Phenotypic, Genetic, and Epigenetic Variation among Diverse Sweet Cherry Gene Pools
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf Phenotyping and Image Analysis
2.3. AFLP Analysis
2.4. MSAP Analysis
2.5. Scoring of AFLP Markers
2.6. Scoring of MSAP Markers
2.7. Correlations Between Inter-Population Genetic, Epigenetic and Phenotypic Distance
3. Results
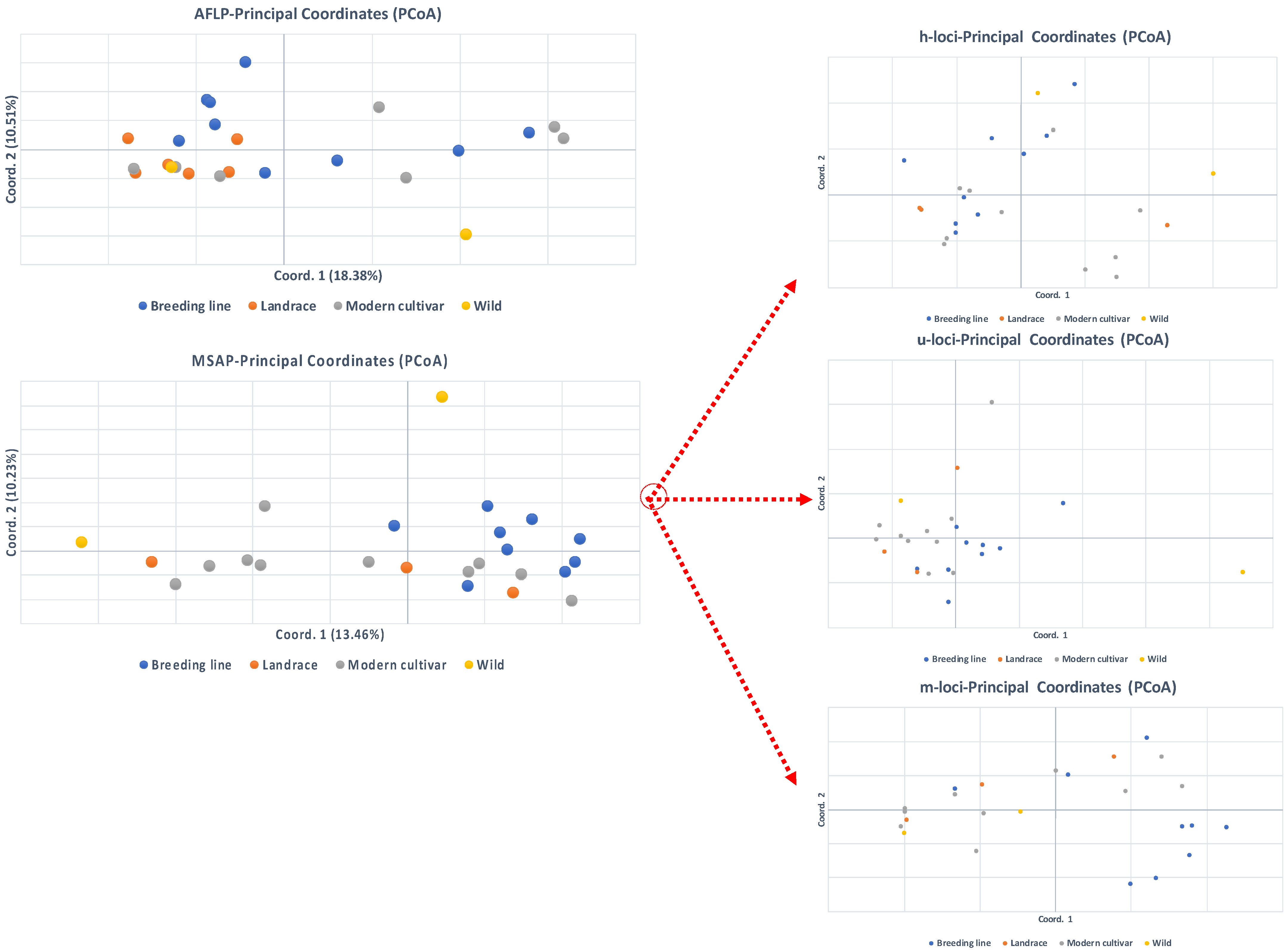
3.1. AFLP Genetic Diversity
3.2. MSAP Epigenetic Diversity
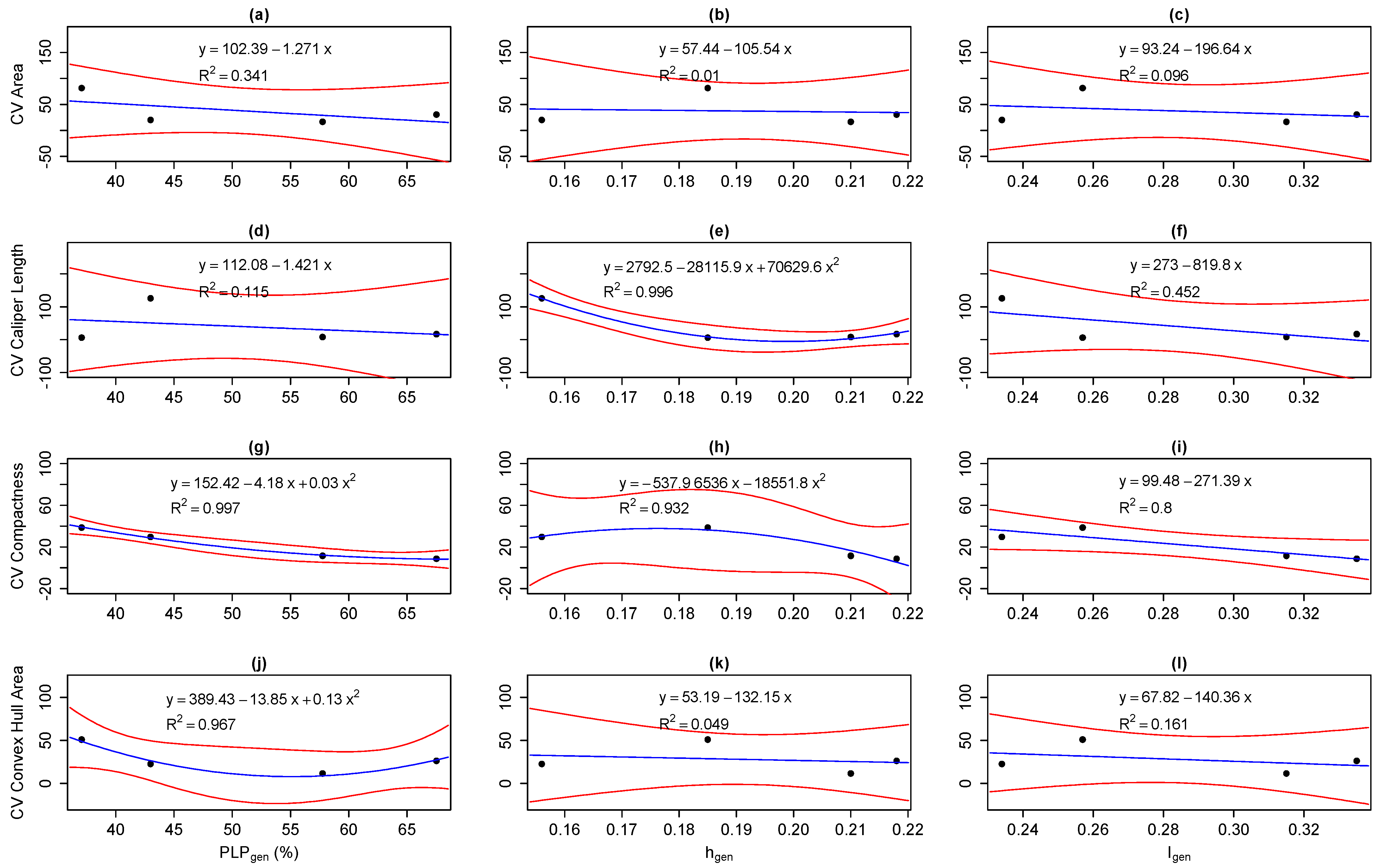
3.3. Correlations Between Intra-Population Genetic, Epigenetic and Phenotypic Variation
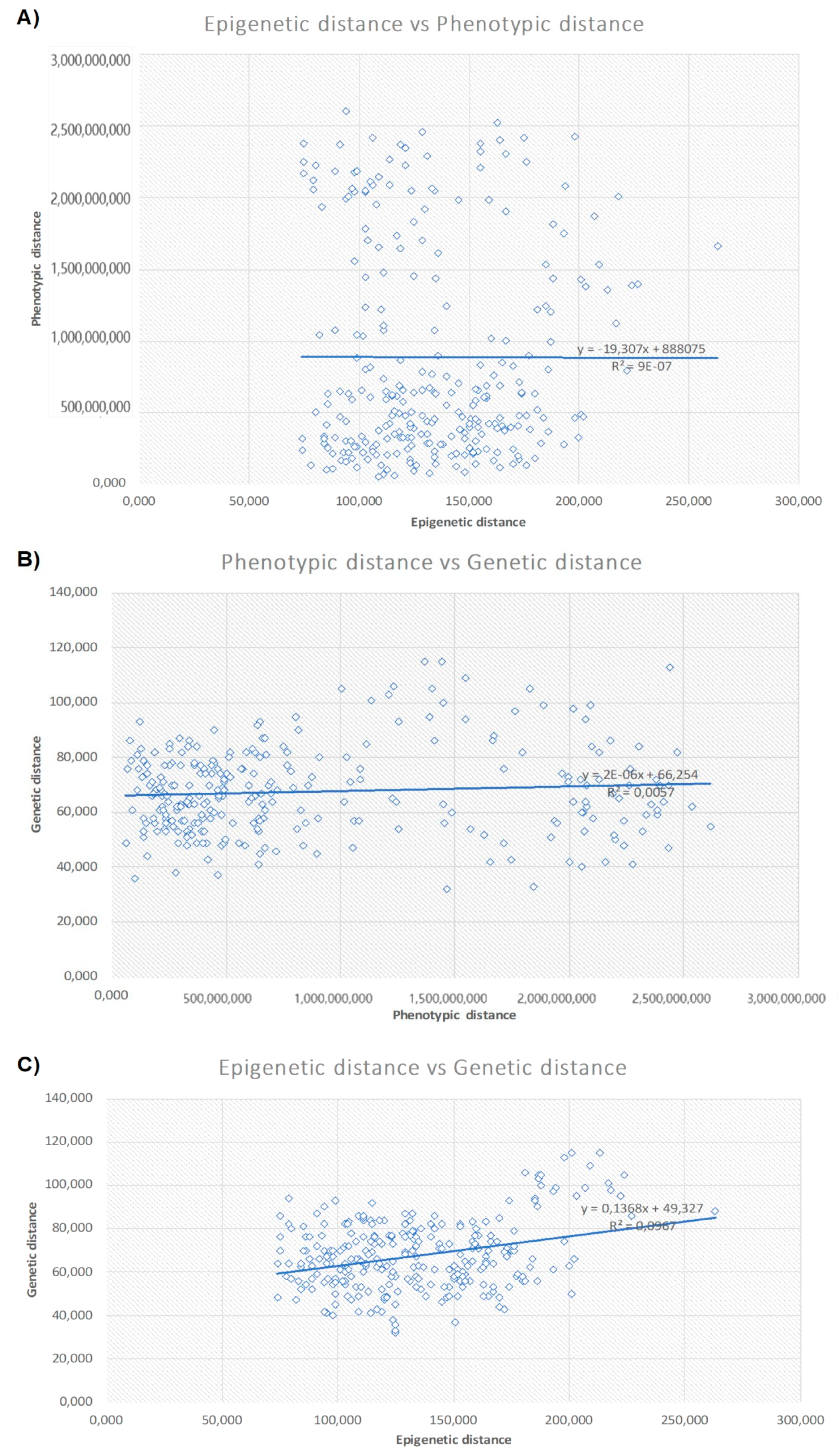
3.4. Correlations Between Inter-Population Genetic, Epigenetic, and Phenotypic Distance
4. Discussion
4.1. Correlations Between Epigenetic and Genetic Variation
4.2. Correlations of Phenotypic Variation with Genetic and Epigenetic Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zambounis, A.; Ganopoulos, I.; Avramidou, E.; Aravanopoulos, F.A.; Tsaftaris, A.; Madesis, P. Evidence of extensive positive selection acting on cherry (Prunus avium L.) resistance gene analogs (RGAs). Aust. J. Crop Sci. 2016, 10, 1324. [Google Scholar] [CrossRef]
- Jarausch, W.; Eyquard, J.; Mazy, K.; Lansac, M.; Dosba, F. High level of resistance of sweet cherry (Prunus avium L.) towards European stone fruit yellows phytoplasmas. Adv. Hortic. Sci. 1999, 13, 108–112. [Google Scholar]
- Castede, S.; Campoy, J.A.; Le Dantec, L.; Quero-Garcia, J.; Barreneche, T.; Wenden, B.; Dirlewanger, E. Mapping of candidate genes involved in bud dormancy and flowering time in sweet cherry (Prunus avium). PLoS ONE 2015, 10, e0143250. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Farsakoglou, A.-M.; Aravanopoulos, F.; Molassiotis, A.; Michailidis, M.; Malliarou, E.; Avramidou, E.; Tsaftaris, A.; Osanthanunkul, M.; Madesis, P. Towards sweet cherry (Prunus avium L.) breeding: Phenotyping evaluation of newly developed hybrids. Euphytica 2018, 214, 99. [Google Scholar] [CrossRef]
