Application of UV-C Irradiation to Rosa x hybrida Plants as a Tool to Minimise Macrosiphum rosae Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. UV-C Irradiation
2.3. Rose Leaf Bioassays (RLB)
2.4. Experimental Layout of Greenhouse Soil Trials (GST)
2.5. Experimental Layout of Glasshouse Pot Trial (GPT)
2.6. Rose Plant Physiological Responses
2.7. Statistical Analysis
3. Results
3.1. Rose Leaf Bioassays (RLB)
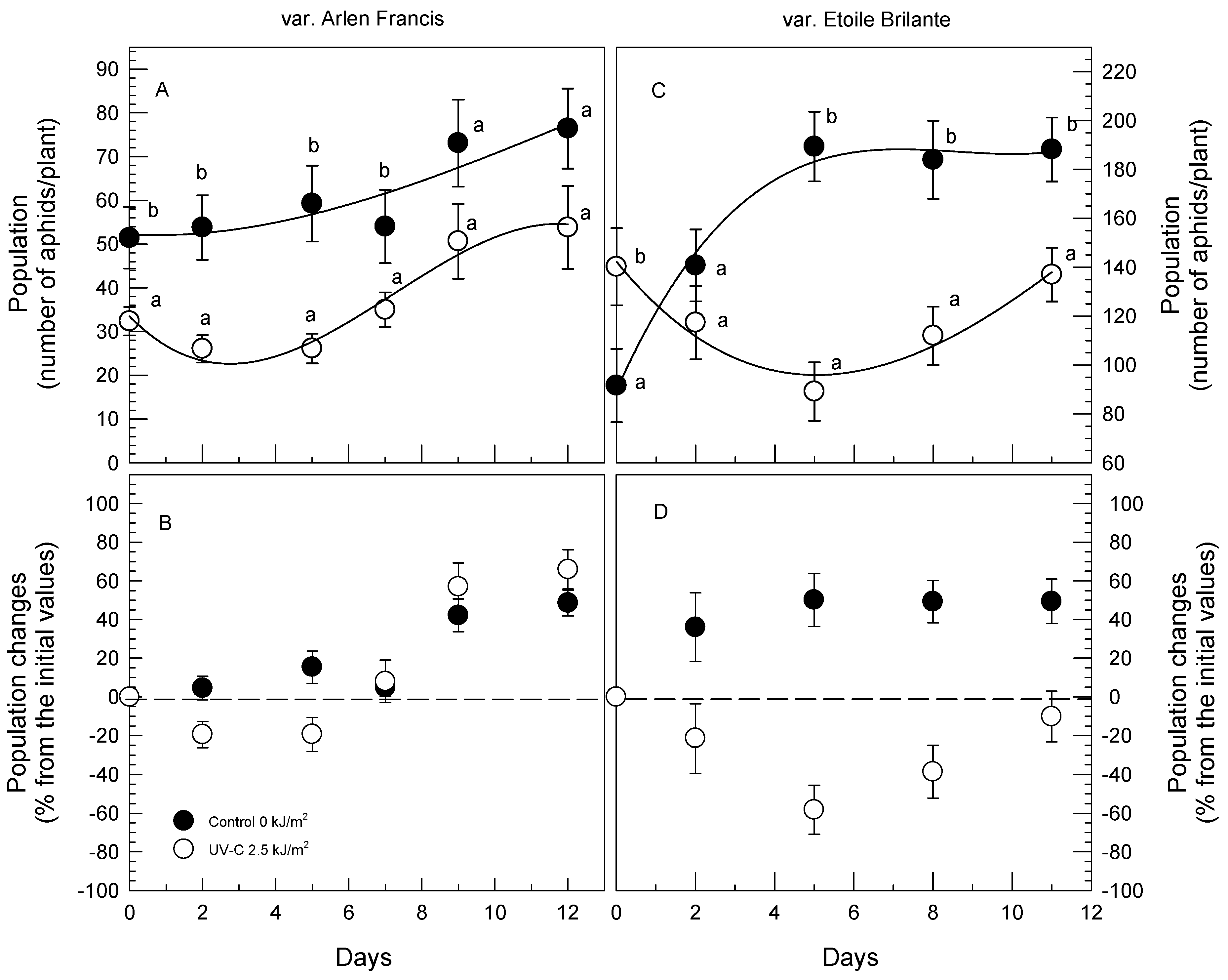
3.2. Greenhouse Soil Trials (GST)
3.3. Greenhouse Pot Trial (GPT)
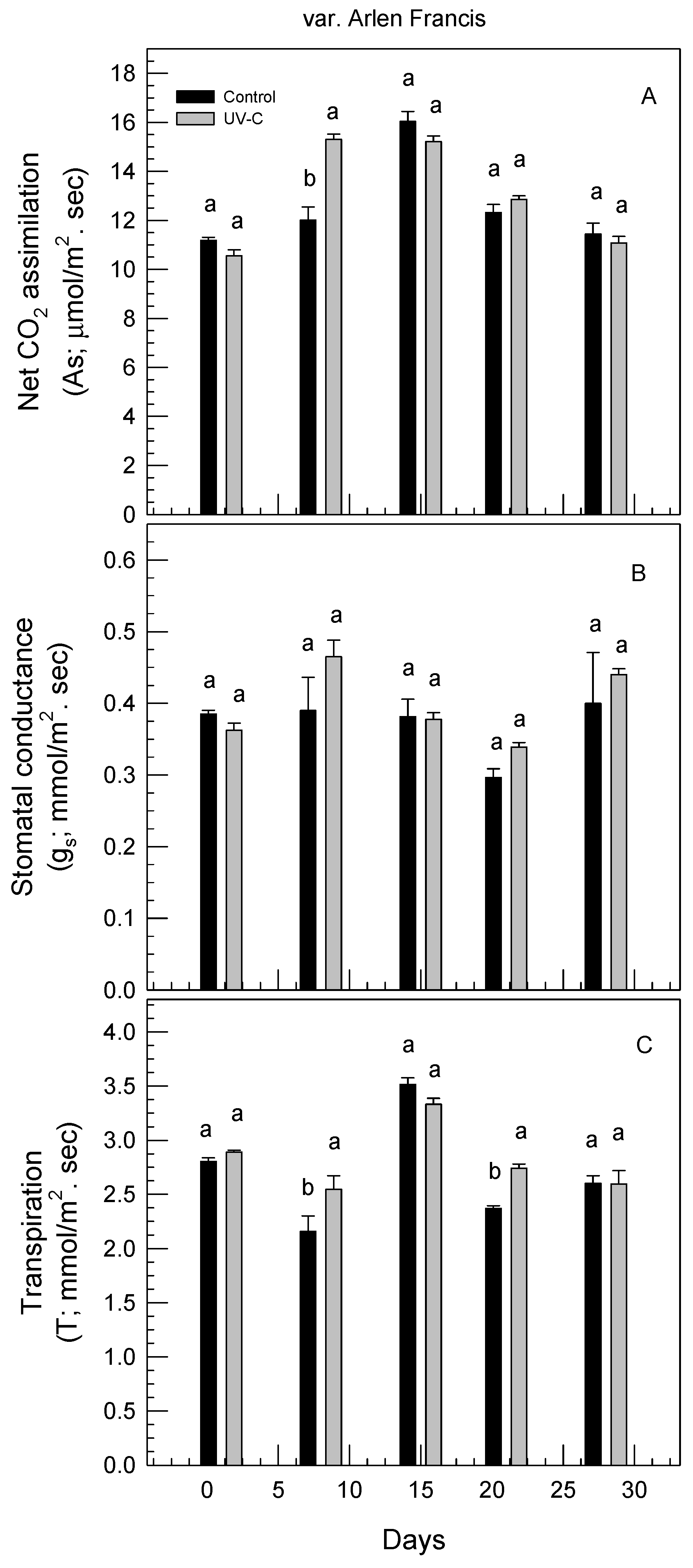
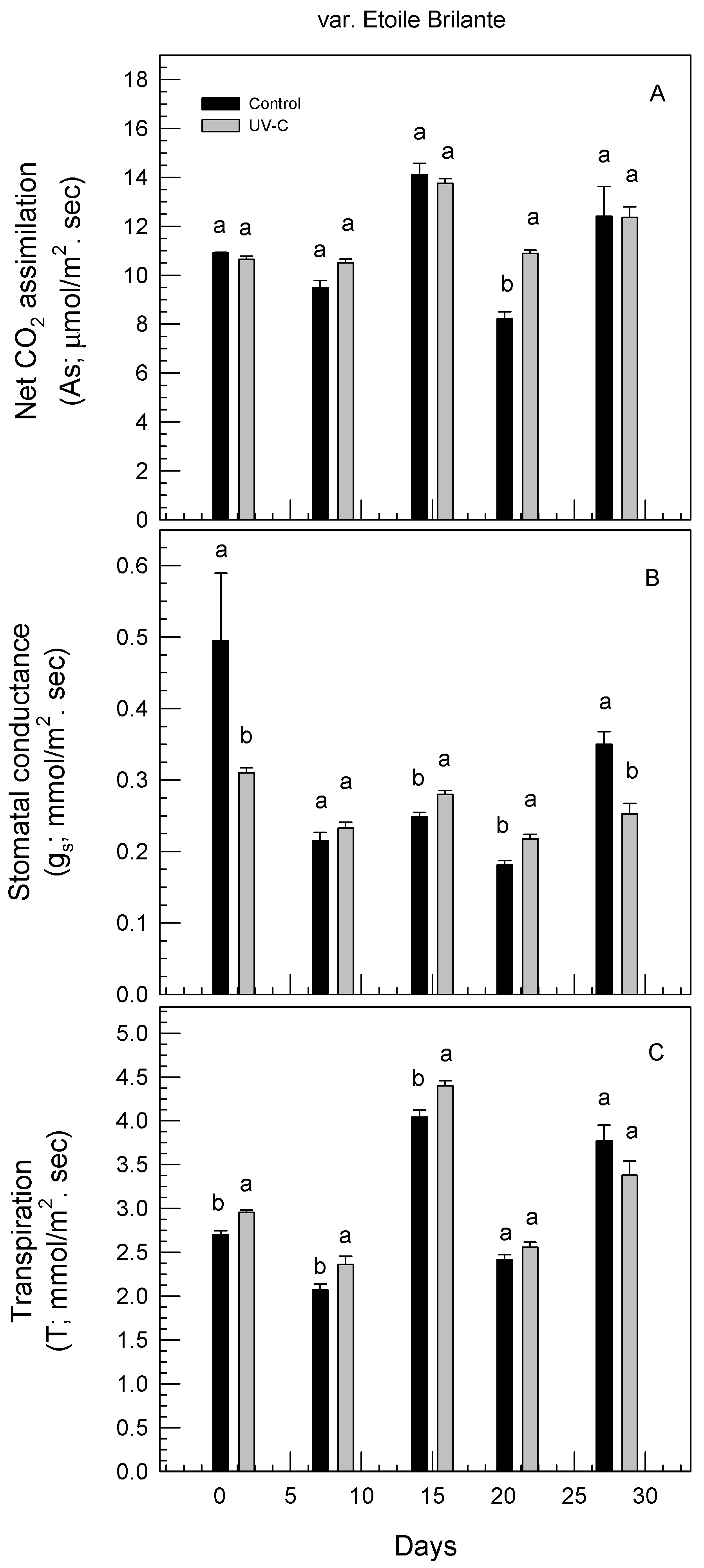
3.4. Effect of UV-C Irradiation on Physiological Responses of Rose Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Darras, A.I. Novel elicitors induce defense responses in cut flowers. In Plant Pathology, 1st ed.; Cumagun, C.J.R., Ed.; InTech Publications: Reijka, Croatia, 2012; pp. 85–116. [Google Scholar]
- Izaguirre, M.M.; Scopel, A.L.; Baldwin, I.T.; Ballaré, C.L. Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol. 2003, 132, 1755–1767. [Google Scholar] [CrossRef]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol. Entomol. 2001, 26, 312–324. [Google Scholar] [CrossRef]
- Stratmann, J. Ultraviolet-B radiation co-opts defense signaling pathways. Trends Plant Sci. 2003, 8, 526–533. [Google Scholar] [CrossRef]
