Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit
Abstract
1. Introduction
2. Materials and Methods
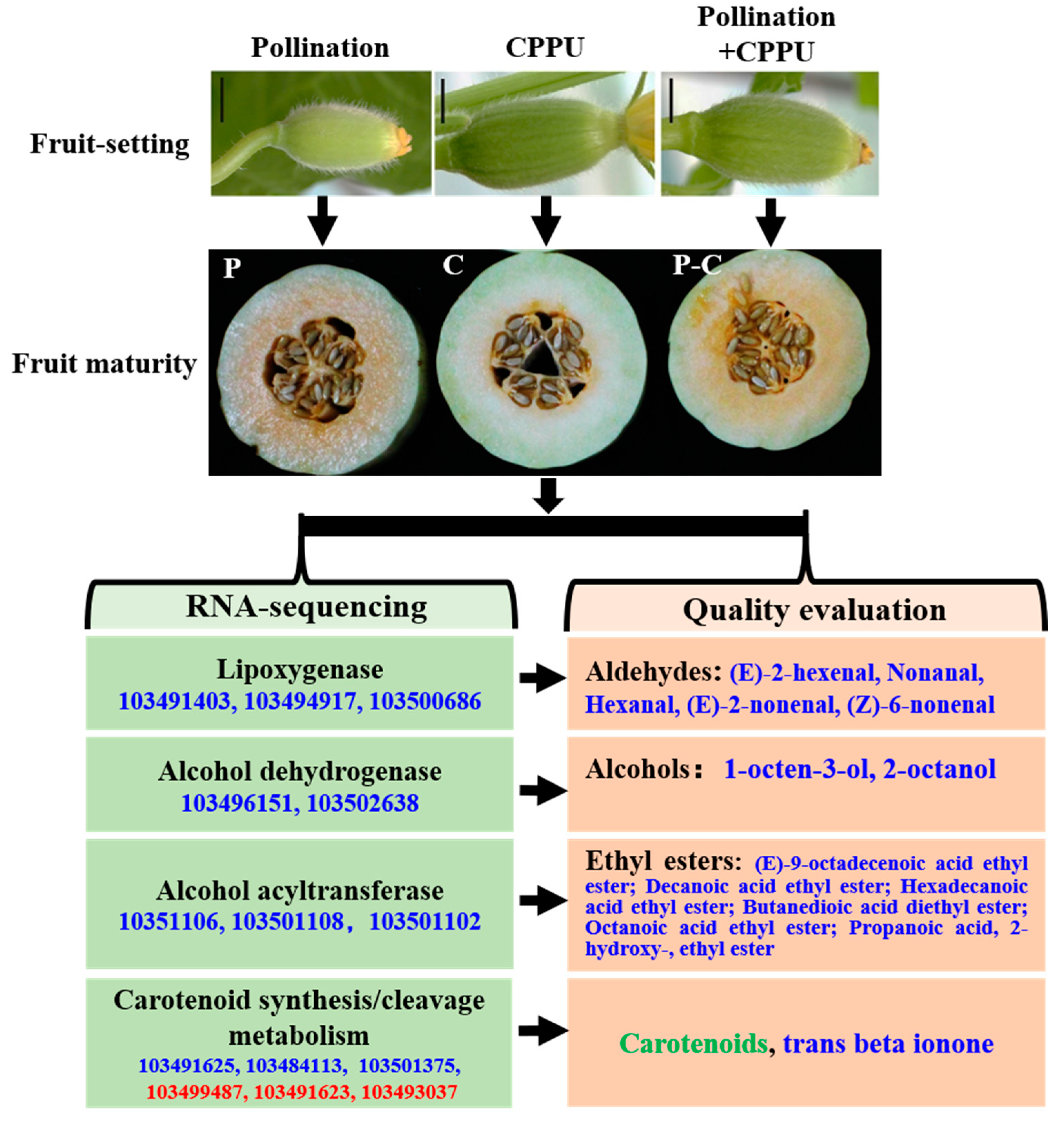
2.1. Plant Materials and Treatment for Fruit Set
2.2. Analysis of Fruit Quality
2.3. Identification of VOCs by GC–MS
2.4. RNA Extraction, Library Construction, and RNA Sequencing
2.5. Mapping of Reads to the Reference Genome and Differential Gene Expression Analysis
2.6. Validation of Gene Expression by qRT-PCR
3. Results
3.1. Effect of CPPU on the Sensorial Qualities of Different Treatments
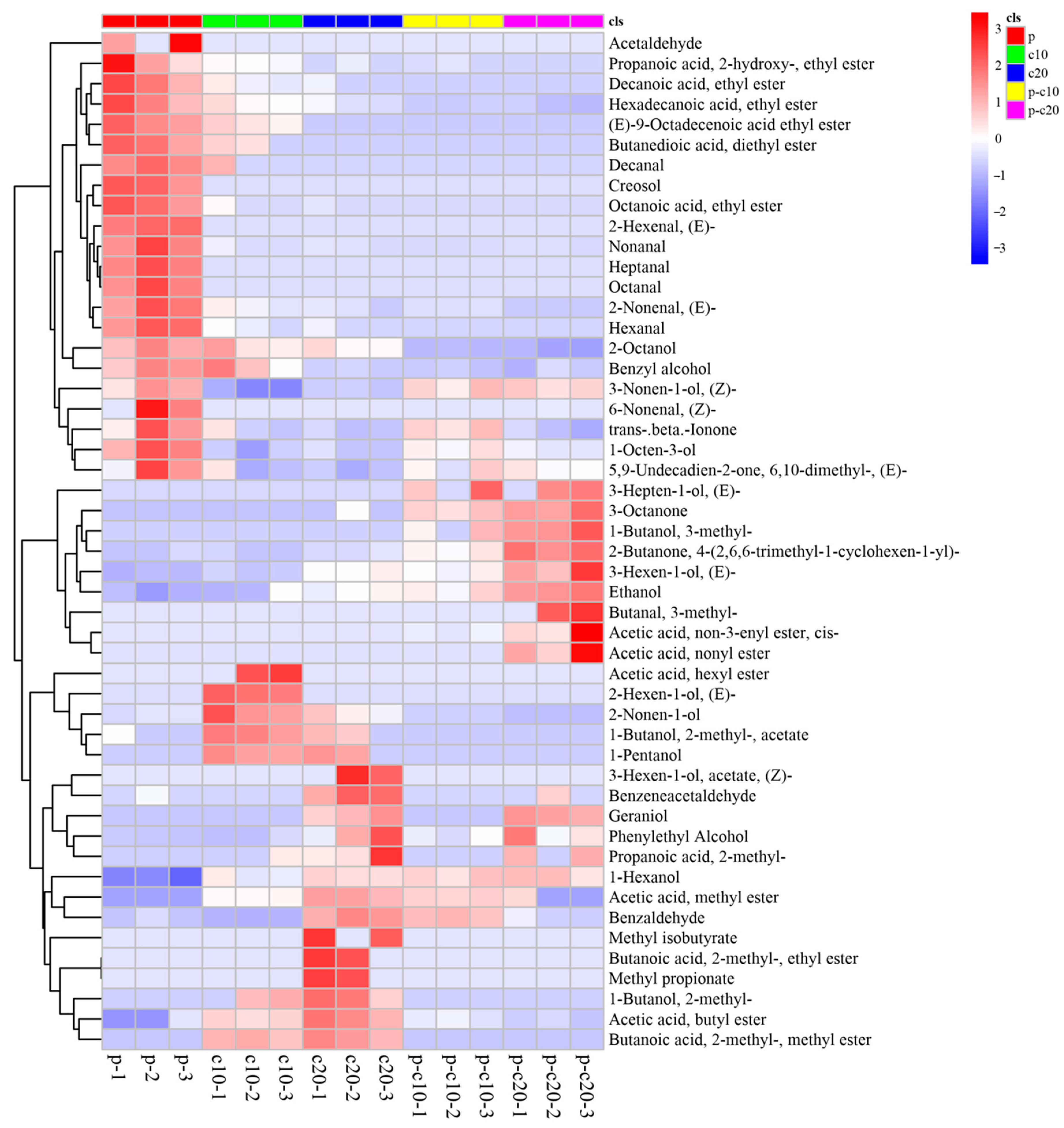
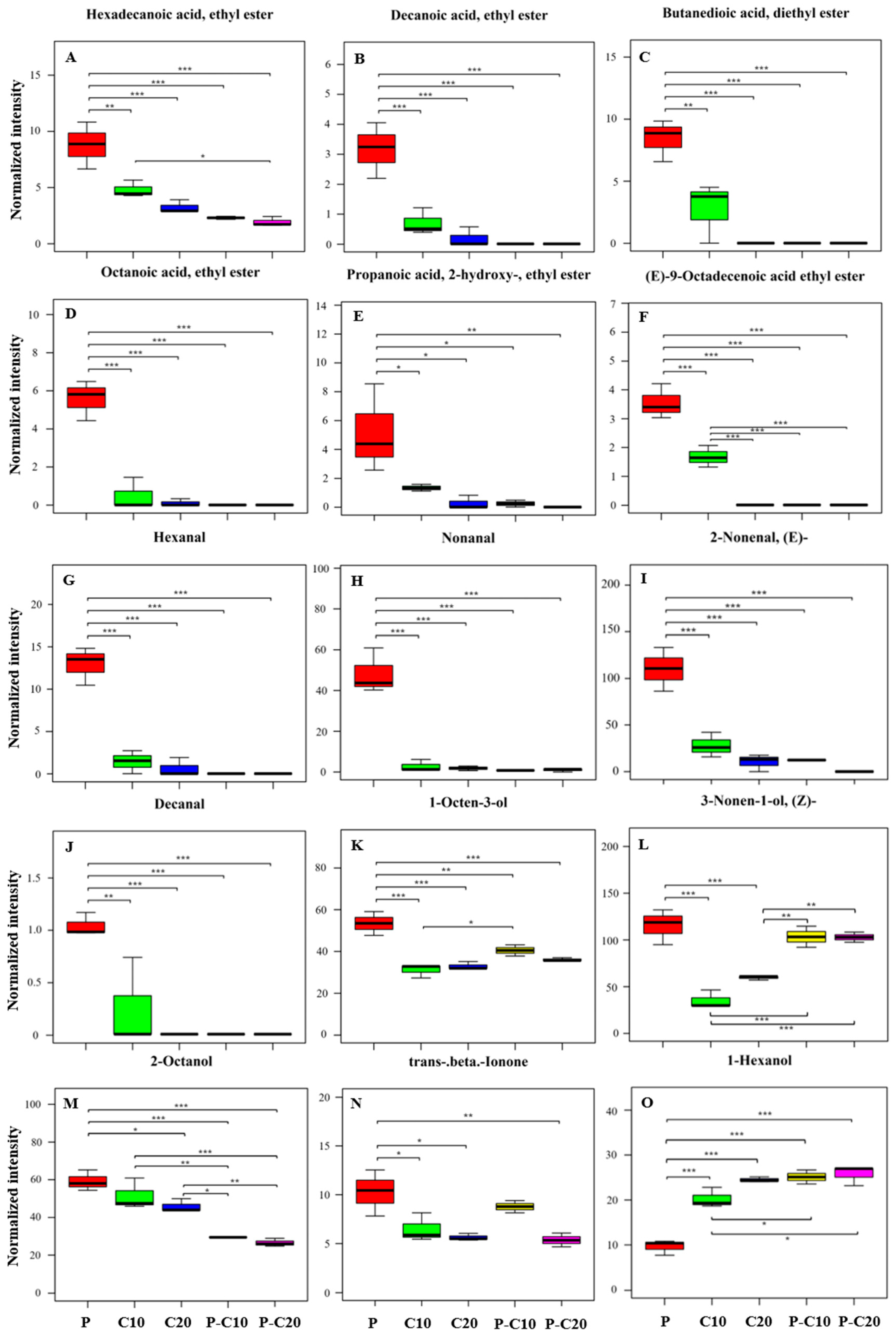
3.2. CPPU Treatment of Ovaries Influences VOCs of Mature Melon Fruits
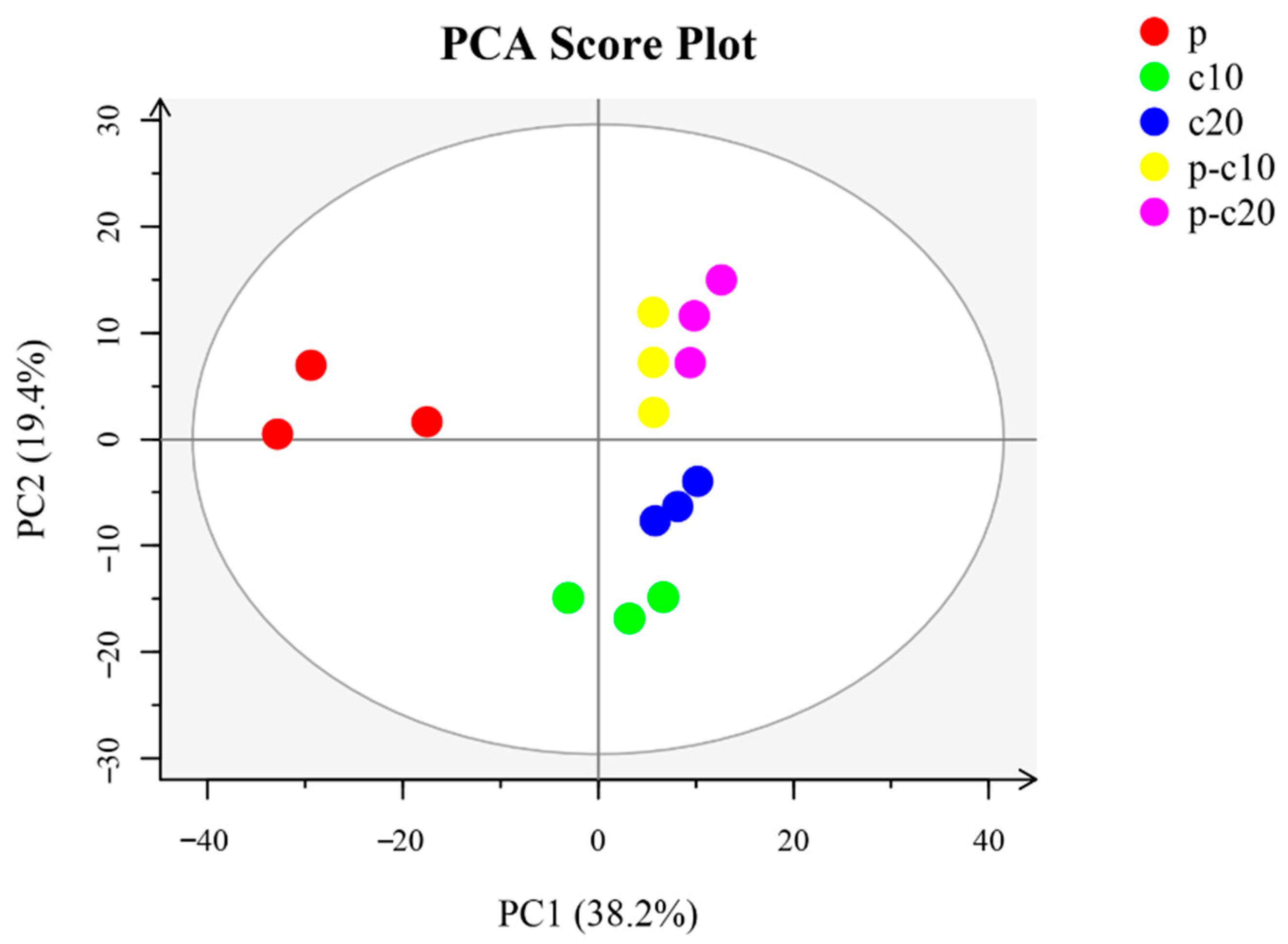
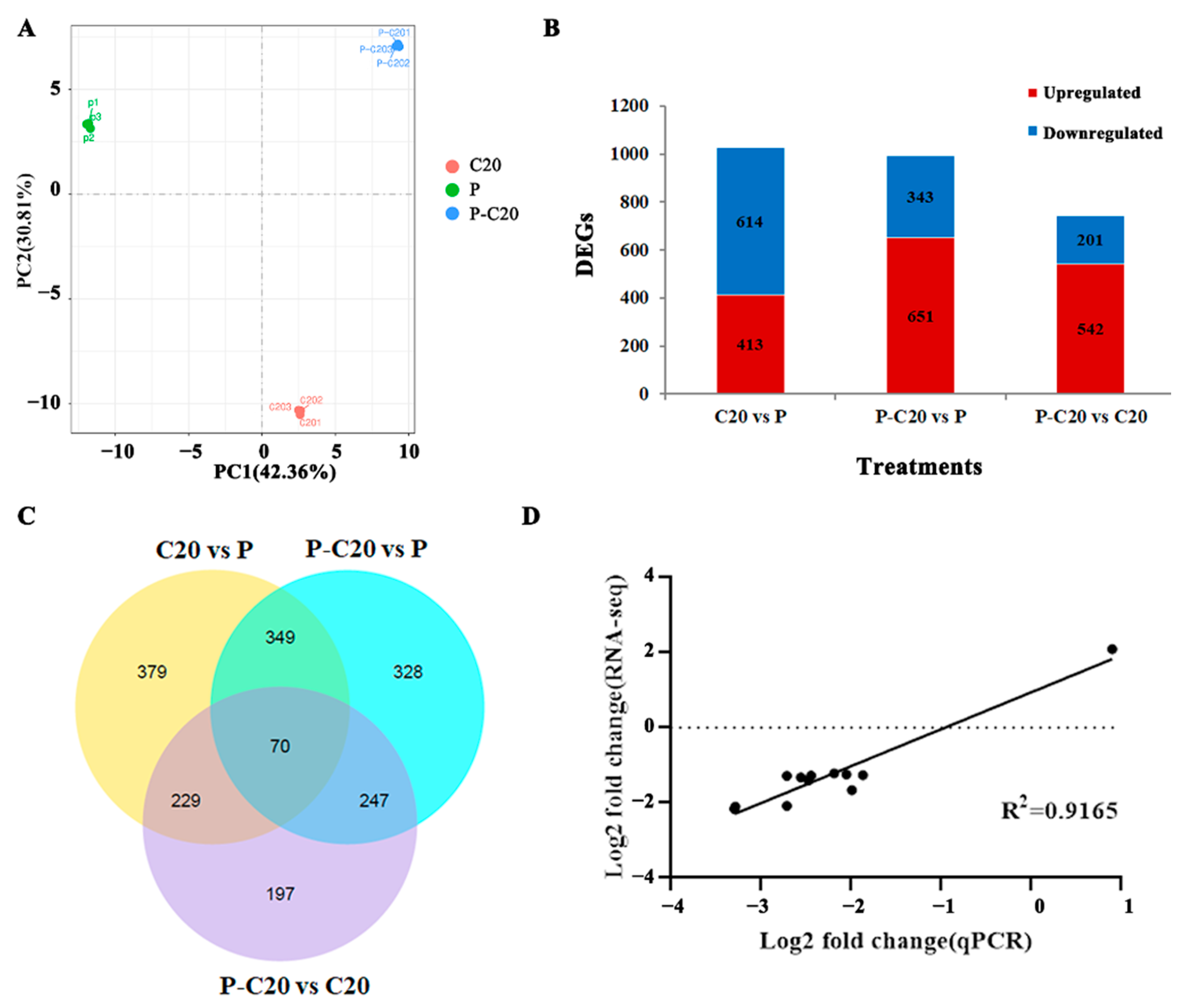
3.3. DEG Analysis between Different Treatments
3.4. GO Enrichment and KEGG Pathway Analysis of DEGs
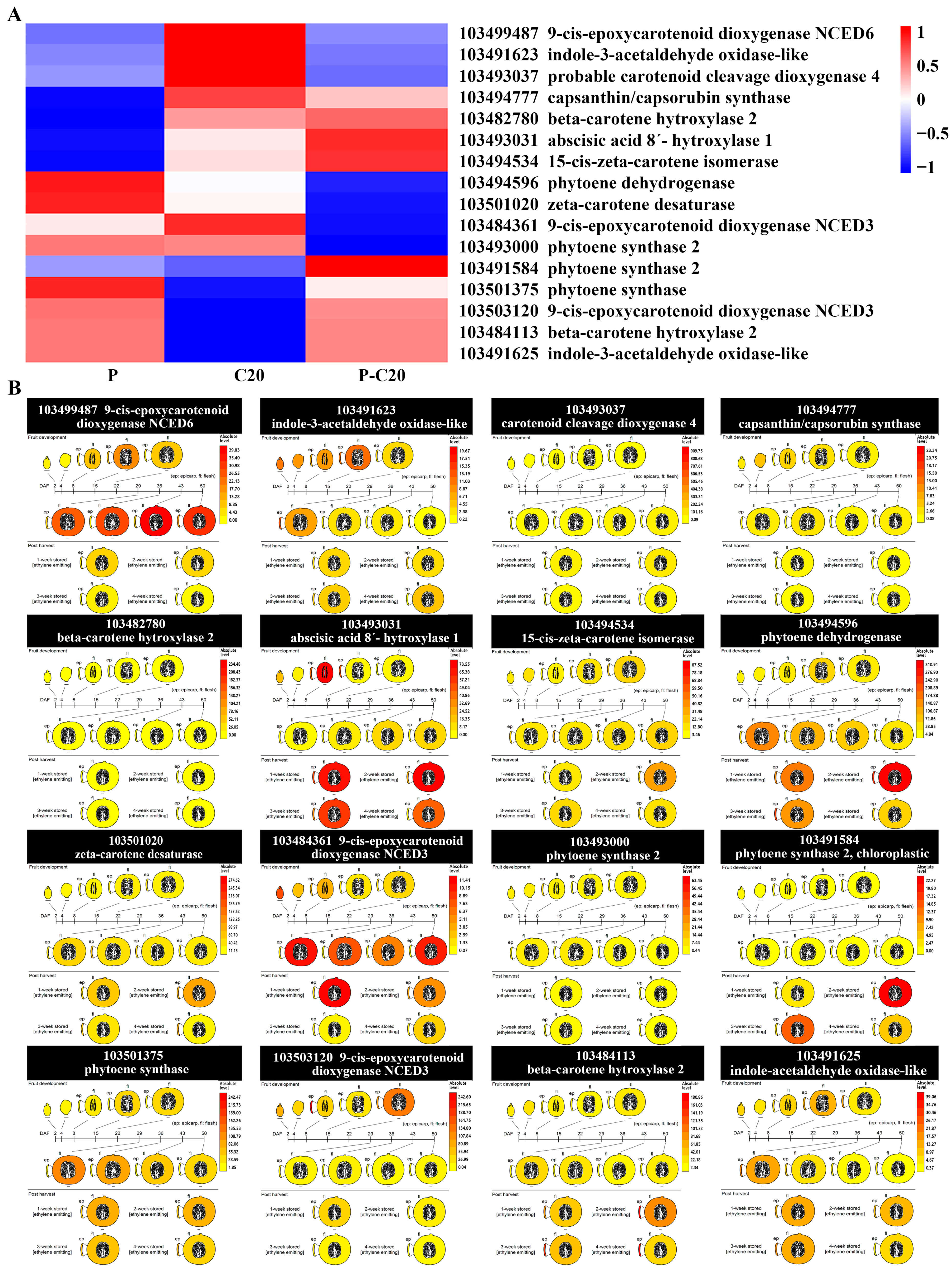
3.5. Expression Pattern of DEGs Involved in Carotenoid Biosynthesis Pathways
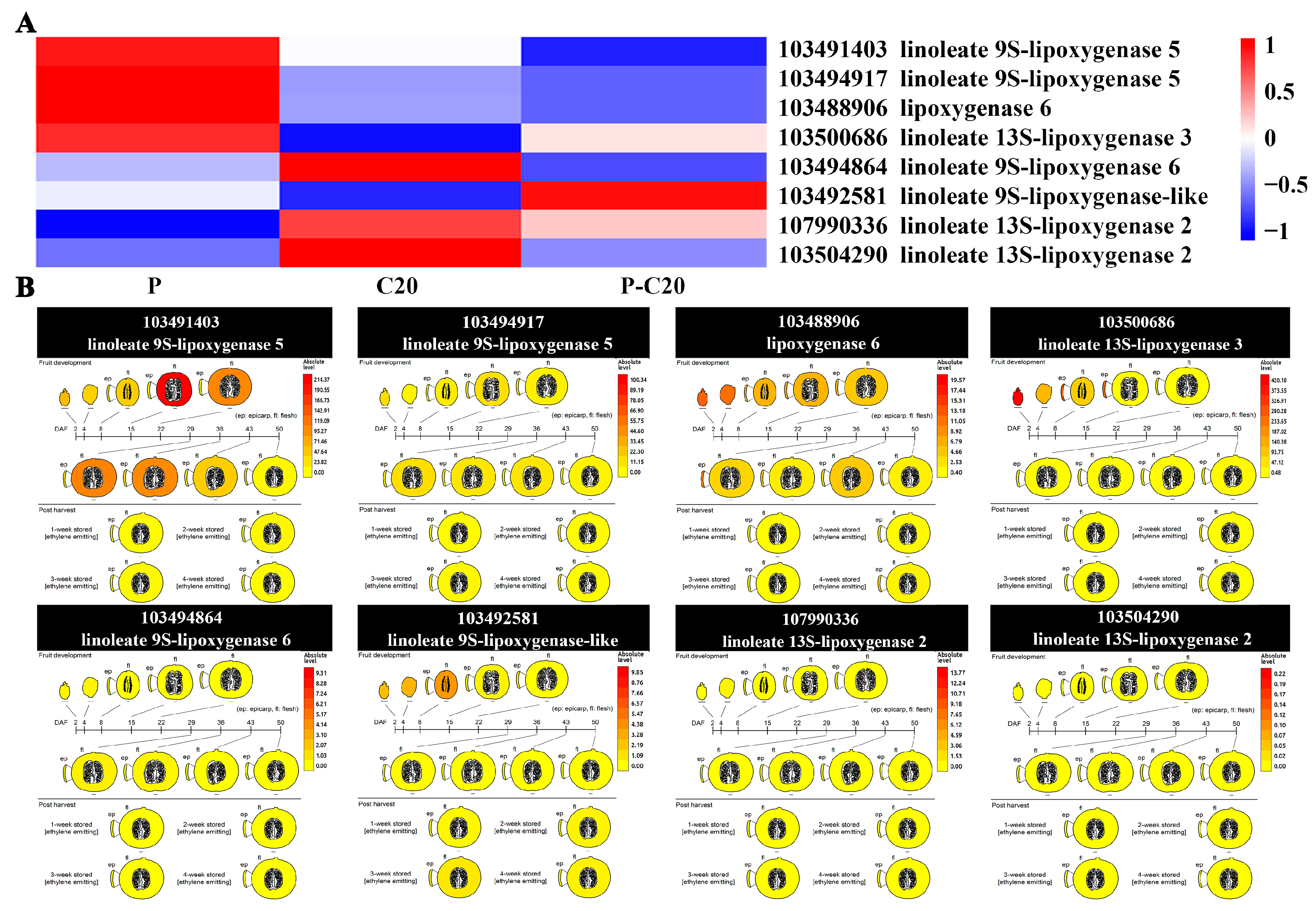
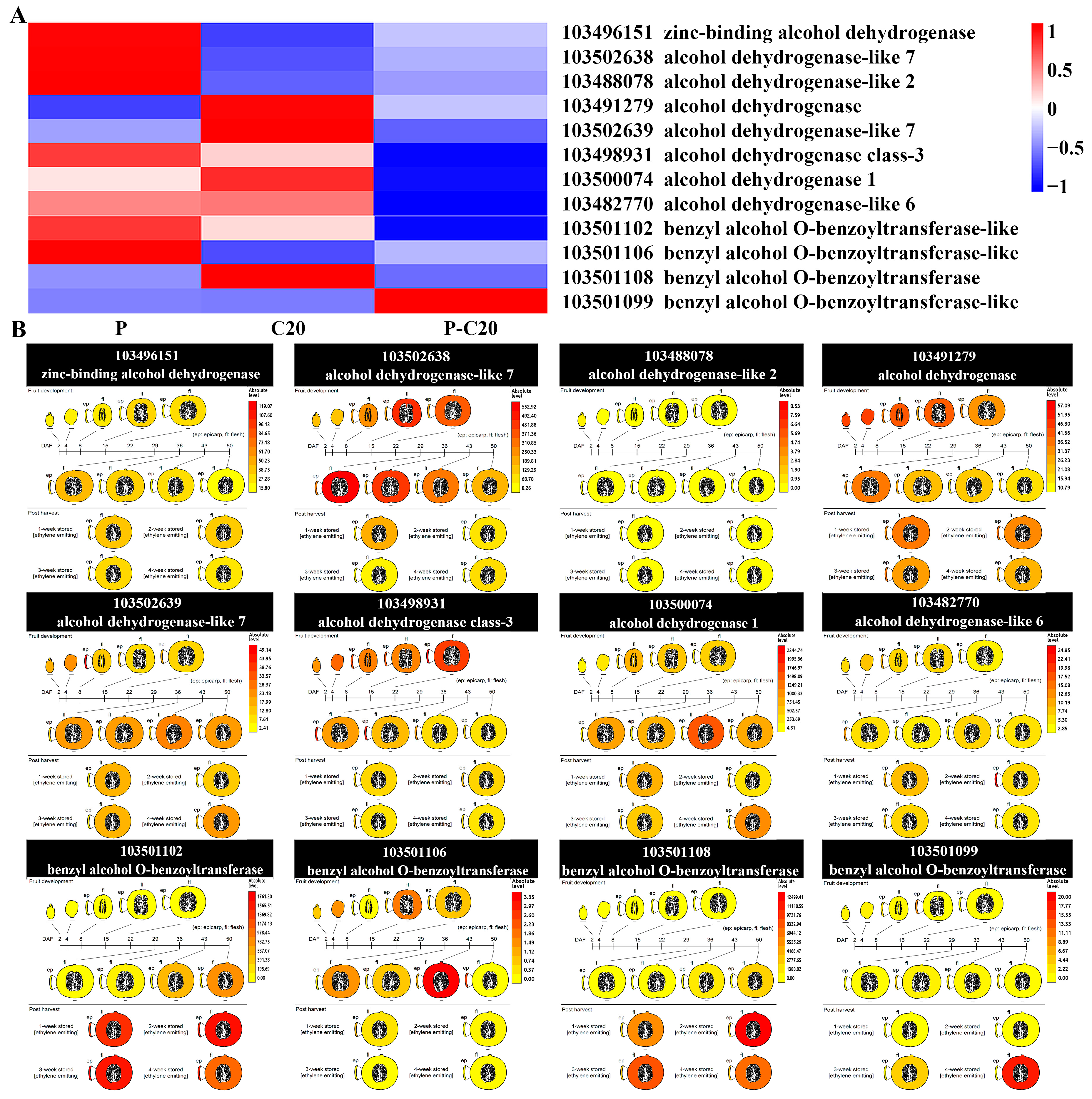
3.6. Expression Pattern of DEGs Involved in Fatty Acid Metabolism Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burger, Y.; Sa’ar, U.; Paris, H.; Lewinsohn, E.; Katzir, N.; Tadmor, Y.; Schaffer, A. Genetic variability for valuable fruit quality traits in Cucumis melo. Israel J. Plant Sci. 2006, 54, 233–242. [Google Scholar] [CrossRef]
- Burger, Y.; Paris, H.S.; Cohen, R.; Katzir, N.; Tadmor, Y.; Lewinsohn, E.; Schaffer, A.A. Genetic Diversity of Cucumis Melo. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 165–198. ISBN 9780470527238. [Google Scholar]
- Lester, G.E.; Arias, L.S.; Gomez-Lim, M. Muskmelon Fruit Soluble Acid Invertase and Sucrose Phosphate Synthase Activity and Polypeptide Profiles during Growth and Maturation. J. Am. Soc. Hortic. Sci. 2001, 126, 33–36. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Hayata, Y.; Li, X.-X.; Osajima, Y. Pollination and CPPU Treatment Increase Endogenous IAA and Decrease Endogenous ABA in Muskmelons during Early Development. J. Am. Soc. Hortic. Sci. 2002, 127, 908–911. [Google Scholar] [CrossRef]
- Kawasaki, Y. Fruit Set and Temperature Stress. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 87–99. ISBN 978-4-431-55250-5. [Google Scholar]