- Schüller, E.; Fernández, F.F.; Antanaviciute, L.; Anhalt-Brüderl, U.; Spornberger, A.; Forneck, A. Autochthonous Austrian Varieties of Prunus avium L. Represent a Regional Gene Pool, Assessed Using SSR and AFLP Markers. Genes 2021, 12, 322. [Google Scholar] [CrossRef]
- El Baji, M.; Hanine, H.; En-Nahli, S.; Socias I Company, R.; Kodad, O. Morphological and Pomological Characteristics of Sweet Cherry (Prunus Avium L.) Grown In-situ under South Mediterranean Climate in Morocco. Int. J. Fruit Sci. 2021, 1–14. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Aravanopoulos, F.A.; Argiriou, A.; Kalivas, A.; Tsaftaris, A. Is the genetic diversity of small scattered forest tree populations at the southern limits of their range more prone to stochastic events? A wild cherry case study by microsatellite-based markers. Tree Genet. Genomes 2011, 7, 1299–1313. [Google Scholar] [CrossRef]
- Campoy, J.A.; Lerigoleur-Balsemin, E.; Christmann, H.; Beauvieux, R.; Girollet, N.; Quero-García, J.; Dirlewanger, E.; Barreneche, T. Genetic diversity, linkage disequilibrium, population structure and construction of a core collection of Prunus avium L. landraces and bred cultivars. BMC Plant Biol. 2016, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, A.; Manioudaki, M.; Bazakos, C.; Kissoudis, C.; Farsakoglou, A.-M.; Karagiannis, E.; Michailidis, M.; Polychroniadou, C.; Zambounis, A.; Kazantzis, K. Whole genome re-sequencing of sweet cherry (Prunus avium L.) yields insights into genomic diversity of a fruit species. Hortic. Res. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Avramidou, E.V.; Ganopoulos, I.V.; Doulis, A.G.; Tsaftaris, A.S.; Aravanopoulos, F.A. Beyond population genetics: Natural epigenetic variation in wild cherry (Prunus avium). Tree Genet. Genomes 2015, 11, 95. [Google Scholar] [CrossRef]
- Heyn, H.; Moran, S.; Hernando-Herraez, I.; Sayols, S.; Gomez, A.; Sandoval, J.; Monk, D.; Hata, K.; Marques-Bonet, T.; Wang, L. DNA methylation contributes to natural human variation. Genome Res. 2013, 23, 1363–1372. [Google Scholar] [CrossRef]
