Abstract
Two pot experiments were conducted to evaluate the response of radish crops against different plant growth regulators, biostimulants, and leaf extracts at Yale University, USA. The first experiment examined the marginal effect of vitamin B12 when added to the Berlyn Laboratory’s proprietary biostimulant formula (GPB Core). Increasing concentrations of vitamin B12 were added, as investigated in groups SL (0 mg/L), SB1 (0.5 mg/L), SB2 (1.0 mg/L), and SB3 (1.5 mg/L). The addition of vitamin B12 conferred no significant incremental benefit over the GPB Core. However, the GPB Core formula (SL) increased fresh shoot biomass by 172.9%, dry shoot biomass by 136.4%, fresh root biomass by 64.7%, and dry root biomass by 29.1% over plant treated with inorganic fertilizer alone (p < 0.01). The second experiment examined the combined marginal effect of vitamin B12 and coenzyme Q10 (CoQ10) when added to the GPB Core. The three experimental groups included the GPB Core plus inorganic fertilizer (S+); GPB Core, vitamin B12, CoQ10, and inorganic fertilizer (SBQ+); and GPB Core, vitamin B12, CoQ10, and no inorganic fertilizer (SBQ0). SBQ0 outperformed the inorganic fertilizer control in fresh shoot, dry shoot, fresh root, and dry root biomass by 190.3%, 127.1%, 128.5%, and 41.3%, respectively (p < 0.01), indicating that inorganic fertilizer can be replaced by biostimulants while simultaneously increasing yield. Additionally, the differences between SBQ+ and SBQ0 in the biomass metrics were statistically insignificant, indicating that in the presence of biostimulants, inorganic fertilizers confer a slight marginal benefit. There was no evidence, however, that the addition of CoQ10 and vitamin B12 conferred benefits over S+. Overall, the application of biostimulants statistically significantly improves radish biomass. Both experiments indicate that under low stress conditions, biostimulants can replace inorganic fertilizer while simultaneously increasing yield.
1. Introduction
Acknowledged as an important crop due to its global disposition, radish (Raphanus sativus L.) belongs to the Brassicaceae family and is grown in tropical and sub-tropical climates including Europe, South America, and Southeast Asia [1,2]. Moreover, it is an economically important crop for the United Kingdom, as 5800 tons of radish are grown annually [3]. Predominately grown for its swollen tap root, it has multiple uses as a leafy vegetable, oil crop, root vegetable, and cover plant [4]. As a cover crop, it is used to minimize nitrogen loss, improve soil fertility, and mitigate soil erosion [4,5].
Plant biostimulants are bioactive mixtures containing organic and inorganic materials that when exogenously applied to a plant improves growth, vigor, productivity, nutrient uptake efficiency, and overall crop quality [6,7,8]. Plants experience biotic and abiotic stressors, which result in the production of reactive oxygen species (ROS) due to a bottlenecking in the photoelectron chain from a lack of oxidized electron acceptors. To mitigate the damage that the toxic ROS has on cell infrastructure, plants have evolved antioxidant pathways, including superoxide dismutase (SOD) and the ascorbic acid glutathione chain. Even in seemingly optimal environments, plants experience stress and are unable to invest in the carbon-intensive synthesis of antioxidant compounds, and exogenous application of antioxidant substances and amino acids has been demonstrated to increase stress tolerance and overall yield [9]. As a result, biostimulant production is common in some EU countries, including France, Spain, and Italy [10]. Biostimulants are not nutrients [11] and cannot replace inorganic fertilizers under all scenarios, but they can minimize necessary mineral nutrient application, reduce nutrient deficiencies, and ameliorate abiotic stress when applied in small amounts [10,12].
Biostimulants increase root biomass, nutrient translocation, enzyme activity, and promote nutrient uptake [13]. Current biostimulant formulas commonly include vitamins, minerals, amino acids, polysaccharides, oligosaccharides, and other natural substances like humic acids, seaweed (kelp) extracts, mycorrhizal spores, and bacteria. They improve soil conditions and positively affect crop physiology and plant metabolism [10,11]. Moreover, they are also capable of increasing plants’ nutrient uptake and efficiency by changing soil conditions, nutrient solubility, and root morphology [10]. Plant biostimulants can reduce the level of required inorganic fertilizer to optimize yield, thereby reducing water pollution and improving the efficiency of conventional agricultural practices, which currently involve high levels of costly fertilizers, fungicides, soil amendments, and genetic modification of crops. Currently, half of the inorganic fertilizer added to crops rapidly leaches down into the water table [14,15]. An increase of 25% in radish yield was observed in an organic production system with biostimulants application [16].
