Abstract
There is evidence that chloride (Cl―) can lead to both an improved hydration and water use efficiency in plants due to its osmotic properties. The potato crop is widely assumed to be sensitive to Cl―. This is based on studies which found tuber yield or tuber starch reductions following a Cl― fertilization. However, there are also contradictory reports which could not find any detrimental effect of Cl― fertilization on potato plant development. As potato is inefficient in the use of water, we aimed to test if it is possible to improve the hydration status of potato without reducing tuber yield and dry matter by means of Cl― fertilization. We conducted a pot experiment with four different Cl― doses and investigated soil–plant water relations, biomass, tuber yield and dry matter development. Our findings deliver an indication that the potato crop is much less sensitive to Cl― than previously assumed and, more importantly, that a Cl― supply can indeed improve the potato shoot water status. This happened without impairing tuber yield and dry matter. Since potato is very sensitive to drought stress, we assume that Cl― fertilization is a promising measure to improve the drought resilience of potato.
1. Introduction
With regard to chloride (Cl―), glycophytic plants are subdivided into Cl― tolerant, intermediate Cl― tolerant, and Cl― sensitive plants [1]. The potato (Solanum tuberosum L.) is widely assumed to be sensitive to Cl― [2,3]. Typical Cl― toxicity symptoms in glycophytic plants are reductions in biomass of the whole plant, leaf necrosis or even leaf burning [4]. Such Cl― toxicity symptoms are frequently reported for glycophytic plants exceeding a Cl― shoot concentration of 33 mg g dw−1 [1,4]. However, in a study by Hütsch et al. [5], potato plants did not show any green biomass reductions, leaf necrosis or leaf burning while reaching Cl― shoot concentrations of 65–74 mg Cl― g dw−1. The prevailing assumption that the potato is sensitive to Cl― is derived from reports which showed that Cl―-containing fertilizers reduce tuber yield [6], tuber dry matter [7] and specific gravity or tuber starch concentrations [8]. However, there are also reports which did not find any Cl―-related effect of a potassium-chloride (KCl) fertilization on tuber yield, dry matter or starch when compared to a potassium-sulphate (K2SO4) fertilization [5,9,10]. One reason why some studies reported about tuber yield, dry matter or starch reductions and others did not could be the use of different cultivars that may respond differently to salt stress [11]. Moreover, divergent outcomes could be related to different agricultural management practices used in the studies, especially with regard to the water supply of the plant. Chloride is easily prone to leaching [12], especially in sandy soils. Thus, it is likely that differences in irrigation and differences between soil types may lead to different results with respect to the impact of Cl― on potato. In addition, it matters if Cl― is applied in the immediate vicinity of the plant, or whether it is distributed rather inaccurately over the field [13]. While the authors found a decrease in tuber yield and starch concentrations (as estimated by specific gravity measurements) from plots fertilized with KCl in the row, they could not detect any effect of broadcast KCl fertilizer application. The plant certainly absorbed more Cl― if the Cl― delivering fertilizer has been applied in its immediate vicinity. Each of the mentioned factors are likely to influence the final amount of Cl― that is taken up by the plant. However, all studies have in common that the above-ground biomass is not negatively affected by Cl―.
Chloride is an essential micronutrient, thus, being required in very small quantities [14]. However, Cl― can accumulate in the vacuole of plant cells reaching concentrations of a macronutrient, where it acts as an osmoticum [4]. In this context, there are reports of beneficial functions of Cl― in a concentration range of a macronutrient, which is why Cl― has even been referred to as beneficial macronutrient [15,16]. Franco-Navarro et al. [17] reported about highly increased Cl― leaf concentrations (51.08 mg Cl― g dw−1) upon a Cl― treatment in tobacco plants (Nicotiana tabacum L. var. Habana). This Cl― accumulation resulted in a lower osmotic potential, increased water contents, and a lower transpiration rate (without showing a decrease in CO2 assimilation) of leaves of these plants. Besides, they found increased leaf sizes and shoot biomasses. These increases may be attributable to the lower osmotic potential in the Cl― treated plants. An increase in turgor and water content likely led to increased cell expansion in the leaves caused by the osmotic properties of Cl― that accumulated in these leaves. Finally, the authors reported an improved water use efficiency (WUE) defined as the increase in plant fresh weight over time per accumulated water consumption (g FW mL H2O–1).