- Hayata, Y.; Niimi, Y.; Iwasaki, N. Synthetic Cytokinin-1-(2=chloro=4=pyridyl)-3-phenylurea (CPPU)-Promotes Fruit Set and Induces Parthenocarpy in Watermelon. J. Am. Soc. Hortic. Sci. 1995, 120, 997–1000. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.; Xiang, X.; Xiang, H.; Jin, Z.; Khan, S.; Liu, Y. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Ainalidou, A.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Diamantidis, G.; Matsi, T. CPPU treatment and pollination: Their combined effect on kiwifruit growth and quality. Sci. Hortic. 2015, 193, 147–154. [Google Scholar] [CrossRef]
- Williamson, J.G.; NeSmith, D.S. Effects of CPPU Applications on Southern Highbush Blueberries. HortScience 2007, 42, 1612–1615. [Google Scholar] [CrossRef]
- Greene, D.W. CPPU Influences Fruit Quality and Fruit Abscission of McIntosh’ Apples. HortScience 2001, 36, 1292–1295. [Google Scholar] [CrossRef]
- Flaishman, M.; Amihai Shargal, A.S.; Raphael, S. The synthetic cytokinin CPPU increases fruit size and yield of Spadona’ and Costia’ pear (Pyrus communis L.). J. Hortic. Sci. Biotechnol. 2001, 76, 145–149. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M. Plant growth regulators improve sweet cherry fruit quality without reducing endocarp growth. Sci. Hortic. 2013, 150, 73–79. [Google Scholar] [CrossRef]
- Wang, W.; Khalil-Ur-Rehman, M.; Feng, J.; Tao, J. RNA-seq based transcriptomic analysis of CPPU treated grape berries and emission of volatile compounds. J. Plant Physiol. 2017, 218, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Hayata, Y.; Niimi, Y.; Inoue, K.; Kondo, S. CPPU and BA, with and without Pollination, Affect Set, Growth, and Quality of Muskmelon Fruit. HortScience 2000, 35, 868–870. [Google Scholar] [CrossRef]
- Hou, L.; Hao, H.; Yu, X. Applicaiton of CPPU on muskmelon alters fruit size and quality. Fresh Prod. 2011, 5, 26–29. [Google Scholar]
- Huang, Y.; Li, W.; Zhao, L.; Shen, T.; Sun, J.; Chen, H.; Kong, Q.; Nawaz, M.A.; Bie, Z. Melon fruit sugar and amino acid contents are affected by fruit setting method under protected cultivation. Sci. Hortic. 2017, 214, 288–294. [Google Scholar] [CrossRef]
- Allwood, J.W.; Cheung, W.; Xu, Y.; Mumm, R.; De Vos, R.C.H.; Deborde, C.; Biais, B.; Maucourt, M.; Berger, Y.; Schaffer, A.A.; et al. Metabolomics in melon: A new opportunity for aroma analysis. Phytochemistry 2014, 99, 61–72. [Google Scholar] [CrossRef]