It is also important to identify the right time of application and exact dose of biostimulant to gain appropriate results and minimize production costs. Biostimulants can be applied at different stages of plant growth (at sowing, early development, blooming, or fruiting stage). It can be applied as a foliar spray or root drench. They also can promote growth in the following spring even if applied late in the growing season. Biostimulants also improve the green pigment in leaf vegetables, improve plant growth, and enhance the antioxidant potential in plants [17]. In one experiment, Russo and Berlyn (1992) demonstrated that green beans had increased thiamine levels in proportion to the thiamine content of the applied biostimulant [9].
Biostimulants are gaining popularity because they significantly improve plant physiological, metabolic, and molecular processes, including nutrient and water use efficiency [10]. Plant biostimulants are also added to the rhizosphere and protect against environmental stress including soil salinity, drought, and sub-optimal temperatures [11]. To this end, they are used for horticulture and agronomic crops grown in drought-prone regions. The biostimulant-induced increase in root biomass enables plants to explore the deeper soil layers during drought and stimulates the synthesis of compatible solutes to re-establish favorable water potential gradients for efficient water uptake. Similarly, microbial biostimulants create absorption surfaces around the root systems and sequester soil water to improve plant growth [11]. The addition of plant-derived protein hydrolysates improves the fresh and dry biomass of lettuce (Lactuca sativa L.) and increases the concentration of glucosinolates, osmolytes, sterols, and terpenes [18]. Additionally, amino acids act against the degradation of plant cell architecture, specifically chlorophyll, thereby increasing cell lifespan. They also improve the quality and nutritional value of food crops [19]. It is reported that the effects of protein hydrolysates are directly linked with the metabolism of biosynthetic products that promote crop growth and yield and also induce tolerance against abiotic stress [20].
The purpose of this study was to understand the impact of biostimulants on radish yield and growth. With this aim, greenhouse experiments with different combinations of biostimulants and leaf extract were performed. Hence the objectives are to (i) explore the influential effect of biostimulants on yield and growth of radish, (ii) determine the marginal effects of vitamin B12 (first experiment) and of vitamin B12 and CoQ10 (second experiment) on the GPB Core, (iii) enhance the available data for scientific community to better understand biostimulants activity, and (iv) to minimize chemical fertilization while simultaneously increasing greenhouse vegetables’ yields. To the extent of the authors’ knowledge, prior experiments examining the impact of exogenous application of vitamin B12 and vitamin B12 in tandem with CoQ10 on radish plant growth in low-stress environments do not exist in literature.
2. Materials and Methods
Experimental site
To evaluate the impacts of biostimulants on radish productivity, two sets of pot experiments were conducted from July to November of 2019 at the Yale School of the Environment. Five-inch pots were filled with 200 g of Metromix 510 soil (now Sungro), and the field capacity was determined by saturating and reweighing each pot.
Experiment 1
The experiment was comprised of five treatments with 40 replications in a completely randomized design (CRD). The tested treatments were:
- Nutri-leaf inorganic fertilizer (1.0 g/L) (I)
- GPB Core (8.7 g/L) (SL)
- GPB Core (8.7 g/L) + 0.5 mg/L B12 (SB1)
- GPB Core (8.7 g/L) + 1.0 mg/L B12 (SB2)
- GPB Core (8.7 g/L) + 1.5 mg/L B12 (SB3)
Radish seeds were washed in running water overnight and two seeds per pot were sowed on 1 August 2019. The smaller seedling was later removed from the pot. After seedling establishment, 25 mL of spray were applied as a soil drench weekly. Four applications were done on 12 August, 19 August, 26 August, and 2 September. The crop was harvested on 9 September 2019. Shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight were recorded in grams.