These described osmotic properties, which lead to beneficial effects in plants, such as an improved plant hydration status, may on the other hand also be the reason for the above described negative effects such as lower tuber yields and tuber starch concentrations reported in connection with Cl― fertilization in potato. Beringer et al. [18] stated that only the KCl treatment lead to an alteration of sink and source strengths in the form of an increased sink strength of the shoot over the tubers when comparing a fertilization of potato with KCl and K2SO4. The authors found a lower osmotic potential in leaf cell sap, an increased leaf water content as well as an increased shoot growth in Cl― treated potato plants, similar to the findings as reported by Franco-Navarro et al. [17]. The authors suggested that the increased shoot growth, which was induced by an increased cell expansion due to the osmotic properties of Cl―, results in an increased consumption of photo-assimilates of the shoots. This finally led to a lack of sucrose export to the tubers and could be the reason for lower tuber yields or lower tuber starch concentrations upon a Cl― supply. Tuber yield and tuber starch reductions, however, seems to depend on the amount of Cl― taken up, as demonstrated by divergent outcomes of previously presented studies which investigated the impact of Cl― fertilization on potato tuber and starch yields.
Restricted water availability results in severe tuber yield and quality reductions [19]. In 2018 in Germany, for instance, the tuber yields were reduced by 16% due to lacking precipitation compared to the mean tuber yields of the years 2013–2017 [20]. In the course of climate change, more frequent dry spells are expected in central Europe [21]. Thus far, however, there are no sensible measures available that guarantee stable potato yields and quality despite limited water availability. Therefore, motivated by the study by Franco-Navarro et al. [17], which demonstrated that a Cl― supply can improve the WUE of tobacco plants, the aim of the present study was to find out if a Cl― fertilization may also result in an improved plant hydration status in potato. The study by Franco-Navarro et al. [17] is based on an artificial semi-hydroponic system and only considers the effects of Cl― on green biomass. Therefore, we extended our study to a soil-based system and focused on a below-ground sink organ, namely the potato tuber, in addition to the green biomass development. We aimed to answer whether it is possible to improve the plant hydration status of potato with the help of Cl― fertilization but without reducing tuber yield and tuber dry matter. Thus, we conducted a pot experiment with four different Cl― supplies (0, 200, 400, and 800 mg Cl― kg−1 soil). We hypothesized that a moderate increased Cl― supply (200 or 400 mg Cl― kg−1 soil) to potato would (i) result in improved plant hydration status without reduction in tuber yield and tuber dry matter, while (ii) a high Cl― supply (800 mg Cl― kg−1 soil) still improves the plant hydration status but causes tuber yield and tuber dry matter reductions.
2. Materials and Methods
2.1. Experimental Setup and Plant Growth Conditions
Potato plants of the cultivar Afra were grown individually in 6-L Mitscherlich pots with an air dried and sieved (2 mm mesh) sandy soil (WRB-type: Albic Luvisol) in a covered but translucent outdoor installation for 87 days. Further soil physical and chemical parameters are given in Supplementary Table S1. Before planting, the soil was treated with 0 mg, 200 mg, 400 mg or 800 mg Cl― kg soil−1 (á 4 replicates). Chloride was added via CaCl2. The supply of further nutrients was 50 mg of potassium kg soil−1 via K2SO4, 100 mg of magnesium kg soil−1 via MgSO4, 400 mg of nitrogen kg soil−1 via KNO3, 200 mg of phosphorus kg soil−1 via CaHPO4 × 2H2O, 2 mg of boron kg soil−1 via H3BO3, 2 mg of zinc kg soil−1 via ZnSO4 × 7H2O, 6 mg of manganese kg soil−1 via MnSO4 × H2O, 3 of mg iron kg soil−1 via Fe(III) EDTA, and 0.01 of mg molybdenum kg soil−1 via Na2MoO4. For planting, mother tubers were pre-sprouted until sprouts had a length of approximately 1 cm. Only tuber pieces with one sprout were taken with the help of a melon spoon. Two tuber pieces per pot were planted and a few days after plant emergence the weaker plant was removed. The plants grew in a completely randomized design.