- Fallik, E.; Alkali-Tuvia, S.; Horev, B.; Copel, A.; Rodov, V.; Aharoni, Y.; Ulrich, D.; Schulz, H. Characterisation of Galia’ melon aroma by GC and mass spectrometric sensor measurements after prolonged storage. Postharvest Biol. Technol. 2001, 22, 85–91. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Ling, L.C.; Soderstrom, E.L.; Ogawa, J.M.; Turnbaugh, J.G. Additional aroma components of honeydew melon. J. Agric. Food Chem. 1982, 30, 1208–1211. [Google Scholar] [CrossRef]
- Shi, J.; Wu, H.; Xiong, M.; Chen, Y.; Chen, J.; Zhou, B.; Wang, H.; Li, L.; Fu, X.; Bie, Z.; et al. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020, 316, 126342. [Google Scholar] [CrossRef]
- Fredes, A.; Sales, C.; Barreda, M.; Valcárcel, M.; Roselló, S.; Beltrán, J. Quantification of prominent volatile compounds responsible for muskmelon and watermelon aroma by purge and trap extraction followed by gas chromatography-mass spectrometry determination. Food Chem. 2016, 190, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Li, Q.; Yu, L.; Wang, C.; Qi, H. High concentrations of CPPU promotes cucurbitacin B accumulation in melon (Cucumis melo var. makuwa Makino) fruit by inducing transcription factor CmBt. Plant Physiol. Biochem. 2020, 154, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Grauwet, T.; Carle, R. Influence of harvest maturity and fruit logistics on pineapple (Ananas comosus L. Merr.) volatiles assessed by headspace solid phase microextraction and gas chromatography-mass spectrometry (HS-SPME-GC/MS). Food Chem. 2014, 150, 382–391. [Google Scholar] [CrossRef]
- Oberacher, H.; Whitley, G.; Berger, B. Evaluation of the sensitivity of the Wiley registry of tandem mass spectral data, MSforID’ with MS/MS data of the NIST/NIH/EPA mass spectral library’. J. Mass Spectrom. 2013, 48, 487–496. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Liu, L.; Yang, K.; Xiao, B.; Zhou, G.; Tian, Z.; Luo, C.; Xia, T.; Dong, L.; et al. Perigestational low-dose BDE-47 exposure alters maternal serum metabolome and results in sex-specific weight gain in adult offspring. Chemosphere 2019, 233, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Martin, J.A.; Wang, Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011, 12, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Bhat, N.R.; Desai, B.B.; Suleiman, M.K. Flavor in Grapes: Its Characterization and Commercial Applications. In Handbook of Fruit and Vegetable Flavors; Hui, Y.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 279–302. ISBN 9780470622834. [Google Scholar]