Experiment 2
The experiment was comprised of five treatments with 40 replications in a completely randomized design. The tested treatments were:
- Control
- Nutri-leaf inorganic fertilizer (1.0 g/L) (I)
- GPB Core (8.7 g/L) + 0.25 g/L Nutri-leaf (S+)
- GPB Core (8.7 g/L) + 1.5 mg/L B12 + 400 mg/L CoQ10 + 0.25 g/L Nutri-leaf (SBQ+)
- GPB Core (8.7 g/L) + 1.5 mg/L B12 + 400 mg/L CoQ10 (SBQ0)
Identical treatment application methods and doses were used. The seeds were planted on 14 October 2019, and germination started on 17 October. The four applications were done on 28 October, 4 November, 12 November, and 18 November. The crop was harvested on 24 November 2019. Shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight were recorded in grams. The details of both experiments, their treatments details, and differences are given in Table 1 below.

Table 1.
Experimental details, treatments plan and their differences.
Statistical Analysis
One-way Analysis of Variance (ANOVA) tests were used to evaluate the statistical significance of the results. For pairwise comparisons between the differences in means, the results were further evaluated with planned orthogonal contrast tests. Two-tailed tests were used to account for possible growth inhibitory effects, and normality and independent error terms were assumed. The Bartlett’s Test of Homogeneity of Variances was used to test the equality of variances assumption for the contrast tests. The software package R (version 4.0.3) was used.
3. Results
3.1. Experiment 1
The biostimulants significantly improved radish biomass over the group treated with inorganic fertilizer. The measurement with the largest percent increase as a result of the biostimulant application was fresh shoot biomass, which statistically significantly increased in SL (172.9%), B1 (163.3%), B2 (167.9%), and B3 (171.2%) (Figure 1A). The metric with the second largest percent increase was dry shoot biomass, which increased in SL (136.4%), B1 (132.7%), B2 (136.2%), and B3 (144.6%) (Figure 2A). Similarly, in SL, B1, B2, and B3, fresh root biomass was increased by 64.7%, 60.2%, 45.8%, and 50.4%, respectively (Figure 1B). Dry root biomass was the metric with the lowest percent increase, but the measurement was statistically significantly improved in SL (29.1%), B1 (29.5%), B2 (19.8%), and B3 (27.1%) (Figure 2B). All different letters are significant at p < 0.01.
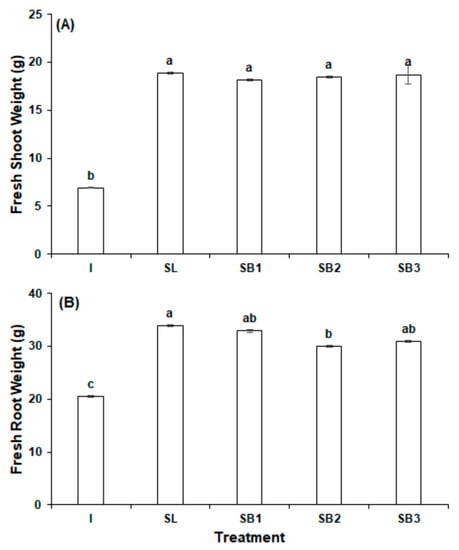
Figure 1.
Fresh weight of radish in experiment 1: (A) shoot weight (B) root weight. All different letters are significant at p < 0.05.
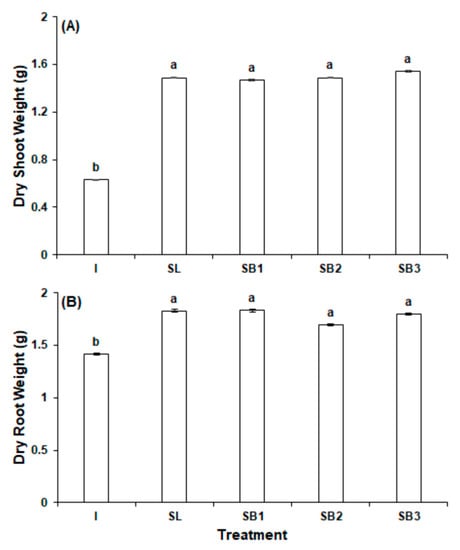
Figure 2.
Dry weight of radish in experiment 1: (A) shoot weight (B) root weight. All different letters are significant at p < 0.05.