2.2. Measurements Describing Soil-Water Plant Relations
The whole plant transpiration, the relative leaf water content (RWC), and the soil moisture were analyzed 40 and 47 days after planting (DAP). All measurements were examined after irrigation which was carried out according to demand (meaning, when the soil of the control plants was dry) with de-ionized water every second to third day. Hereby, the given water amount was orientated on control plants and each plant received the same amount of water. The water amount ranged from 100 to 300 mL per pot. The whole plant transpiration was quantified by weighing each pot at three time points (9:30 a.m., 12:30 p.m., and 15:30 p.m.). Prior to taking the weight of each pot, the soil was covered with cling film and weighted down with gravel in order to exclude water loss from the soil via evaporation. It was assumed that the weight loss of the pots between the measurement times was caused by water loss through transpiration. The determination of RWC was conducted as described in González and González-Vilar [22] based on the original method by Barrs and Weatherley [23]. The method can be used as an indicator for a water deficit in leaves as it compares the initial and the turgid water content. For the RWC determination, leaf discs of older fully developed but not senescent leaves (please also see section “Relative leaf chlorophyll concentrations” for how older leaves were identified in this study) were taken.
The soil moisture was recorded with a HH2 Moisture Meter (Delta-T Devices Ltd., Cambridge, UK).
Finally, the whole plant water content was determined at harvest (87 DAP) by taking the weight difference of fresh and dry weight of the whole shoots.
2.3. Biomass Recording, Tuber Yield and Tuber Dry Matter
During the plant growth period, the plant height was determined with a folding ruler at seven different times from 21 until 64 DAP. The plant height was noted at the highest point of the plant starting from the soil surface of the used Mitscherlich pots. The plant was measured as it had grown, so it was not elongated.
At harvest (87 DAP), shoots were cut directly above the soil, the fresh weight was taken and the shoots were dried at 105 °C for three days. Following this, the shoot dry matter was assessed.
The tuber yield and tuber number were determined by separating the tubers from the soil, stolones and roots. Subsequently, the tubers were counted and their fresh weights were recorded.
For the assessment of the root biomass, the roots were washed out of the soil using a sieve with a mesh size of 0.63 mm. The coarser roots were removed from the sieve using tweezers. The residue was re-suspended in water and finer roots were separated with tweezers. The obtained roots were dried at 105 °C for three days and dry root weights were taken.
For the determination of dry matter, three medium-sized tubers of each pot were taken, cut into equally large pieces, and their fresh weight was examined. Subsequently, the tuber pieces were dried at 60 °C for 24 h and then at 105 °C for 4 h in a drying chamber and the dry matter (%) was calculated.
2.4. Leaf Chloride Analyses
Chloride was measured in older and fully developed, but not senescent, leaves 52 DAP. The leaves were dried at 60 °C for three days in a drying chamber and grounded in a ball mill (Schwingmühle MM 400, Retsch, Haan, Germany) at a frequency of 30 for 60 s. 20 mg of dried and homogenized material was dissolved in 1 mL of 0.1 M HCl. The samples were centrifuged at 23 °C and 25,000× g for 30 min. The supernatant was transferred to a centrifugal filter (PES, MWCO 3 kD, VWR, Monroeville, PA, USA) and centrifuged at 8000× g and 23 °C for 60 min. After filtration, the samples were diluted 20 times with deionized water and analyzed using the Metrohm ECO IC system, with a Na2CO3/NaHCO3 gradient over a Metrosep A Supp 17 150/4.0 column with a flow of 0.7 mL/min.
2.5. Relative Leaf Chlorophyll Concentrations
The relative leaf chlorophyll concentrations were assessed using a portable chlorophyll meter (SPAD-502, Minolta, Japan) in young and old leaves at four different times from 35 until 56 DAP. With regard to the division into young and old leaves, it should be noted that no generally valid division into young and old plant parts is possible in the case of potato, since new leaves are emerging in each part of the plant throughout the whole growth period. Finally, older as well as younger leaves can basically be found in each part of the plant. However, most of the time, old fully developed leaves are mainly located in the lower part, while young still developing leaves are usually rather located in the upper part of the plant [24].
