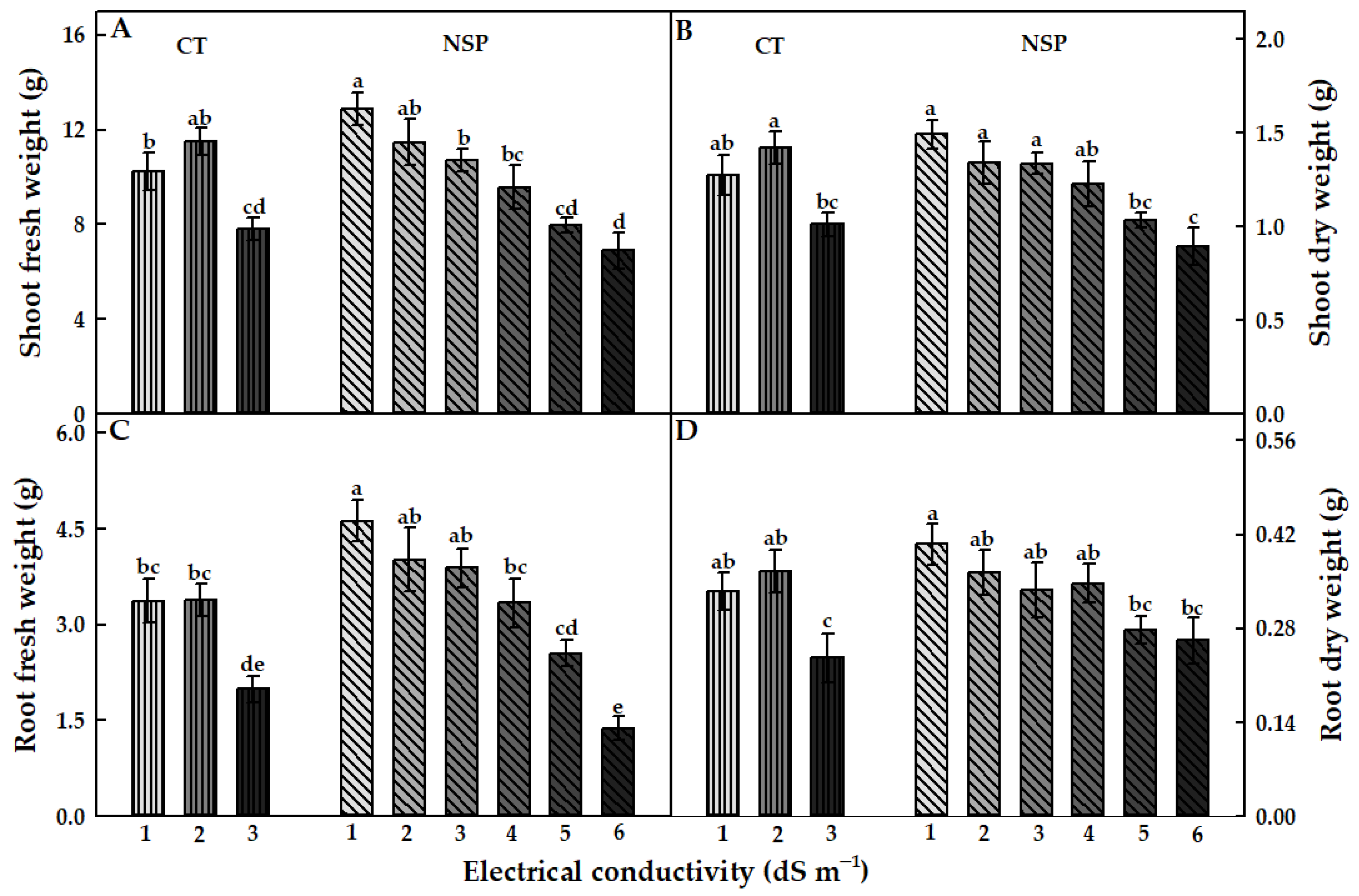
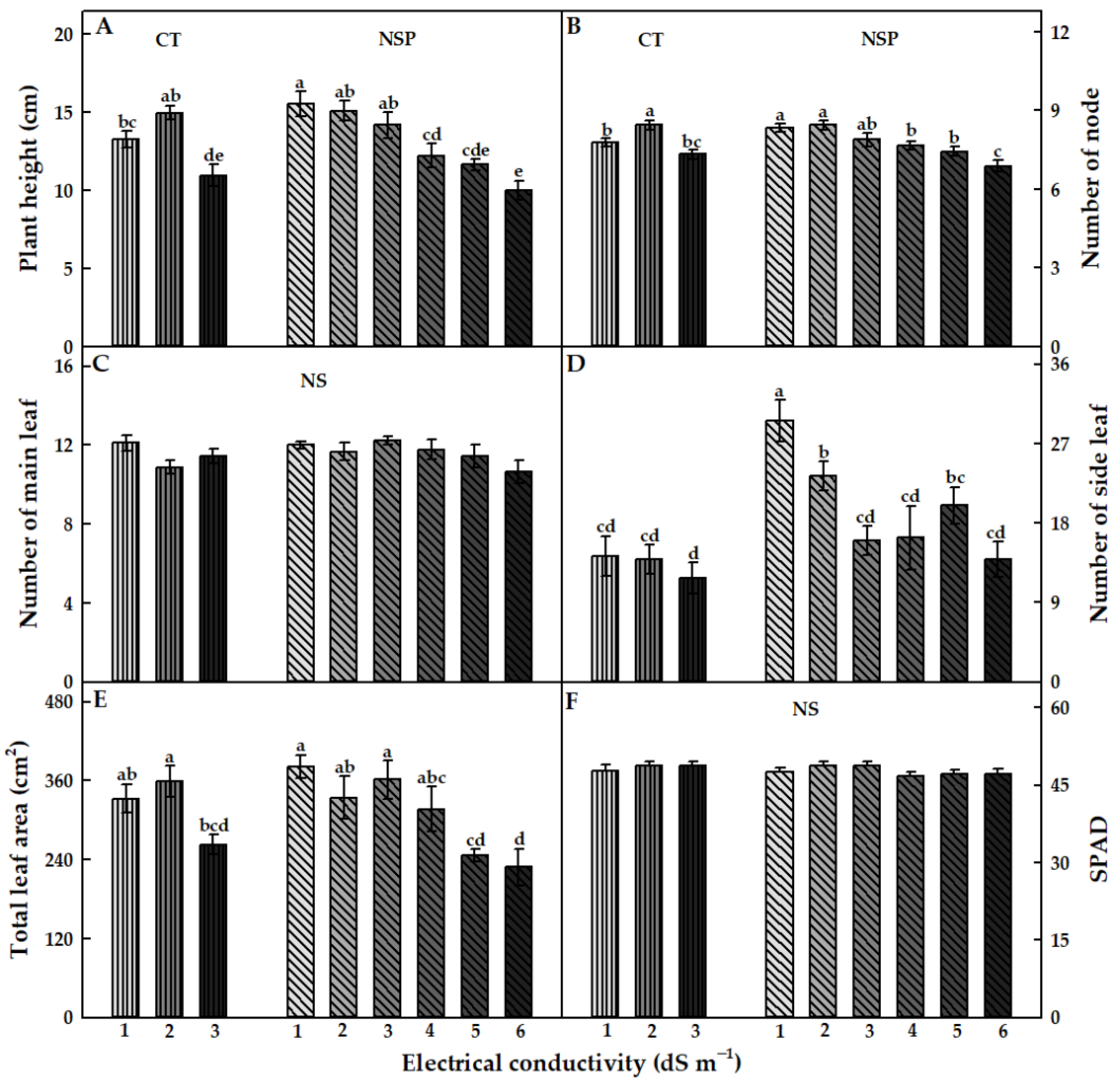
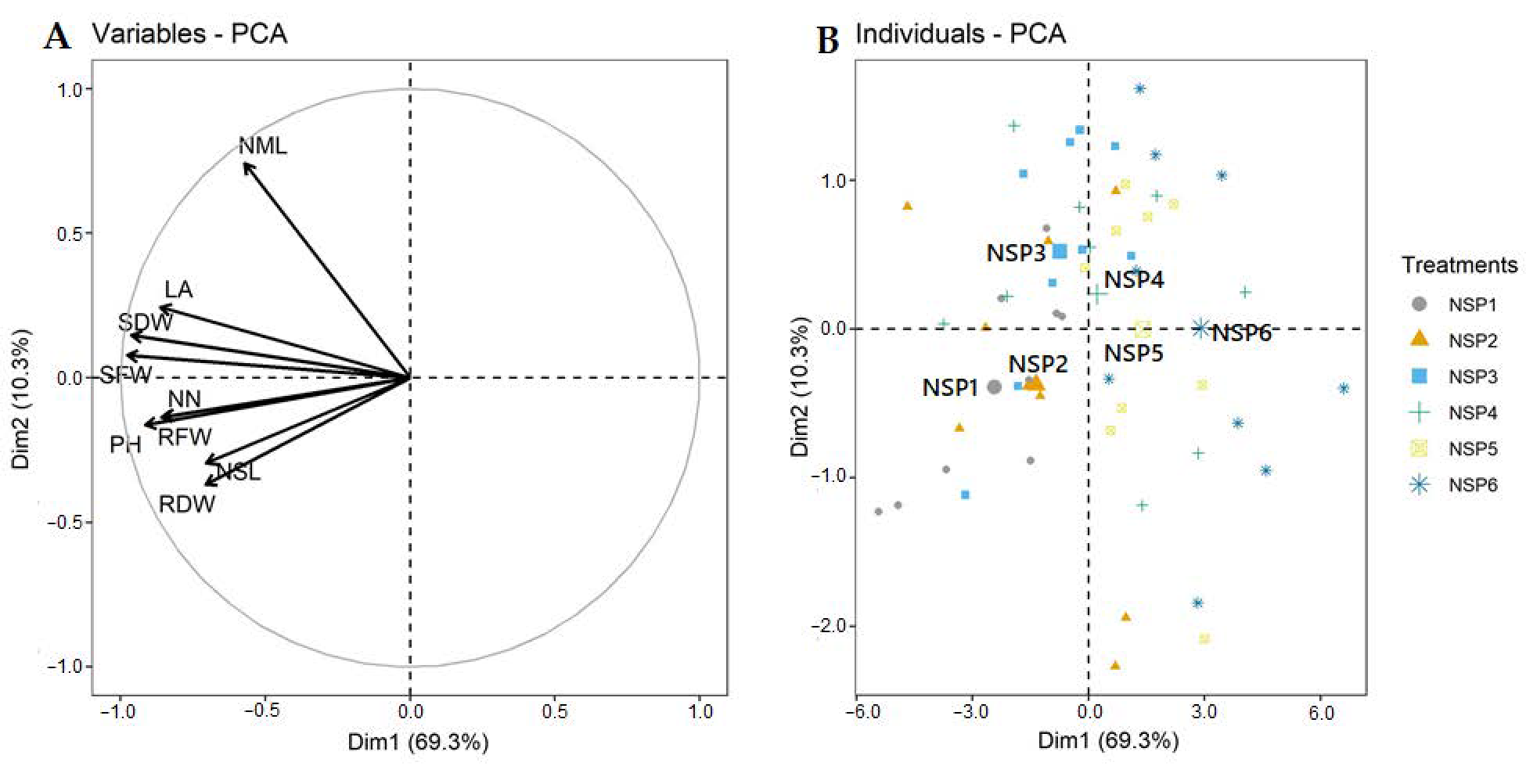
| Treatment | Electrical Conductivity (dS m−1) | Macro Elements (mg L−1) | Micro Elements (mg L−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Fe | Zn | Mo | Mn | B | ||
| CT | 1 | 109.3 | 24.76 | 132.8 | 83.38 | 28.8 | 44.57 | 1.33 | 0.07 | 0.035 | 0.347 | 0.16 |
| NSP | 131.17 | 27.86 | 189.1 | 72.09 | 6.49 | 9.33 | 0.91 | 0.21 | 0.03 | 0.335 | 0.21 | |
| Treatment | EC (dS m−1) | Macronutrient Concentration (mg g−1) | Macronutrient Content (mg) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | N | P | K | Ca | Mg | S | ||||||||||||||
| 1 | 47.04 | b | 9.32 | b | 39.28 | d | 15.66 | ab | 4.80 | a | 2.38 | cd | 68.22 | abc | 13.47 | ab | 57.05 | bcd | 22.75 | ab | 7.00 | a | 3.46 | ab | |
| CT | 2 | 47.92 | b | 9.70 | ab | 40.37 | cd | 15.34 | ab | 4.24 | b | 2.55 | ab | 73.30 | a | 15.01 | a | 62.24 | ab | 23.68 | ab | 6.58 | a | 3.96 | a |
| 3 | 51.27 | ab | 10.31 | a | 36.76 | e | 13.43 | cd | 4.36 | b | 2.66 | ab | 57.12 | c | 11.50 | bc | 41.07 | e | 14.98 | de | 4.83 | b | 2.97 | b | |