- Massicotte, R.; Whitelaw, E.; Angers, B. DNA methylation: A source of random variation in natural populations. Epigenetics 2011, 6, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Mirouze, M.; Paszkowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Bossdorf, O.; Verhoeven, K.J. Understanding natural epigenetic variation. New Phytol. 2010, 187, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.; Kiselev, K. Age-associated alterations in the somatic mutation and DNA methylation levels in plants. Plant Biol. 2016, 18, 185–196. [Google Scholar] [CrossRef]
- Thorstensen, T.; Grini, P.E.; Aalen, R.B. SET domain proteins in plant development. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2011, 1809, 407–420. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Zhou, D.-X. Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2011, 1809, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Lee, Y.-S.; Sung, S. Epigenetic regulation by long noncoding RNAs in plants. Chromosome Res. 2013, 21, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic reprogramming in plant and animal development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jacobsen, S.E. Epigenetic modifications in plants: An evolutionary perspective. Curr. Opin. Plant Biol. 2011, 14, 179–186. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Ecker, J.R. Epigenetic and epigenomic variation in Arabidopsis thaliana. Trends Plant Sci. 2012, 17, 149–154. [Google Scholar] [CrossRef]
- Vekemans, X.; Beauwens, T.; Lemaire, M.; Roldán-Ruiz, I. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol. 2002, 11, 139–151. [Google Scholar] [CrossRef]
- Yeh, F.; Yang, R.; Boyle, T.; Ye, Z.; Xiyan, J.; Yang, R.; Boyle, T. PopGene32, Microsoft Windows-Based Freeware for Population Genetic Analysis; Version 1.32; Molecular Biology and Biotechnology Centre, University of Alberta: Edmonton, Alberta, 2000. [Google Scholar]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification. Trans. Am. Microsc. Soc. 1974, 93, 303–304. [Google Scholar]