2.6. Statistics
Statistical analyses were performed using the R software version 4.0.2 2020. First, a suitable statistical mixed model was defined. The data were assumed to be normally distributed and to be heteroscedastic due to the different levels of “chloride treatment”, “sampling date”, and “leaf age”. This assumption is based on a graphical residual analysis. The statistical model included “chloride treatment”, “sampling date”, and “leaf age” as well as all their interaction terms (two-fold and three-fold) as fixed factors. The plant (and the sampling date, nested in plant) was regarded as a random factor. Based on this model, a Pseudo R2 was calculated and an analysis of variances (ANOVA) with following multiple contrast tests was performed [25,26].
3. Results
3.1. Soil–Plant Water Relations
Plants with a Cl― supply of 800 mg Cl― kg soil−1 transpired significantly less compared to both plants supplied with 200 mg Cl― kg soil−1 and control plants (0 mg Cl― kg soil−1) (Figure 1A). Nevertheless, these plants (supplied with 800 mg Cl― kg soil−1) had a relative leaf water content similar to that of all other Cl― treatments, including the control plants (Figure 1B). In addition, pots which received a Cl― fertilization of 800 mg Cl― kg soil−1 showed a significantly higher soil moisture (Figure 1C). Furthermore, at harvest, the plants supplied with 800 mg Cl― kg soil−1 had significantly higher shoot water contents (Figure 2A).
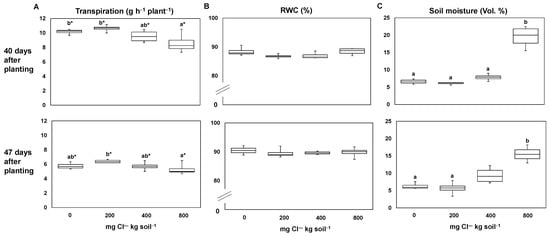
Figure 1.
The effect of increasing chloride (Cl―)-fertilization on plant and soil water relations. Transpiration per plant (A), RWC (B), and soil moisture (C) at 40 days (above) and 47 days (below) after planting. Plants were treated with four Cl― supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. Different lowercase letters indicate significant differences between Cl― treatments determined by multiple contrast test. No given letter means there was no significant effect. Levels of significance: p < 0.05; * = p < 0.1.
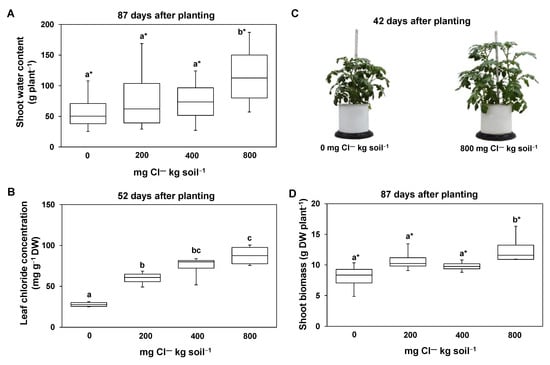
Figure 2.
The effect of increasing chloride (Cl―)-fertilization on leaf chloride concentrations, shoot biomass, and shoot water content. Shoot water content at harvest (87 days after planting) (A), leaf Cl― concentrations 52 days after planting (B), representative phenotypes of plants being treated by 0 or 800 mg Cl― kg soil−1 (folding rule: 62 cm) at 42 days after planting (C), and shoot biomass at harvest (D). Plants were treated with four Cl― supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. Different lowercase letters indicate significant differences between Cl― treatments determined by multiple contrast test. Levels of significance: p < 0.05; * = p < 0.1.