- Demkura, P.V.; Abdala, G.; Baldwin, I.T.; Ballaré, C.L. Jasmonate-dependent and-independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Nederpel, C.; Naranjo, S.; Kim, H.K.; Rodríguez-López, M.J.; Chen, G.; Gaétan, G.; Leiss, K.A.; Klinkhamer, P.G. Ultraviolet radiation modulates both constitutive and inducible plant defenses against thrips but is dose and plant genotype dependent. J. Pest Sci. 2021, 94, 69–81. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, M.; Takeda, M. UV tolerance in the two-spotted spider mite, Tetranychus urticae. J. Insect Physiol. 2009, 55, 649–654. [Google Scholar] [CrossRef]
- Short, B.D.; Janisiewicz, W.; Takeda, F.; Leskey, T.C. UV-C irradiation as a management tool for Tetranychus urticae on strawberries. Pest Manag. Sci. 2018, 74, 2419–2423. [Google Scholar] [CrossRef]
- Dader, B.; Gwynn-Jones, D.; Moreno, A.; Winters, A.; Fereres, A. Impact of UV-A radiation on the performance of aphids and whiteflies and on the leaf chemistry of their host plants. J. Photochem. Photobiol. B Biol. 2014, 138, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, H.Y.; Thieme, T. Probing behaviors of Sitobion avenae (Hemiptera: Aphididae) on enhanced UV-B irradiated plants. Arch. Biol. Sci. 2013, 65, 247–254. [Google Scholar]
- Hu, Z.Q.; Zhao, H.Y.; Thieme, T. The effects of enhanced ultraviolet-B radiation on the biology of green and brown morphs of Sitobion avenae (Hemiptera: Aphididae). Environ. Entomol. 2013, 42, 578–585. [Google Scholar] [CrossRef]