- Li, J.; Lin, T.; Ma, G. A new Hami melon cultivar ‘Dongfangmi 3’. Acta Hortic. Sin. 2020, 47, 3017–3018. (In Chinese) [Google Scholar] [CrossRef]
- Wyllie, S.G.; Leach, D.N. Aroma volatiles of Cucumis melo cv. Golden Crispy. J. Agric. Food Chem. 1990, 38, 2042–2044. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Ruiz, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Aroma profile of a collection of near-isogenic lines of melon (Cucumis melo L.). Food Chem. 2010, 118, 815–822. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Ohmiya, A. Diversity of Carotenoid Composition in Flower Petals. Jpn. Agric. Res. Q. JARQ 2011, 45, 163–171. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horváth, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Lewinsohn, E.; Rosati, C. Study of Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef]
- Falchi, R.; Vendramin, E.; Zanon, L.; Scalabrin, S.; Cipriani, G.; Verde, I.; Vizzotto, G.; Morgante, M. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013, 76, 175–187. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gómez, M.D.; Orzaez, D.; Granell, A.; Gómez-Gómez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Kato, M.; Fleischmann, P.; Watanabe, N. Biosynthesis of α- and β-ionone, prominent scent compounds, in flowers of Osmanthus fragrans. Acta Biochim. Pol. 2012, 59, 79–81. [Google Scholar] [CrossRef]
- Kemp, T.R.; Knavel, D.E.; Stoltz, L.P. cis-6-Nonenal: A flavor component of muskmelon fruit. Phytochemistry 1972, 11, 3321–3322. [Google Scholar] [CrossRef]
- Schieberle, P.; Ofner, S.; Grosch, W. Evaluation of Potent Odorants in Cucumbers (Cucumis sativus) and Muskmelons (Cucumis melo) by Aroma Extract Dilution Analysis. J. Food Sci. 1990, 55, 193–195. [Google Scholar] [CrossRef]
- Oh, S.H.; Lim, B.S.; Hong, S.J.; Lee, S.K. Aroma volatile changes of netted muskmelon (Cucumis melo L.) fruit during developmental stages. Hortic. Environ. Biotechnol. 2011, 52, 590–595. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Moreno, E.; García-Mas, J.; Nicolai, B.; Lammertyn, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Climacteric or non-climacteric behavior in melon fruit. Postharvest Biol. Technol. 2008, 49, 27–37. [Google Scholar] [CrossRef]