- Schulz, B.; Eckstein, R.L.; Durka, W. Epigenetic variation reflects dynamic habitat conditions in a rare floodplain herb. Mol. Ecol. 2014, 23, 3523–3537. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Eckstein, R.L.; Durka, W. Scoring and analysis of methylation-sensitive amplification polymorphisms for epigenetic population studies. Mol. Ecol. Resour. 2013, 13, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Culhane, A.C.; Thioulouse, J.; Perrière, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- He, S.; Xu, W.; Li, F.; Wang, Y.; Liu, A. Intraspecific DNA methylation polymorphism in the non-edible oilseed plant castor bean. Plant Divers. 2017, 39, 300–307. [Google Scholar] [CrossRef]
- Ma, K.; Sun, L.; Cheng, T.; Pan, H.; Wang, J.; Zhang, Q. Epigenetic variance, performing cooperative structure with genetics, is associated with leaf shape traits in widely distributed populations of ornamental tree Prunus mume. Front. Plant Sci. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Z.; Li, H.-L.; Li, J.-M.; Yu, F.-H. Correlations between genetic, epigenetic and phenotypic variation of an introduced clonal herb. Heredity 2020, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; House, M.; Booker, H.; Tanino, K. Exploiting epigenetic variation for plant breeding. CAB Rev. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Richards, E.J. Inherited epigenetic variation—revisiting soft inheritance. Nat. Rev. Genet. 2006, 7, 395–401. [Google Scholar] [CrossRef]
- Richards, E.J. Population epigenetics. Curr. Opin. Genet. Dev. 2008, 18, 221–226. [Google Scholar] [CrossRef]
- Dubin, M.J.; Zhang, P.; Meng, D.; Remigereau, M.-S.; Osborne, E.J.; Casale, F.P.; Drewe, P.; Kahles, A.; Jean, G.; Vilhjalmsson, B. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. eLife 2015, 4, e05255. [Google Scholar] [CrossRef]
- Herrera, C.M.; Bazaga, P. Epigenetic differentiation and relationship to adaptive genetic divergence in discrete populations of the violet Viola cazorlensis. New Phytol. 2010, 187, 867–876. [Google Scholar] [CrossRef]