3.2. Above- And Belowground Biomass
The plants supplied with 800 mg Cl― kg soil−1 showed mean Cl― leaf concentrations of 88 ± 13 mg Cl― g dw−1 (Figure 2B). These Cl― leaf concentrations were significantly higher compared to control plants which exhibited on average 28 ± 3 mg Cl― g dw−1. Since no Cl― was fertilized, this Cl― originates from the soil. No plants had any leaf necrosis or growth reductions (Figure 2C). Of note, plants supplied with 800 mg Cl― kg soil−1 exhibited a significantly higher shoot biomass compared to all other Cl―-treated plants (Figure 2C,D). However, plants supplied with 800 mg Cl― kg soil−1 showed lower biomasses until approximately day 30 after planting. This is witnessed by the lower heights of these plants compared to all other Cl―-treated plants (Figure 3A). Later, plants supplied with 800 mg Cl― kg soil−1 were the highest. The relative leaf chlorophyll concentrations (estimated by the SPAD value, Figure 3B,C) showed an opposite trend. Plants supplied with 800 mg Cl― kg soil−1 had the lowest relative chlorophyll concentrations. Significant differences in the relative chlorophyll concentrations between young and old leaves only occurred in the control and in 200 mg Cl― kg soil−1-supplied plants (Figure 3B,C).
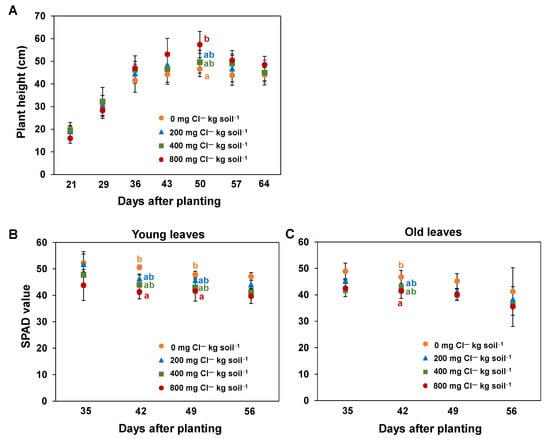
Figure 3.
The effect of increasing chloride (Cl―)-fertilization on plant height and relative leaf chlorophyll concentrations. Plant height at seven different times during the growth period from day 21 to day 64 after planting (A) and relative chlorophyll concentrations indicated by the dimensionless SPAD value in young (B) and old leaves (C) at four different times during plant growth from 35 until 56 days after planting. Plants were treated with four Cl― supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. Different lowercase letters indicate significant differences between Cl― treatments at one time point and one plant age (young or old leaves) determined by multiple contrast test. Levels of significance: p < 0.05.
The different Cl― treatments did not show a significant effect on root biomass (Supplementary Figure S1).
3.3. Tuber Yield, Dry Matter and Tuber Number
The tuber yield, the tuber dry matter, and the tuber number were not significantly affected by the Cl― treatment (Figure 4). However, there was a trend of a smaller number of tubers in plants supplied with 400 mg Cl― kg soil−1 and 800 mg Cl― kg soil−1 when compared to the other two treatments (Figure 4C).
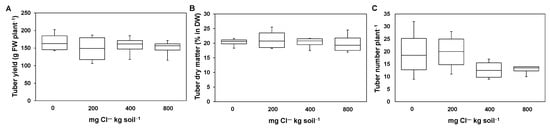
Figure 4.
The effect of increasing chloride (Cl―)-fertilization on tuber yield, tuber dry matter and tuber number. Tuber yield (A), tuber dry matter (B), and tuber number at harvest (C) (87 days after planting). Plants were treated with four Cl― supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. No indication means there was no significant effect.
4. Discussion
Our findings can confirm the initially formulated hypotheses that a Cl― fertilization can lead to an improved plant hydration status in potato. However, contrary to our expectation, this was only true in plants supplied with the highest amount of Cl―, namely plants supplied with 800 mg Cl― kg soil−1. These plants transpired significantly less compared to the control plants and plants supplied with 200 mg Cl― kg soil−1 (Figure 1A). Still, plants supplied with 800 mg Cl― kg soil−1 had a RWC similar to that of all other Cl―-treated plants (Figure 1B). Furthermore, at harvest, the plants supplied with 800 mg Cl― kg soil−1 had the significantly highest shoot water contents (Figure 2A), which further demonstrates that these plants had an improved hydration status. In addition, the high Cl― fertilization significantly increased soil moisture (Figure 1C). This also suggests that the highly Cl―-fertilized plants (800 mg Cl― kg soil−1) transpired less because they still had a sufficient hydration status, as evidenced by the relative leaf water contents (Figure 1B). The lower transpiration rate is likely the result of an increased synthesis of abscisic acid as a consequence of a Cl―-induced apoplastic alkalinization [27]. This in turn leads to a stomata closure and thus to a lower transpiration rate [27]. The fact that plants supplied with 800 mg Cl― kg soil−1 still had a sufficient hydration status can be explained by the osmotic properties of Cl―: the Cl― accumulation found in the leaves of the higher Cl―-fertilized plants probably resulted in a decrease in the osmotic potential leading to an increase in the water content in leaves, which is an often-reported observation caused by Cl― in plants [4]. Due to this, the plants supplied with 800 mg Cl― kg soil−1 were able to use the available soil water more efficiently compared to the other treated plants, while they exhibited a higher soil moisture. These data indicate that a Cl― fertilization in advance of an expected soil water shortage could be effective in maintaining plant water status. However, this needs to be tested and this could also depend on the cultivar.