Despite the outperformance of the four experimental groups over the inorganic fertilizer control, the addition of vitamin B12 to the GPB Core formula conferred no detectable benefit in any biomass metric. The most pronounced changes were in fresh root biomass, which with respect to SL decreased by 2.7% in B1 (p = 0.66), 11.5% in B2 (p = 0.04), and 8.7% in B3 (p = 0.14). Dry root biomass was largely unchanged in B1, which slightly increased by 0.2% (p = 0.97), and down by 7.3% in B2 (p = 0.24) and by 1.6% in B3 (p = 0.79). Fresh and dry shoot biomass were all slightly down or unchanged.
3.2. Experiment 2
Biostimulants played a significant role in the development of the roots and shoots. Compared to C, which received no biostimulant nor inorganic fertilizer, the four biomass measurements statistically significantly improved (p < 0.01). In I, S+, SBQ+, and SBQ0, respectively, fresh shoot biomass was increased by 327.3%, 1250.8%, 1229.2%, and 1140.5%; dry shoot by 190.7%, 607.8%, 617.8%, and 560.1%; fresh root by 389.0%, 973.5%, 1090.1%, and 1017.1%; and dry root by 215.0%, 336.9%, 358.6%, and 345.2%. These substantial increases show the importance of fertilizer, soil amendments, and biostimulants to agriculture.
Compared to I, the biostimulant treatments statistically significantly (p < 0.01) increased biomass. The largest increase was in fresh shoot biomass, which increased by 216.2% in S+, 211.1% in SBQ+, and 190.3% in SBQ0 (Figure 3A). Consequentially, dry shoot biomass also increased by 143.5% in S+, 146.9% in SBQ+, and 127.1% in SBQ0 (Figure 4A). Root biomass also experienced significant benefits, but to a lesser degree than the shoot biomass metrics: Respectively, fresh root biomass (Figure 1B) and dry root biomass (Figure 2B) increased by 119.6% and 38.7% in S+, 143.4% and 45.6% in SBQ+, and 128.5% and 41.3% in SBQ0. The biostimulants markedly increased all biomass metrics.
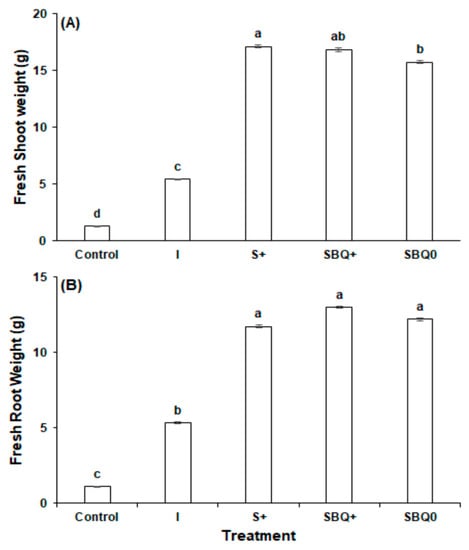
Figure 3.
Fresh weight of radish in experiment 2: (A) Shoot weight (B) root weight. All different letters are significant at p < 0.05.
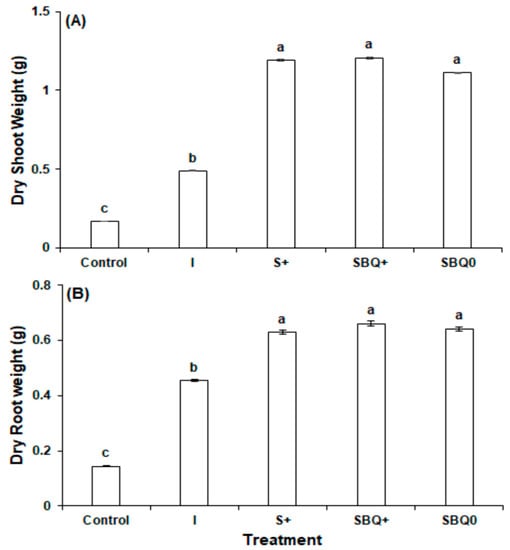
Figure 4.
Dry weight of radish in experiment 2: (A) Shoot weight (B) root weight. All different letters are significant at p < 0.05.