| 1 | 48.40 | b | 9.22 | b | 44.63 | a | 16.20 | a | 2.45 | c | 2.31 | d | 77.64 | a | 14.72 | a | 71.50 | a | 26.00 | a | 3.90 | c | 3.69 | a | |
| 2 | 48.89 | b | 10.37 | a | 43.65 | ab | 15.17 | b | 2.29 | cd | 2.50 | bc | 74.27 | a | 15.59 | a | 66.02 | ab | 22.88 | ab | 3.43 | c | 3.77 | a | |
| NSP | 3 | 50.29 | ab | 10.53 | a | 41.55 | bcd | 14.00 | c | 2.29 | cd | 2.58 | ab | 72.33 | ab | 15.17 | a | 59.87 | bc | 20.20 | bc | 3.29 | c | 3.73 | a |
| 4 | 50.73 | ab | 10.31 | a | 42.00 | bc | 12.84 | d | 2.16 | cd | 2.65 | ab | 71.80 | ab | 14.66 | a | 59.68 | bc | 18.22 | cd | 3.08 | c | 3.75 | a | |
| 5 | 54.33 | a | 9.94 | ab | 45.16 | a | 11.73 | e | 2.04 | d | 2.68 | a | 59.53 | bc | 10.90 | c | 49.62 | cde | 12.85 | e | 2.23 | d | 2.94 | b | |
| 6 | 54.15 | a | 10.37 | a | 45.11 | a | 11.64 | e | 2.08 | d | 2.70 | a | 56.30 | c | 10.78 | c | 46.92 | de | 12.09 | e | 2.17 | d | 2.82 | b | |
| Treatment | EC (dS m−1) | Macronutrient Concentration (mg g−1) | Macronutrient Content (mg) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | N | P | K | Ca | Mg | S | ||||||||||||||