The increased water content in leaves in turn stimulates cell extension growth, leading to larger leaf cell sizes [17]. This cell growth stimulating effect of Cl― was probably the reason that resulted in increased shoot growth of the plants in the present study (Figure 2C,D and Figure 3A). Besides, the lower relative chlorophyll concentrations in plants supplied with 800 mg Cl― kg soil−1 (Figure 3B,C) may be attributed to this cell growth stimulating effect as cell expansion driven by Cl― may dilute chloroplast concentration. Franco-Navarro et al. [17,28] 2019) likewise attributed the increased WUE in their study to this anatomical change caused by Cl―. They observed a lower stomatal density due to enlarged leaf cells, resulting in a lower stomatal conductance, which also may cause a decrease in transpiration rate. Although the authors found a lower transpiration rate in Cl―-treated plants, they did not observe a decrease in CO2 assimilation. This is explained by an increased mesophyll diffusion conductance to CO2 due to an increased surface area of chloroplast. Finally, the improved mesophyll diffusion conductance to CO2 was able to compensate the lower stomatal conductance [28]. Similar findings were reported by Cui et al. [29] recently in Pugionium cornutum—a xerophytic desert plant being widely distributed in Central Asia and belonging to the family of Brassicaceae. We assume that similar osmotic adjustments took place in potato in the present study, causing a decreased transpiration (Figure 1A) but without impairing photosynthesis and the partitioning of photoassimilates to the tubers as we could not find significant reductions in tuber yield or tuber dry matter (Figure 4A,B). The dry matter and the starch concentration of a tuber are usually closely related as starch is the major component of the dry matter in a potato tuber [30]. Thus, the dry matter of a tuber is indicative for its starch concentrations.
Plants, supplied with 800 mg Cl― kg soil−1, had lower biomasses shown by the lower plant heights (Figure 3A) in the first weeks of plant growth. A delayed crop emergence and plant growth following a Cl― fertilization was also reported by Van Loon and Van den Berg [7]. In addition, the authors reported about reduced dry matter in tubers of Cl―-treated plants. However, in the present study, the initial delay in crop growth seems to have been compensated by the later increased biomass development (Figure 2C,D and Figure 3A) as we did not observe any reduction in tuber yield or dry matter (Figure 4A,B).
Finally, the present study can show that a Cl― fertilization can not only improve the plant hydration status in potato but can also achieve this without reducing tuber yield, dry matter and likely also starch concentration. However, in contrast to initial expectations, both were only the case under the highest Cl― supply. Likewise, Hütsch et al. [5] did not find any reduction in tuber yield or tuber starch after Cl― supply. However, the authors treated their plants with a considerably lower Cl― dose, as in the present study. Hütsch et al. [5] supplied their plants at maximum with 150 mg Cl― kg soil−1.
The plants supplied with 800 mg Cl― kg soil−1 had mean Cl― leaf concentrations of 88 ± 13 mg Cl― g dw−1 (Figure 2B) without showing any toxicity symptoms (Figure 2C). Thus, similar to Hütsch et al. [5] and Franco-Navarro et al. [17] the Cl― leaf concentrations of plants supplied with 800 mg Cl― kg soil−1 were above Cl― leaf concentrations (33 mg g dw−1) often believed to cause toxicity in glycophytic plants [1,4]. These results further indicate that the general opinion that potato is sensitive to Cl― must be questioned. Still, it cannot be excluded that a higher Cl― supply above 800 mg Cl― kg soil−1 would have caused a reduction in tuber yield or tuber dry matter.