- Nguyen, T.T.A.; Michaud, D.; Cloutier, C. A proteomic analysis of the aphid Macrosiphum euphorbiae under heat and radiation stress. Insect Biochem. Mol. Biol. 2009, 39, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Tiniakou, C. Photomorphogenic reactions in geranium (Pelargonium x hortorum) plants stimulated by brief exposures of ultraviolet-C irradiation. Plant Growth Regul. 2012, 68, 343–350. [Google Scholar] [CrossRef]
- Balestrazzi, A.; Locato, V.; Bottone, M.G.; De Gara, L.; Biggiogera, M.; Pellicciari, C.; Botti, D.; Di Gesù Donà, M.; Carbonera, D. Response to UV-C radiation in topo I-deficient carrot cells with low ascorbate levels. J. Exp. Bot. 2010, 61, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.C.; Joshi, P.K.; Grimm, B.; Arora, S. Alleviation of ultraviolet-C-induced oxidative damage through overexpression of cytosolic ascorbate peroxidase. Biologia 2011, 66, 1052–1059. [Google Scholar] [CrossRef]
- Barcelo, J.A. Photoeffects of visible and ultraviolet radiation on the two-spotted spider mite, Tetranychus urticae. Photochem. Photobiol. 1981, 33, 703–706. [Google Scholar] [CrossRef]
- Sakai, Y.; Osakabe, M. Spectrum-specific damage and solar ultraviolet radiation avoidance in the two-spotted spider mite. Photochem. Photobiol. 2010, 86, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.A. Effectiveness of ultraviolet radiation as a physical method in controlling the stored product mite, Tyrophagus putrescentiae (Acari: Acaridae). J. Entomol. 2013, 10, 43–48. [Google Scholar] [CrossRef]