The addition of both CoQ10 and vitamin B12 did not confer a statistically significant benefit over the GPB Core. Compared to S+, SBQ+ fresh shoot biomass decreased by 1.6% (p = 0.75), dry shoot biomass increased by 1.4% (p = 0.82), fresh root biomass increased by 10.9% (p = 0.39), and dry root biomass increased by 5.0% (p = 0.60). Although SBQ+ does not confer a yield increase, the GPB Core, vitamin B12, and CoQ10 formula performs well in the absence of fertilizer: The differences in fresh shoot, dry shoot, fresh root, and dry root biomass of SBQ+ and SBQ0, which is identical to SBQ+ except for a lack of inorganic fertilizer, were 7.1% (p = 0.18), 8.7% (p = 0.17), 6.5% (p = 0.56), and 3.0% (p = 0.76), respectively. This signifies that although SBQ+ did have higher yields, inorganic fertilizer confers a statistically insignificant marginal benefit when added to SBQ0.
4. Discussion
The application of the GPB formula greatly increased the roots and shoot biomass in radish, indicating that biostimulants may play an important role in improving agricultural productivity and sustainability. As a growth blend of amino acids, co-enzymes, peptides, vitamins, humic acid, polypeptides, and enzymatic proteins, biostimulants can achieve this increase in yield through several interconnected pathways.
The biostimulant’s ability to increase yield may be partially attributable to the amino acids in the GPB formula. Amino acids are a source of nitrogen for plant uptake, thereby improving plant shoot and root growth [19,21]. Moreover, they improve plant nutrient management, particularly in horticulture crops [19]. For example, previous studies reported that the application of glycine, the simplest amino acid with well-documented biostimulant properties, improved the fresh and dry weight of root and shoot of coriander [22] and soya bean [23] in addition to increasing biomass and leaf greenness in lettuce [24]. This amino acid plays a function in thylakoid membrane production, improves photosynthetic efficiency, and is a chlorophyll precursor [25,26,27].
In general, amino acids reduce to different compounds that contain nitrogen such as ammonium and minimize inorganic nitrogen required by plants. Different studies revealed that the application of biostimulants and growth regulators rich in amino acids significantly improve the fresh and dry yield of crops [28,29]. Furthermore, [30] showed that amino acids, which can function as osmolytes, significantly enhance biomass, nitrogen metabolism, and chlorophyll biosynthesis in lettuce (Lactuca sativa L.). The effect of amino acids and biostimulants in general on plant yield and development can also be attributed to enhanced gibberellin and auxin production capabilities [29,31]. Bioactive substances such as amino acids, peptides, and vitamins present in biostimulants act similarly to auxins and gibberellins [32]. The presence of specific peptides, phytohormones, and protein hydrolysates affects the hormonal balance of plants and stimulates growth [33]. Furthermore, the improved plant yield can be associated with the availability of other phytohormones, specifically cytokinin, which is present in kelp extract. The GPB Core formula contains no plant hormones; however, amino acids may act as a precursor of phytohormones and are involved in vitamin, enzyme, amine, terpene, alkaloids, purine, and pyrimidine synthesis. Amino acids can enhance levels of carotenoids, polyphenols, flavonoids, and ascorbic acid as well as decrease the nitrate concentration [33]. Generally, biostimulants have capability to improve the vitamin C contents, antioxidant activity, and phenolic contents in fruits and vegetables, which results in improved production of specimen [34]. In addition, combining biostimulants with organic humic substances has resulted in elongated and thicker cucumber, thereby increasing yield [35,36].
In the first experiment, vitamin B12, also known as cobalamin, was investigated because of its antioxidant properties and since it can improve leaf area and root growth [37]. Although the results show that the exogenous application of biostimulants markedly enhances radish yield and productivity, they also indicate that the addition of vitamin B12 to the GPB Core formula confers no incremental benefit. Additionally, the results indicate that biostimulants can reduce or completely replace inorganic fertilizer while simultaneously increasing yield. The four experimental groups had mean values higher than that of inorganic fertilizer (p < 0.01), and compared to IF, SL, which contained no inorganic fertilizer, had higher values of fresh shoot, dry shoot, fresh root, and dry root biomass by 172.9%, 136.4%, 64.7%, and 29.1%, respectively. Biostimulants help enhance plant growth, as they improve soil system for better nutrient availability. They are all-natural and environmentally safe, and they can help mitigate atmospheric pollution resulting from chemical fertilization [31].