- Tressl, R.; Drawert, F. Biogenesis of banana volatiles. J. Agric. Food Chem. 1973, 21, 560–565. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; van der Goot, W.; van Kooij, A.J.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F.G. Development of a biocatalytic process for the production of c6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef]
- Kuroda, H.; Oshima, T.; Kaneda, H.; Takashio, M. Identification and functional analyses of two cDNAs that encode fatty acid 9-/13-hydroperoxide lyase (CYP74C) in rice. Biosci. Biotechnol. Biochem. 2005, 69, 1545–1554. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, S.; Jin, Y.; Ju, L.; Chen, Q.; Xing, Q.; Qi, H. Melon13-lipoxygenase CmLOX18 may be involved in C6 volatiles biosynthesis in fruit. Sci. Rep. 2017, 7, 2816. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Lee, G.I.; Itoh, A.; Li, L.; DeRocher, A.E. Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol. 2000, 123, 711–724. [Google Scholar] [CrossRef]
- Shalit, M.; Katzir, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M.; et al. Acetyl-coa: Alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J. Agric. Food Chem. 2001, 49, 794–799. [Google Scholar] [CrossRef]
- Beaulieu, J.C. Volatile Changes in Cantaloupe during Growth, Maturation, and in Stored Fresh-cuts Prepared from Fruit Harvested at Various Maturities. J. Am. Soc. Hortic. Sci. 2006, 131, 127–139. [Google Scholar] [CrossRef]
- Qian, Z.W.; Tang, X.W.; Wu, Z.; Yang, M.H.; Liu, M.C. Comparison of the aromatic compounds and nutritional quality among different types of melon fruits. Chin. Agric. Sci. Bull. 2009, 25, 165–171. (In Chinese) [Google Scholar]
- Kemp, T.R.; Knavel, D.E.; Stoltz, L.P. Volatile Cucumis melo components: Identification of additional compounds and effects of storage conditions. Phytochemistry 1973, 12, 2921–2924. [Google Scholar] [CrossRef]
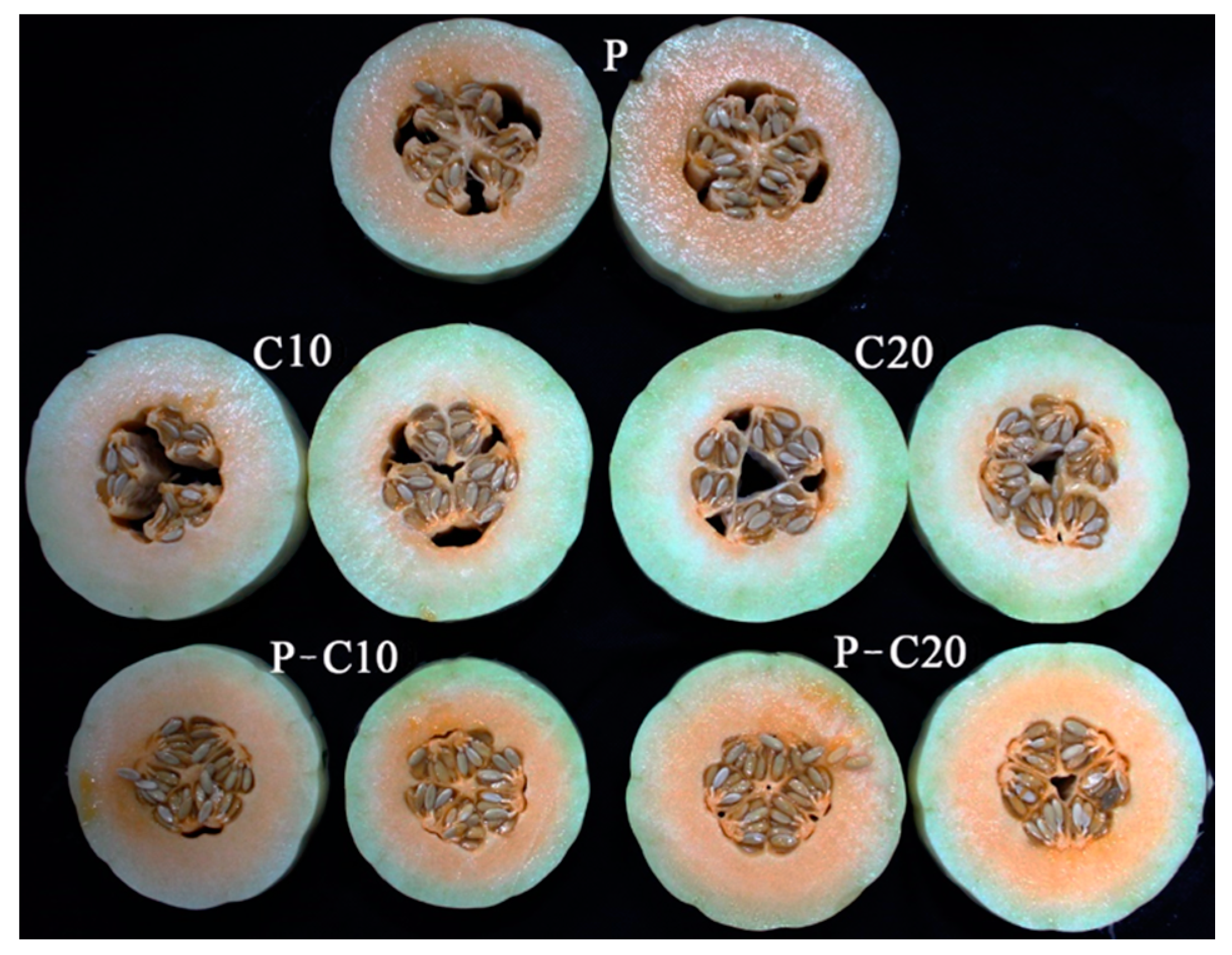
| Treatment | Seeds/Fruit | TA (%) | SS (%) | SS/TA | Pulp Color Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Null | Plump | Center | Edge | L* | a* | b* | |||
| P | 53 a | 712 c | 0.023 b | 12.27 a | 6.83 a | 540.86 b | 67.79 a | 9.00 b | 22.42 b |
| C10 | 550 c | 0 a | 0.026 b c | 12.00 a | 6.00 a | 475.47 a b | 67.77 a | 7.56 a | 19.78 a |
| C20 | 685 d | 0 a | 0.030 c | 12.26 a | 6.27 a | 409.74 a | 68.03 a | 7.52 a | 19.89 a |
| P-C10 | 243 b | 516 b | 0.015 a | 13.33 a | 5.51 a | 877.45 c | 68.17 a | 9.64 b | 23.37 b |
| P-C20 | 307 b | 543 b | 0.015 a | 13.83 a | 7.17 a | 879.71 c | 68.12 a | 9.58 b | 23.99 b |