From previous studies it is known that high Cl― concentrations in soil solution lead to antagonistic uptake interactions with other anions such as nitrate or phosphate, thus resulting in lower nitrate or phosphate concentrations in the plant [15,31]. This leads to a reduced use efficiency of the respective anion as it is available in lower concentrations. In contrast, Rosales et al. [32] suggest that high Cl― concentrations may increase the nitrate use efficiency in plants. The explanation for this is that both Cl― and nitrate share few similar physiological functions in plants and under high concentrations both nutrients accumulate in the vacuole [15]. Contrary to nitrate, Cl― is not metabolized in plants thus showing lower energy costs [32]. Therefore, Rosales et al. [32] argue that higher Cl― concentrations and vacuolar storage reduce the vacuolar compartmentation of nitrate. This may lead to a higher availability of nitrate for other metabolic processes and thus increase its use efficiency. Likewise, in the present study, the nitrate leaf concentrations revealed a decreasing tendency with increasing Cl― supply (Supplementary Figure S2; this was not statistically approved). The phosphate concentrations did not show a clear tendency (Supplementary Figure S2).
Plants, supplied with 800 mg Cl― kg soil−1, showed in tendency a lower number of tubers per plant (Figure 4C). Besides, the number of tubers per plant showed a lower variation. As there was no change in tuber yield in dependence of the Cl― treatment in the present study (Figure 4A), the lower number of tubers indicates that tubers of plants supplied with 800 mg Cl― kg soil−1 turned out bigger. Smaller tuber sizes are often reported when potato tubers experienced a period of drought stress [33]. This is, for instance, a problem for the potato processing industry such as for fries, where specific tuber sizes are required. Thus, a Cl― fertilization may be a tool to overcome such quality losses as a consequence of drought stress periods.
5. Conclusions
Our hypotheses that increased Cl― supply improves plant hydration without impairing tuber yield is confirmed. Moreover, results first show that the assumption that the potato is sensitive to high amounts of Cl― is not always true. In contrast, it can positively improve potato shoot water status without impairing tuber yield. This could be positive under progressive soil drying. This, however, awaits experimental clarification.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11040736/s1, Table S1: Soil physical and chemical parameters. Potassium (K), magnesium (Mg), and phosphorus (P) are given in mg 100 g−1 soil. Total carbon (Ct) and total nitrogen (Nt) are given in %., Figure S1: The effect of increasing chloride (Cl―)-fertilization on root biomass. Plants were treated with four Cl―-supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. No indication means there was no significant effect. Figure S2: The effect of increasing chloride (Cl―)-fertilization on leaf nitrate (NO3−; left side) and phosphate (PO43−; right side) concentrations. Plants were treated with four Cl―-supplies: 0 mg Cl― kg soil−1 (control plants); 200 mg Cl― kg soil−1; 400 mg Cl― kg soil−1; 800 mg Cl― kg soil−1. Mean ± SE values; n = 4. No indication means there was no significant effect.