In the second experiment, CoQ10 was examined as a compound with a possible marginal benefit when added to the GPB Core and vitamin B12. CoQ10 is a membrane-soluble antioxidant and an electron carrier cofactor. Outperforming the fertilizer control in fresh shoot (190.3%), dry shoot (127.1%), fresh root (128.5%), and dry root biomass (41.3%), SBQ0 indicates that under optimal conditions, biostimulants can replace fertilizer while also increasing yield and sustainability. Furthermore, the performance of SBQ0 relative to SBQ+, identical except for 0.25 g/L of Nutri-leaf, indicates that inorganic fertilizer confers a minimal marginal yield increase over biostimulants. SBQ+ outperformed SBQ0 by 7.1% in fresh shoot biomass (p = 0.18), 8.7% in dry shoot biomass (p = 0.17), 6.5% in fresh root biomass (p = 0.56), and 3.0% in dry root biomass (p = 0.76). To this end, under greenhouse conditions, biostimulants can completely replace inorganic fertilizer with a minimal loss of yield.
However, the addition of CoQ10 and vitamin B12 to the GPB Core conferred no significant marginal benefit. It is also important to note that these experiments were conducted under optimal greenhouse conditions. Biostimulants are not proven to replace careful soil nutrient analysis nor remediate acute mineral deficiencies, and CoQ10 may confer an additional stress-mitigation benefit if applied to plants under higher stress levels. Regardless, it is clear that biostimulants are likely to play a role in promoting sustainable agricultural practices while also increasing crop yields.
5. Conclusions
The study concludes that biostimulants have a vital role in increasing radish productivity and reducing chemical fertilization in greenhouse vegetable production. The results indicated that the fresh and dry weights of radish roots and shoots were significantly enhanced with the application of biostimulants even in the absence of fertilizer. Low doses of biostimulants alongside inorganic fertilizers have potential valuable impacts on radish yield and productivity, and biostimulants may be able to replace inorganic fertilizers outright under certain conditions. Although further studies examining site conditions, crop species, timing considerations, and environmental factors are needed to further ascertain the exact role that biostimulants may play in modern agriculture, it is clear that they are a promising technology to increase crop yield, reduce fertilizer use, and facilitate stewardship of the environment.
Author Contributions
Conceptualization, A.R., K.G. and G.P.B.; Data curation, A.R., M.A.B. and G.P.B.; Formal analysis, A.R. and Q.-U.-A.R.; Investigation, A.R. and K.G.; Project administration, G.P.B.; Resources, G.P.B.; Software, M.A.B. and Q.-U.-A.R.; Visualization, G.P.B.; Writing—original draft, A.R.; Writing—review & editing, M.A.B., Q.-U.-A.R., K.G. and G.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wojcieszek, J.; Jiménez-Lamana, J.; Bierła, K.; Ruzik, L.; Asztemborska, M.; Jarosz, M.; Szpunar, J. Uptake, translocation, size characterization and localization of cerium oxide nanoparticles in radish (Raphanus sativus L.). Sci. Total Environ. 2019, 683, 284–292. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Pauletti, G.F.; Baldasso, C. Fodder radish (Raphanus sativus L.) seed cake as a feedstock for pyrolysis. Ind. Crops Prod. 2020, 154. [Google Scholar] [CrossRef]
- Musarika, S.; Atherton, C.E.; Gomersall, T.; Wells, M.J.; Kaduk, J.; Cumming, A.M.J.; Page, S.E.; Oechel, W.C.; Zona, D. Effect of water table management and elevated CO2 on radish productivity and on CH4 and CO2 fluxes from peatlands converted to agriculture. Sci. Total Environ. 2017, 584–585, 665–672. [Google Scholar] [CrossRef]
- Nishio, T. Economic and Academic Importance of Radish; Springer: Cham, Switzerland, 2017; pp. 1–10. [Google Scholar]
- Weil, R.; Kremen, A. Thinking across and beyond disciplines to make cover crops pay. J. Sci. Food Agric. 2007, 87, 551–557. [Google Scholar] [CrossRef]