| 1 | 47.04 | b | 9.32 | b | 39.28 | d | 15.66 | ab | 4.80 | a | 2.38 | cd | 68.22 | abc | 13.47 | ab | 57.05 | bcd | 22.75 | ab | 7.00 | a | 3.46 | ab | |
| CT | 2 | 47.92 | b | 9.70 | ab | 40.37 | cd | 15.34 | ab | 4.24 | b | 2.55 | ab | 73.30 | a | 15.01 | a | 62.24 | ab | 23.68 | ab | 6.58 | a | 3.96 | a |
| 3 | 51.27 | ab | 10.31 | a | 36.76 | e | 13.43 | cd | 4.36 | b | 2.66 | ab | 57.12 | c | 11.50 | bc | 41.07 | e | 14.98 | de | 4.83 | b | 2.97 | b | |
| 1 | 48.40 | b | 9.22 | b | 44.63 | a | 16.20 | a | 2.45 | c | 2.31 | d | 77.64 | a | 14.72 | a | 71.50 | a | 26.00 | a | 3.90 | c | 3.69 | a | |
| 2 | 48.89 | b | 10.37 | a | 43.65 | ab | 15.17 | b | 2.29 | cd | 2.50 | bc | 74.27 | a | 15.59 | a | 66.02 | ab | 22.88 | ab | 3.43 | c | 3.77 | a | |
| NSP | 3 | 50.29 | ab | 10.53 | a | 41.55 | bcd | 14.00 | c | 2.29 | cd | 2.58 | ab | 72.33 | ab | 15.17 | a | 59.87 | bc | 20.20 | bc | 3.29 | c | 3.73 | a |
| 4 | 50.73 | ab | 10.31 | a | 42.00 | bc | 12.84 | d | 2.16 | cd | 2.65 | ab | 71.80 | ab | 14.66 | a | 59.68 | bc | 18.22 | cd | 3.08 | c | 3.75 | a | |
| 5 | 54.33 | a | 9.94 | ab | 45.16 | a | 11.73 | e | 2.04 | d | 2.68 | a | 59.53 | bc | 10.90 | c | 49.62 | cde | 12.85 | e | 2.23 | d | 2.94 | b | |
| 6 | 54.15 | a | 10.37 | a | 45.11 | a | 11.64 | e | 2.08 | d | 2.70 | a | 56.30 | c | 10.78 | c | 46.92 | de | 12.09 | e | 2.17 | d | 2.82 | b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.K.L.; Yeom, M.-S.; Oh, M.-M. Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa. Agronomy 2021, 11, 932. https://doi.org/10.3390/agronomy11050932
Nguyen TKL, Yeom M-S, Oh M-M. Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa. Agronomy. 2021; 11(5):932. https://doi.org/10.3390/agronomy11050932
Chicago/Turabian StyleNguyen, Thi Kim Loan, Moon-Sun Yeom, and Myung-Min Oh. 2021. "Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa" Agronomy 11, no. 5: 932. https://doi.org/10.3390/agronomy11050932
APA StyleNguyen, T. K. L., Yeom, M.-S., & Oh, M.-M. (2021). Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa. Agronomy, 11(5), 932. https://doi.org/10.3390/agronomy11050932