- Murata, Y.; Osakabe, M. The Bunsen–Roscoe reciprocity law in ultraviolet-B-induced mortality of the two-spotted spider mite Tetranychus urticae. J. Insect Physiol. 2013, 59, 241–247. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshioka, Y.; Tsarsitalidou, O.; Ntalia, V.; Ohno, S.; Ohyama, K.; Kitashima, Y.; Gotoh, T.; Takeda, M.; Koveos, D.S. An LED-based UV-B irradiation system for tiny organisms: System description and demonstration experiment to determine the hatchability of eggs from four Tetranychus spider mite species from Okinawa. J. Insect Physiol. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.D.; Moore, J.P.; McPherson, M.; Lambourne, C.; Croft, P.; Heaton, J.C.; Wargent, J.J. Ecological responses to UV radiation: Interactions between the biological effects of UV on plants and on associated organisms. Physiol. Plant. 2012, 145, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takashima, T.; Izawa, N.; Watanabe, M.; Takeda, M. UV radiation elevates arylalkylamine N-acetyltransferase activity and melatonin content in the two-spotted spider mite, Tetranychus urticae. J. Insect Physiol. 2008, 54, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Urban, L.; Sari, D.C.; Orsal, B.; Lopes, M.; Miranda, R.; Aarrouf, J. UV-C light and pulsed light as alternatives to chemical and biological elicitors for stimulating plant natural defenses against fungal diseases. Sci. Hortic. 2018, 235, 452–459. [Google Scholar] [CrossRef]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Katsiloulis, O.; Kratimenou, E. Brief exposures of ultraviolet-C (UV-C) irradiation improves flowering of ornamental plants. Acta Hortic. 2013, 1002, 95–102. [Google Scholar] [CrossRef]