- Banta, J.A.; Richards, C.L. Quantitative epigenetics and evolution. Heredity 2018, 121, 210–224. [Google Scholar] [CrossRef]
- Slatkin, M. Epigenetic inheritance and the missing heritability problem. Genetics 2009, 182, 845–850. [Google Scholar] [CrossRef]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 1999, 401, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Herrera, C.M.; Bazaga, P. Epigenetic variation predicts regional and local intraspecific functional diversity in a perennial herb. Mol. Ecol. 2014, 23, 4926–4938. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Choi, J.-Y.; Lim, M.-J.; Lee, S.-I.; Choi, H.-J.; Kim, N.-S. Genetic and epigenetic diversity among dent, waxy, and sweet corns. Genes Genom. 2015, 37, 865–874. [Google Scholar] [CrossRef]



| Cultivar | Predefined Population | Common Name | Surface Area | Caliper Length | Compactness | Convex Hull Area |
|---|---|---|---|---|---|---|
| BxS5 | Breeding line | - | 994,345 | 2.187.903 | 0.540 | 1,841,280 |
| BxS33 | Breeding line | - | 456,843 | 1.264.413 | 0.543 | 841,147 |
| HGxS11 | Breeding line | - | 1,293,434 | 2.314.375 | 0.614 | 2,105,830 |
| BxS21 | Breeding line | - | 1,642,672 | 2.395.099 | 0.677 | 2,424,134 |
| BxS14 | Breeding line | - | 1,421,852 | 2.214.082 | 0.646 | 2,200,543 |
| BxS19 | Breeding line | - | 1,169,272 | 2.341.492 | 0.580 | 2,015,571 |
| BxS17 | Breeding line | - | 1,163,390 | 2.030.177 | 0.610 | 1,904,195 |
| PtrTrAch | Breeding line | Petrokeraso Tragano Achaias | 897,861 | 1.965.073 | 0.580 | 1,545,661 |
| HGxS30 | Breeding line | 990,953 | 1.929.465 | 0.679 | 1,457,352 | |
| ChalkAn | Landrace | Chalkidos Anonimo | 1,211,613 | 2.042.699 | 0.590 | 2,051,944 |
| Chi | Landrace | Chiou | 959,994 | 1.941.165 | 0.546 | 1,756,523 |
| Mz | Landrace | Mieza | 1,334,036 | 2.177.894 | 0.630 | 2,116,484 |
| PrKld | Landrace | Proimo Kolindrou | 1,235,747 | 2.180.397 | 0.598 | 2,065,893 |
| TrRd | Landrace | Tragana Rodohoriou | 1,192,488 | 2.144.086 | 0.585 | 2,036,052 |
| BxS22 | Landrace | - | 1,443,198 | 2347.44 | 0.706 | 2,042,288 |