Author Contributions
M.T.K. performed the measurements, processed the experimental data, performed the statistical analysis, drafted the manuscript and designed the figures. T.K. and E.P. were involved in planning the work and in drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Ronja Biermann for her support and help during the plant cultivation and data collection. We thank Arne Gull for the performance of the chloride analyses. We acknowledge support by the German Research Foundation (DFG) and the Open Access Publication Fund of Humboldt-Universität zu Berlin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Bergmann, W. Ernährungsstörungen bei Kulturpflanzen: Entstehung, Visuelle und Analytische Diagnose, 3rd ed.; Fischer: Jena, Germany, 1999. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Geilfus, C.-M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Keipp, K.; Glaser, A.-K.; Schubert, S. Potato plants (Solanum tuberosum L.) are chloride-sensitive: Is this dogma valid? J. Sci. Food Agric. 2018, 98, 3161–3168. [Google Scholar] [CrossRef]
- Haeder, H. Einfluss chloridischer und sulfatischer Ernährung auf Assimilation und Assimilatverteilung in Kartoffelpflanzen. Landwirtsch. Forsch. 1976, 32, 122–131. [Google Scholar]
- Van Loon, C.; Van den Berg, W. The effect of chloride fertilization on blackspot susceptibility and other quality characteristics and on yield of potato. Pot Res. 2003, 46, 147–154. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomasiewicz, D.J. Effect of rate and timing of potassium chloride application on the yield and quality of potato (Solanum tuberosum L. ‘Russet Burbank’). Can. J. Plant Sci. 2012, 92, 783–794. [Google Scholar] [CrossRef]
- Davenport, J.R.; Bentley, E.M. Does potassium fertilizer form, source, and time of application influence potato yield and quality in the Columbia Basin? Am. J. Pot. Res. 2001, 78, 311–318. [Google Scholar] [CrossRef]
- Stanley, R.; Jewell, S. The influence of source and rate of potassium fertilizer on the quality of potatoes for french fry production. Pot. Res. 1989, 32, 439–446. [Google Scholar] [CrossRef]
- Bilski, J.J.; Nelson, D.C.; Conlon, R.L. Response of six wild potato species to chloride and sulfate salinity. Am. Potato J. 1988, 65, 605–612. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride in soil: From nutrient to soil pollutant. EEB 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Berger, K.C.; Potterton, F.E.; Hobson, E.L. Yield, quality, and phosphorus uptake of potatoes as influenced by placement and composition of potassium fertilizers. Am. Potato J. 1961, 38, 272–285. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: New roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef] [PubMed]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef]
- Beringer, H.; Koch, K.; Lindhauer, M.G. Source: Sink relationships in potato (Solanum tuberosum) as influenced by potassium chloride or potassium sulphate nutrition. Plant Soil 1990, 124, 287–290. [Google Scholar] [CrossRef]
- Wang-Pruski, G.; Schofield, A. Potato: Improving crop productivity and abiotic stress tolerance. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Tiburcio, A.F., Tuteja, R., Eds.; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2012; pp. 1121–1153. [Google Scholar]
- FAO Food and Agricultural Organization of the United Nation. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 November 2020).
- Sutanto, S.J.; Van Lanen, H.A.; Wetterhall, F.; Llort, X. Potential of pan-european seasonal hydrometeorological drought forecasts obtained from a multihazard early warning system. BAMS 2020, 101, E368–E393. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Roger, M.J.R., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Koch, M.; Winkelmann, M.K.; Hasler, M.; Pawelzik, E.; Naumann, M. Root growth in light of changing magnesium distribution and transport between source and sink tissues in potato (Solanum tuberosum L.). Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R, 1st ed.; Chapman and Hall/CRC: London, UK, 2011. [Google Scholar]
- Schaarschmidt, F.; Vaas, L. Analysis of trials with complex treatment structure using multiple contrast tests. Hortsci 2009, 44, 188–195. [Google Scholar] [CrossRef]
- Geilfus, C.M.; Mithöfer, A.; Ludwig-Müller, J.; Zörb, C.; Muehling, K.H. Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytol. 2015, 208, 803–816. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Li, X.T.; Yuan, J.Z.; Wang, F.Z.; Guo, H.; Xia, Z.R.; Wang, S.M.; Ma, Q. Chloride is beneficial for growth of the xerophyte Pugionium cornutum by enhancing osmotic adjustment capacity under salt and drought stresses. J. Exp. Bot. 2020, 71, 4215–4231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yada, R.; Arul, J. Characterization of thermal properties of potato dry matter–water and starch–water systems. J. Food Sci. 2002, 67, 560–566. [Google Scholar] [CrossRef]
- Papadopoulos, I.; Rendig, V.V. Interactive effects of salinity and nitrogen on growth and yield of tomato plants. Plant Soil 1983, 73, 47–57. [Google Scholar] [CrossRef]
- Rosales, M.A.; Franco-Navarro, J.D.; Peinado-Torrubia, P.; Díaz-Rueda, P.; Álvarez, R.; Colmenero-Flores, J.M. Chloride improves nitrate utilization and NUE in plants. Front. Plant Sci. 2020, 11, 442. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).