- Darras, A.I.; Bali, I.; Argyropoulou, E. Disease resistance and growth responses in Pelargonium × hortorum plants to brief pulses of UV-C irradiation. Sci. Hortic. 2015, 181, 95–101. [Google Scholar] [CrossRef]
- Darras, A.I.; Vlachodimitropoulou, A.; Dimitriadis, C. Regulation of corm sprouting, growth and flowering of pot Freesia hybrida L. plants by cold and UV-C irradiation forcing. Sci. Hortic. 2019, 252, 110–112. [Google Scholar] [CrossRef]
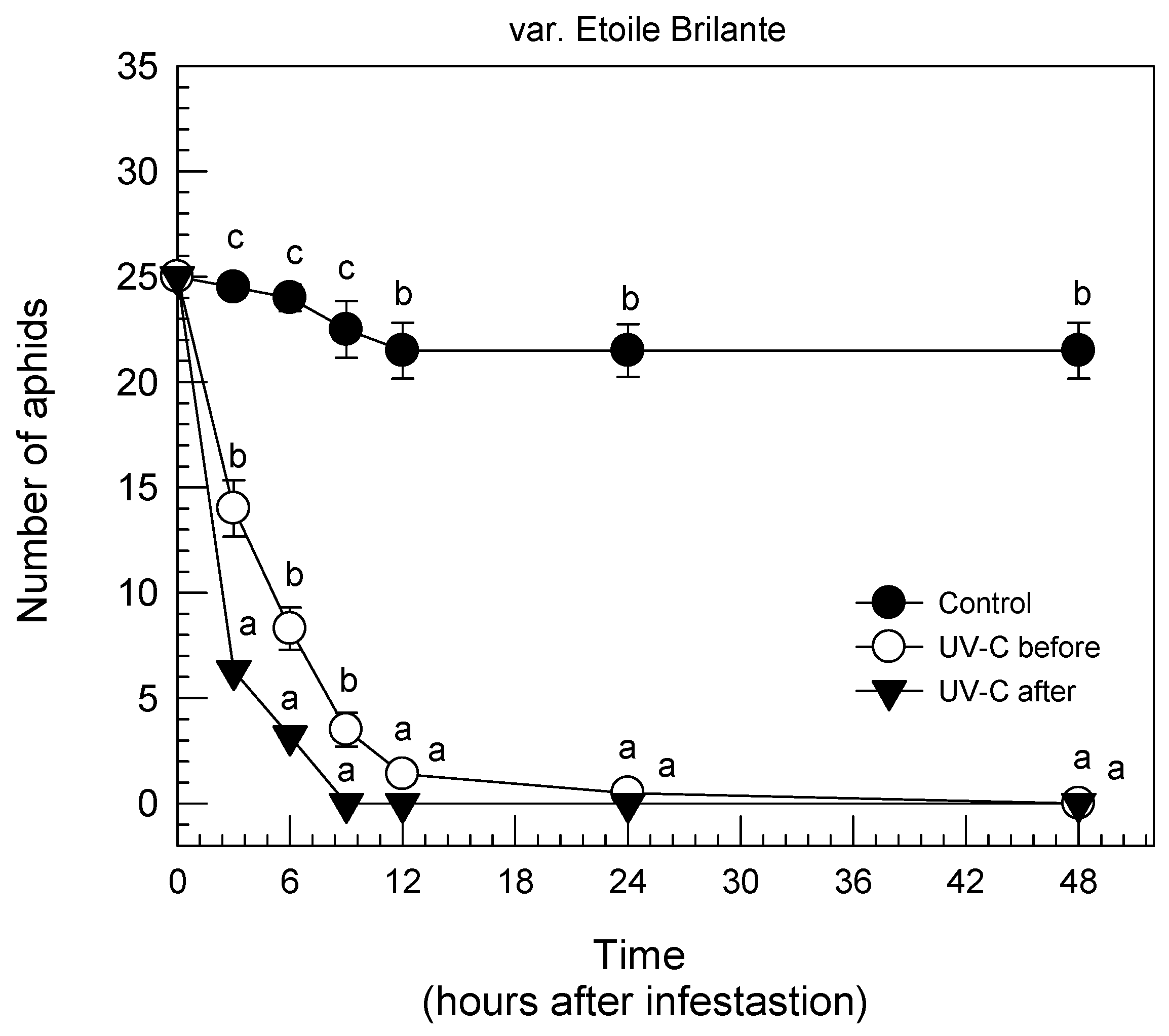
| UV-C Treatments | Parameters | |||
|---|---|---|---|---|
| Aphid Population Changes (%) | Fecundity (New Eggs and 1st Instars) | |||
| Adults | 1st Instar | Eggs | 1st Instar | |
| 1. “Etoile Brilante” | ||||
| 0 kJ/m2 (controls) | −47 | +100 | 4.3 b | 6.7 b |
| 2.5 kJ/m2 before infestation | −87 | 0 | 0.0 a | 0.0 a |
| 2.5 kJ/m2 after infestation | −56 | 0 | 0.0 a | 0.0 a |
| 2. “Aarlen Francis” | ||||
| 0 kJ/m2 (controls) | −40 | +100 | 2.7 b | 14.0 c |
| 2.5 kJ/m2 before infestation | −73 | 0 | 0.4 a | 0.0 a |
| 2.5 kJ/m2 after infestation | −50 | 0 | 1.3 ab | 3.8 b |
| Source | Aphid Number | |||
|---|---|---|---|---|
| “Arlen Francis” | ||||
| Day-0 | 42.35 ab | |||
| Day-2 | 39.95 a | |||
| Day-5 | 42.71 ab | |||
| Day-7 | 44.52 ab | |||
| Day-9 | 61.87 bc | |||
| Day-12 | 65.12 c | |||
| Control | 61.35 b | |||
| UV-C | 37.49 a | |||
| df | Mean squares | F | Sig. | |
| UV-C (U) | 1 | 37,075.63 | 20.37 | 0.000 |
| Time (days) (T) | 5 | 5256.72 | 2.89 | 0.015 |
| U × T | 5 | 347.80 | 0.19 | 0.966 |