| AgLd | Landrace | Agiorgitiko Lilantiou | 970,705 | 2012.02 | 0.602 | 1,611,516 |
| Lmnd | Modern cultivar | Lemonidi | 1,227,253 | 2.396.711 | 0.510 | 2,404,142 |
| TrEds | Modern cultivar | Tragana Edes-sis | 1,564,111 | 2.282.969 | 0.666 | 2,345,886 |
| TrEdsN | Modern cultivar | Tragana Edessis-Naousis | 1,166,668 | 2.049.908 | 0.634 | 1,839,019 |
| Vas | Modern cultivar | Vasiliadi | 1,595,101 | 2.245.907 | 0.710 | 2,245,480 |
| Tsol | Modern cultivar | Tsolakeika | 1,103,878 | 1.965.568 | 0.571 | 1,931,870 |
| Bak | Modern cultivar | Bakirtzeika | 1,184,177 | 2.363.933 | 0.611 | 1,935,432 |
| Mhl | Wild | Prunus mahaleb | 345,525 | 1.892.687 | 0.359 | 960,569 |
| Wild | Wild | - | 1,285,426 | 2.066.577 | 0.629 | 2,041,743 |
| 5′ to 3′ Sequence | |
|---|---|
| EcoRI adapter | CTCGTAGACTGCGTACC AATTGGTACGCAGTC |
| MseI adapter | GACGATGAGTCCTGAG TACTCAGGACTCAT |
| HpaII/MspI adapter | GACGATGAGTCTCGAT CGATCGAGACTCAT |
| Pre-selective EcoRI primer | GACTGCGTACCAATTC-A |
| Pre-selective MseI primer | GATGAGTCCTGAGTAA-C |
| Pre-selective HpaII/MspI primer | ATGAGTCTCGATCGG-A |
| Selective EcoRI primers | GACTGCGTACCAATTC+ATG (FAM) GACTGCGTACCAATTC+ACT (HEX) GACTGCGTACCAATTC+AAC (ROX) GACTGCGTACCAATTC+AAG (TAMRA) |
| Selective MseI primer | GATGAGTCCTGAGTAA-CAA GATGAGTCCTGAGTAA-CAC GATGAGTCCTGAGTAA-CGT GATGAGTCCTGAGTAA-CTC |
| Selective HpaII/MspI primer | ATGAGTCTCGATCGGATC ATGAGTCTCGATCGGACT ATGAGTCTCGATCGGAAT |
| A. Genetic Variation | B. Epigenetic Variation | |||||
|---|---|---|---|---|---|---|
| Pre-Defined Population | PLPgen (%) | hgen | Igen | PLPepi (%) | hepi | Iepi |
| Breeding line | 67.53 | 0.218 | 0.335 | 54.06 | 0.174 | 0.268 |
| Landrace | 42.99 | 0.156 | 0.234 | 35.69 | 0.159 | 0.227 |
| Modern cultivar | 57.75 | 0.210 | 0.315 | 66.08 | 0.204 | 0.317 |
| Wild | 37.08 | 0.185 | 0.257 | 40.64 | 0.230 | 0.282 |
| C. Phenotypic Variation | ||||||
| CV Area | CV Caliper Length | CV Compactness | CV Convex Hull Area | |||
| Breeding line | 30.427 | 16.681 | 8.598 | 26.147 | ||
| Landrace | 20.202 | 125.841 | 29.648 | 22.562 | ||