- Rafiee, H.; Naghdi Badi, H.; Mehrafarin, A.; Qaderi, A.; Zarinpanjeh, N.; Sekara, A.; Zand, E. Application of plant biostimulants as new approach to improve the biological responses of medicinal plants-A critical review. J. Med. Plants 2016, 59, 3–39. [Google Scholar]
- Berlyn, G.P.; Russo, R.O. The use of organic biostimulants in nitrogen fixing trees. Nitrogen Fixing Tree Res. Rep. 1990, 8, 78–80. [Google Scholar]
- Russo, R.O.; Berlyn, G.P. The effect of an organic biostimulant (Roots TM) on the growth of loblolly pine (Pinus taeda) seedlings in greenhouse conditions. Agrociencia Ser. Recur. Nat. Renov. 1992, 2, 7–13. [Google Scholar]
- Russo, R.O.; Berlyn, G.P. Vitamin-Humic-Algal Root Biostimulant Increases Yield of Green Bean. HortScience 1992, 27, 847. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, H.; Luo, J.; Zhang, X.; Liu, Z.; Zhang, Y.; Zhai, L.; Lei, Q.; Ren, T.; et al. Managing irrigation and fertilization for the sustainable cultivation of greenhouse vegetables. Agric. Water Manag. 2018, 210, 354–363. [Google Scholar] [CrossRef]
- Bashir, M.A.; Liu, J.; Geng, Y.; Wang, H.; Pan, J.; Zhang, D.; Rehim, A.; Aon, M.; Liu, H. Co-culture of rice and aquatic animals: An integrated system to achieve production and environmental sustainability. J. Clean. Prod. 2020, 249, 119310. [Google Scholar] [CrossRef]
- Gouveia, N.A.; de Andrade, M.G.O.; de Ávila, J.; de Oliveira, T.R.; Simon, C.A.; de Lima, S.F. Evaluation of organic cotton residue and application of biostimulant in the production of radish (Raphanus sativus). Res. Soc. Dev. 2020, 9, e386974092. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Petropoulos, S.A. Practical applications of plant biostimulants in greenhouse vegetable crop production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Garcia, A.L.; Madrid, R.; Gimeno, V.; Rodriguez-Ortega, W.M.; Nicolas, N.; Garcia-Sanchez, F. The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Span. J. Agric. Res. 2011, 9, 852. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Effects of different levels of glycine in the nutrient solution on the growth, nutrient composition, and antioxidant activity of coriander (Coriandrum sativum L.). Acta Agrobot. 2019, 72. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; de Oliveira, R.S.; Constantin, J.; Kremer, R.J.; Biffe, D.F. Amino acid application can be an alternative to prevent glyphosate injury in glyphosate-resistant soybeans. J. Plant Nutr. 2012, 35, 268–287. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through a hydrolysis. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Yan, B.X.; Sun, Q.Y. Glycine residues provide flexibility for enzyme active sites. J. Biol. Chem. 1997, 272, 3190–3194. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; López-Maside, L.; Donapetry-García, C.; Fernández-Fernández, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef]
- Tantawy, A.S.; Abdel-Mawgoud, A.M.R.; El-Nemr, M.A.; Chamoun, Y.G. Alleviation of salinity effects on tomato plants by application of amino acids and growth regulators. Eur. J. Sci. Res. 2009, 30, 484–494. [Google Scholar]
- Abdelgawad, K.F.; Mhmoud, A.A.; Mohamed, H.F.Y. Foliar spraying with some biostimulants improves growth, chemical constituents, and yield of head lettuce plant. Middle East J. Agric. Res. 2018, 7, 1268–1277. [Google Scholar]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2012, 1009, 29–36. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-a review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Kocira, A.; Lamorska, J.; Kornas, R.; Nowosad, N.; Tomaszewska, M.; Leszczyńska, D.; Kozłowicz, K.; Tabor, S. Changes in Biochemistry and Yield in Response to Biostimulants Applied in Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 189. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 2019, 123, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Magazin, N.; Miodragović, M.; Popara, G. Bioregulators can improve fruit size, yield and plant growth of northern highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2018, 235, 214–220. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).