| “Etoile Brilante” | ||||
| Day-0 | 115.9 a | |||
| Day-2 | 129.1 ab | |||
| Day-5 | 139.3 bc | |||
| Day-8 | 143 bc | |||
| Day-11 | 157.9 c | |||
| Control | 158.8 b | |||
| UV-C | 115.3 a | |||
| df | Mean squares | F | Sig. | |
| UV-C (U) | 1 | 23,674.88 | 5.43 | 0.001 |
| Time (days) (T) | 4 | 2464.28 | 52.15 | 0.000 |
| U × T | 4 | 8672.48 | 19.10 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darras, A.I.; Skouras, P.J.; Assimomitis, P.; Labropoulou, C.; Stathas, G.J. Application of UV-C Irradiation to Rosa x hybrida Plants as a Tool to Minimise Macrosiphum rosae Populations. Agronomy 2021, 11, 702. https://doi.org/10.3390/agronomy11040702
Darras AI, Skouras PJ, Assimomitis P, Labropoulou C, Stathas GJ. Application of UV-C Irradiation to Rosa x hybrida Plants as a Tool to Minimise Macrosiphum rosae Populations. Agronomy. 2021; 11(4):702. https://doi.org/10.3390/agronomy11040702
Chicago/Turabian StyleDarras, Anastasios I., Panagiotis J. Skouras, Panagiotis Assimomitis, Chara Labropoulou, and George J. Stathas. 2021. "Application of UV-C Irradiation to Rosa x hybrida Plants as a Tool to Minimise Macrosiphum rosae Populations" Agronomy 11, no. 4: 702. https://doi.org/10.3390/agronomy11040702
APA StyleDarras, A. I., Skouras, P. J., Assimomitis, P., Labropoulou, C., & Stathas, G. J. (2021). Application of UV-C Irradiation to Rosa x hybrida Plants as a Tool to Minimise Macrosiphum rosae Populations. Agronomy, 11(4), 702. https://doi.org/10.3390/agronomy11040702