| Modern cultivar | 16.465 | 7.816 | 11.437 | 11.490 | ||
| Wild | 81.499 | 6.211 | 38.578 | 50.927 | ||
| AFLP Summary AMOVA Table | MSAP Summary AMOVA Table | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | Est. Var. | df | SS | MS | Est. Var. | % |
| Among populations | 3 | 256,885 | 85,628 | 3776 | 3 | 142,444 | 47,481 | 2955 | 9% |
| Within populations | 20 | 1,286,698 | 64,335 | 64,335 | 20 | 636,056 | 31,803 | 31,803 | 91% |
| Total | 23 | 1,543,583 | 68,111 | 23 | 778,500 | 34,758 | 100% | ||
| Stat | Value | P (rand ≥ data) | Stat | Value | P (rand ≥ data) | ||||
| PhiPT | 0.055 | 0.037 | 0.085 | 0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidou, E.V.; Moysiadis, T.; Ganopoulos, I.; Michailidis, M.; Kissoudis, C.; Valasiadis, D.; Kazantzis, K.; Tsaroucha, E.; Tsaftaris, A.; Molassiotis, A.; et al. Phenotypic, Genetic, and Epigenetic Variation among Diverse Sweet Cherry Gene Pools. Agronomy 2021, 11, 680. https://doi.org/10.3390/agronomy11040680
Avramidou EV, Moysiadis T, Ganopoulos I, Michailidis M, Kissoudis C, Valasiadis D, Kazantzis K, Tsaroucha E, Tsaftaris A, Molassiotis A, et al. Phenotypic, Genetic, and Epigenetic Variation among Diverse Sweet Cherry Gene Pools. Agronomy. 2021; 11(4):680. https://doi.org/10.3390/agronomy11040680
Chicago/Turabian StyleAvramidou, Evangelia V., Theodoros Moysiadis, Ioannis Ganopoulos, Michail Michailidis, Christos Kissoudis, Dimitrios Valasiadis, Konstantinos Kazantzis, Eirini Tsaroucha, Athanasios Tsaftaris, Athanassios Molassiotis, and et al. 2021. "Phenotypic, Genetic, and Epigenetic Variation among Diverse Sweet Cherry Gene Pools" Agronomy 11, no. 4: 680. https://doi.org/10.3390/agronomy11040680
APA StyleAvramidou, E. V., Moysiadis, T., Ganopoulos, I., Michailidis, M., Kissoudis, C., Valasiadis, D., Kazantzis, K., Tsaroucha, E., Tsaftaris, A., Molassiotis, A., Aravanopoulos, F. A., & Xanthopoulou, A. (2021). Phenotypic, Genetic, and Epigenetic Variation among Diverse Sweet Cherry Gene Pools. Agronomy, 11(4), 680. https://doi.org/10.3390/agronomy11040680