| Types | P | C10 | C20 | P-C10 | P-C20 |
|---|---|---|---|---|---|
| All volatiles | 67 | 68 | 73 | 61 | 65 |
| Aldehydes | 12 | 6 | 6 | 5 | 5 |
| Alcohols | 18 | 21 | 22 | 22 | 21 |
| Alkanes | 6 | 6 | 5 | 4 | 5 |
| Esters | 10 | 13 | 13 | 5 | 6 |
| Terpenes | 2 | 3 | 2 | 2 | 3 |
| Acids | 2 | 4 | 4 | 3 | 4 |
| Ketones | 9 | 9 | 12 | 12 | 13 |
| Others | 8 | 6 | 9 | 8 | 8 |
| Type | P | C20 | P-C20 | Total |
|---|---|---|---|---|
| Total clean reads | 47,417,428 | 46,854,420 | 46,704,089 | 140,975,937 |
| Total mapped reads (%) | 40,730,142 (85.9%) | 42,789,550 (91.32%) | 43,752,495 (93.68%) | 127,272,187 |
| Unique mapped reads (%) | 40,291,259 (84.97%) | 42,322,747 (90.33%) | 43,281,516 (92.67%) | 125,895,522 |
| GO Domain | GO ID | Description | p-Value (<0.05) | Upregulated DEGs | Downregulated DEGs |
|---|---|---|---|---|---|
| C20 vs. P | |||||
| CC | GO:0000502 | proteasome complex | 0.000373 | 15 | 0 |
| CC | GO:1905369 | endopeptidase complex | 0.000373 | 15 | 0 |
| CC | GO:0005839 | proteasome core complex | 0.001694 | 13 | 0 |
| CC | GO:0098796 | membrane protein complex | 0.001752 | 46 | 17 |
| CC | GO:0031090 | organelle membrane | 0.005092 | 24 | 4 |
| CC | GO:0000148 | 1,3-beta-D-glucan synthase complex | 0.005436 | 4 | 4 |
| CC | GO:0016469 | proton-transporting two-sector ATPase complex | 0.005773 | 14 | 3 |
| CC | GO:0044433 | cytoplasmic vesicle part | 0.007137 | 11 | 0 |
| CC | GO:0048475 | coated membrane | 0.008665 | 18 | 0 |
| MF | GO:0046982 | protein heterodimerization activity | 1.64 × 10−6 | 31 | 7 |
| P-C20 vs. P | |||||
| BP | GO:0044262 | cellular carbohydrate metabolic process | 0.000112 | 27 | 25 |
| CC | GO:0000502 | proteasome complex | 0.001535 | 13 | 1 |
| CC | GO:1905369 | endopeptidase complex | 0.001535 | 13 | 1 |
| CC | GO:0000148 | 1,3-beta-D-glucan synthase complex | 0.004909 | 3 | 5 |
| CC | GO:0005794 | Golgi apparatus | 0.005856 | 12 | 2 |
| CC | GO:0005839 | proteasome core complex | 0.006268 | 11 | 1 |
| CC | GO:0044433 | cytoplasmic vesicle part | 0.0063 | 10 | 1 |
| CC | GO:0048475 | coated membrane | 0.007316 | 17 | 1 |
| P-C20 vs. C20 | |||||
| MF | GO:0046982 | protein heterodimerization activity | 0.00011 | 6 | 24 |
| MF | GO:0016616 | oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | 0.00014 | 8 | 27 |
| Pathway ID | Description | p-Value | Up-DEGs | Down-DEGs |
|---|---|---|---|---|
| C20 vs. P | ||||
| cmo01200 | Carbon metabolism | 3.79 × 10−7 | 80 | 48 |
| cmo00010 | Glycolysis/gluconeogenesis | 4.57 × 10−5 | 45 | 17 |
| cmo03050 | Proteasome | 0.000106 | 33 | 1 |
| cmo00020 | Citrate cycle | 0.000438 | 24 | 6 |
| cmo00710 | Carbon fixation in photosynthetic organisms | 0.000754 | 26 | 12 |
| cmo04141 | Protein processing in endoplasmic reticulum | 0.003614 | 62 | 26 |
| cmo01230 | Biosynthesis of amino acids | 0.004817 | 64 | 36 |
| cmo00270 | Cysteine and methionine metabolism | 0.005417 | 26 | 22 |
| cmo00620 | Pyruvate metabolism | 0.019647 | 28 | 13 |
| cmo00410 | Beta-alanine metabolism | 0.025858 | 12 | 10 |
| cmo01210 | 2-Oxocarboxylic acid metabolism | 0.025864 | 15 | 8 |
| cmo00520 | Amino sugar and nucleotide sugar metabolism | 0.027494 | 36 | 19 |
| cmo00630 | Glyoxylate and dicarboxylate metabolism | 0.030972 | 18 | 15 |
| cmo00100 | Steroid biosynthesis | 0.036458 | 12 | 3 |
| cmo00906 | Carotenoid biosynthesis | 0.055387 | 8 | 10 |
| cmo00071 | Fatty acid degradation | 0.244985 | 8 | 12 |
| cmo01212 | Fatty acid metabolism | 0.364423 | 15 | 10 |
| cmo00999 | Biosynthesis of secondary metabolites | 0.462116 | 6 | 1 |
| cmo00061 | Fatty acid biosynthesis | 0.741792 | 11 | 2 |
| cmo00592 | Alpha-linolenic acid metabolism | 0.969227 | 5 | 6 |
| P-C20 vs. P | ||||
| cmo00410 | Beta-alanine metabolism | 0.00065 | 5 | 20 |
| cmo01200 | Carbon metabolism | 0.000949 | 47 | 60 |
| cmo04144 | Endocytosis | 0.001829 | 62 | 13 |
| cmo00280 | Valine, leucine and isoleucine degradation | 0.001919 | 5 | 22 |
| cmo03050 | Proteasome | 0.002888 | 27 | 2 |
| cmo00960 | Tropane, piperidine and pyridine alkaloid biosynthesis | 0.003568 | 3 | 9 |
| cmo03030 | DNA replication | 0.00469 | 21 | 6 |
| cmo00270 | Cysteine and methionine metabolism | 0.005184 | 20 | 25 |
| cmo01230 | Biosynthesis of amino acids | 0.012349 | 39 | 53 |
| cmo00020 | Citrate cycle | 0.034884 | 16 | 7 |
| cmo00710 | Carbon fixation in photosynthetic organisms | 0.049507 | 15 | 15 |
| cmo00071 | Fatty acid degradation | 0.153584 | 5 | 15 |
| cmo00592 | Alpha-linolenic acid metabolism | 0.177497 | 6 | 12 |
| cmo01212 | Fatty acid metabolism | 0.188288 | 9 | 16 |
| cmo00906 | Carotenoid biosynthesis | 0.571574 | 3 | 9 |
| cmo00061 | Fatty acid biosynthesis | 0.828661 | 6 | 5 |
| cmo00591 | Linoleic acid metabolism | 0.864514 | 1 | 2 |
| P-C20 vs. C20 | ||||
| cmo01230 | Biosynthesis of amino acids | 9.25 × 10−6 | 27 | 66 |
| cmo00710 | Carbon fixation in photosynthetic organisms | 1.07 × 10−5 | 11 | 25 |
| cmo00280 | Valine, leucine and isoleucine degradation | 2.22 × 10−5 | 2 | 26 |
| cmo01212 | Fatty acid metabolism | 7.77 × 10−5 | 8 | 24 |
| cmo01200 | Carbon metabolism | 0.000115 | 22 | 75 |
| cmo00250 | Alanine, aspartate and glutamate metabolism | 0.00033 | 7 | 21 |
| cmo00630 | Glyoxylate and dicarboxylate metabolism | 0.0004 | 8 | 25 |
| cmo00640 | Propanoate metabolism | 0.000946 | 1 | 18 |
| cmo00400 | Phenylalanine, tyrosine and tryptophan biosynthesis | 0.000996 | 6 | 17 |
| cmo00270 | Cysteine and methionine metabolism | 0.001372 | 13 | 28 |
| cmo00071 | Fatty acid degradation | 0.002424 | 5 | 18 |
| cmo00010 | Glycolysis/Gluconeogenesis | 0.007568 | 9 | 36 |
| cmo00410 | beta-Alanine metabolism | 0.008243 | 4 | 16 |
| cmo00480 | Glutathione metabolism | 0.009575 | 5 | 30 |
| cmo00620 | Pyruvate metabolism | 0.014003 | 5 | 29 |
| cmo00900 | Terpenoid backbone biosynthesis | 0.022365 | 3 | 19 |
| cmo00592 | Alpha-linolenic acid metabolism | 0.026348 | 4 | 15 |
| cmo00520 | Amino sugar and nucleotide sugar metabolism | 0.032696 | 22 | 22 |
| cmo00999 | Biosynthesis of secondary metabolites | 0.043666 | 1 | 8 |
| cmo00061 | Fatty acid biosynthesis | 0.055871 | 4 | 12 |
| cmo00906 | Carotenoid biosynthesis | 0.248993 | 5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lin, T.; Ren, D.; Wang, T.; Tang, Y.; Wang, Y.; Xu, L.; Zhu, P.; Ma, G. Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit. Agronomy 2021, 11, 1007. https://doi.org/10.3390/agronomy11051007
Li J, Lin T, Ren D, Wang T, Tang Y, Wang Y, Xu L, Zhu P, Ma G. Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit. Agronomy. 2021; 11(5):1007. https://doi.org/10.3390/agronomy11051007
Chicago/Turabian StyleLi, Jufen, Tao Lin, Dandan Ren, Tan Wang, Ying Tang, Yiwen Wang, Ling Xu, Pinkuan Zhu, and Guobin Ma. 2021. "Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit" Agronomy 11, no. 5: 1007. https://doi.org/10.3390/agronomy11051007
APA StyleLi, J., Lin, T., Ren, D., Wang, T., Tang, Y., Wang, Y., Xu, L., Zhu, P., & Ma, G. (2021). Transcriptomic and Metabolomic Studies Reveal Mechanisms of Effects of CPPU-Mediated Fruit-Setting on Attenuating Volatile Attributes of Melon Fruit. Agronomy, 11(5), 1007. https://doi.org/10.3390/agronomy11051007







