Impact of Pests on Cereal Grain and Nutrient Yield in Boreal Growing Conditions
Abstract
1. Introduction
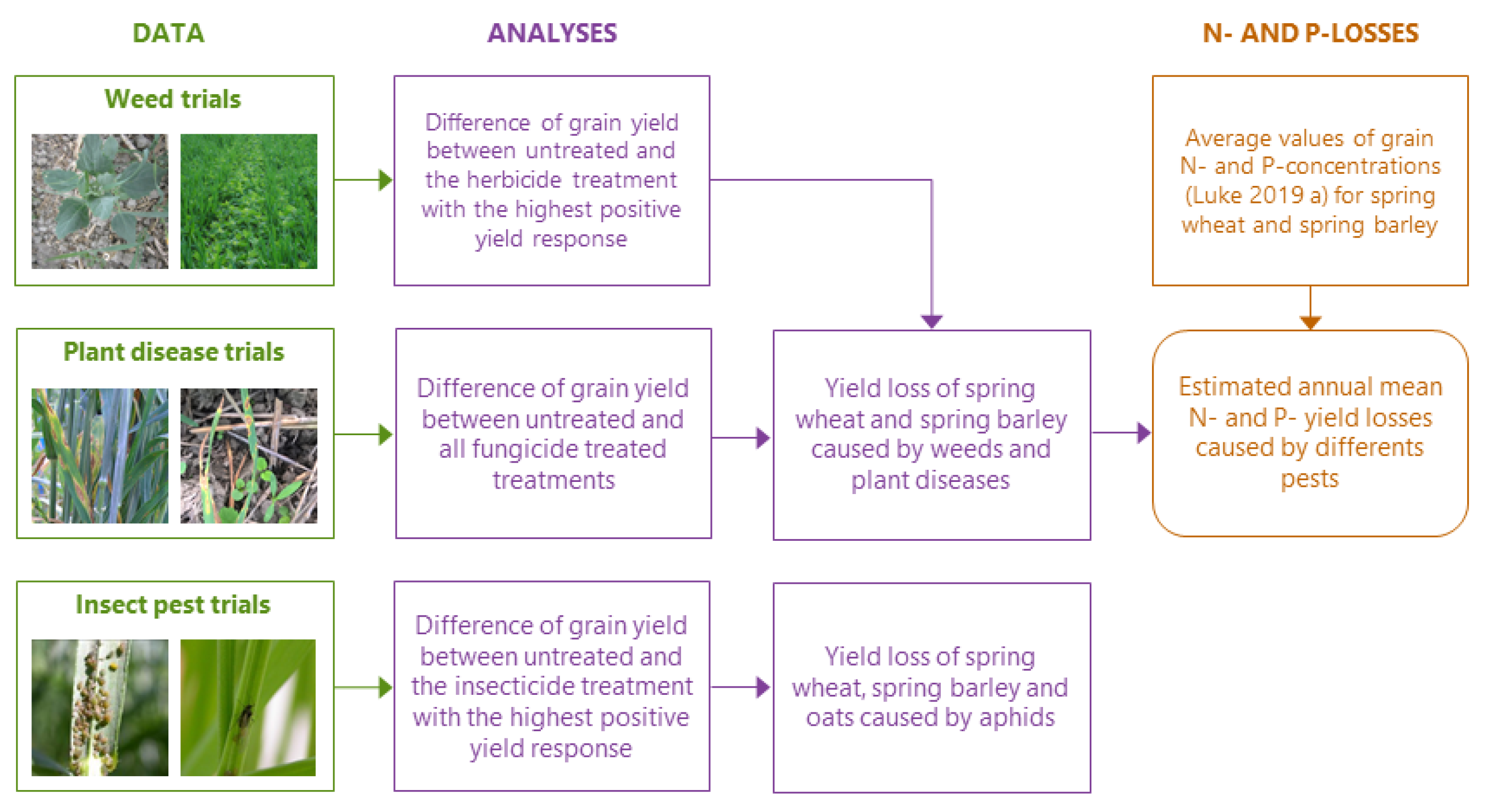
2. Material and Methods
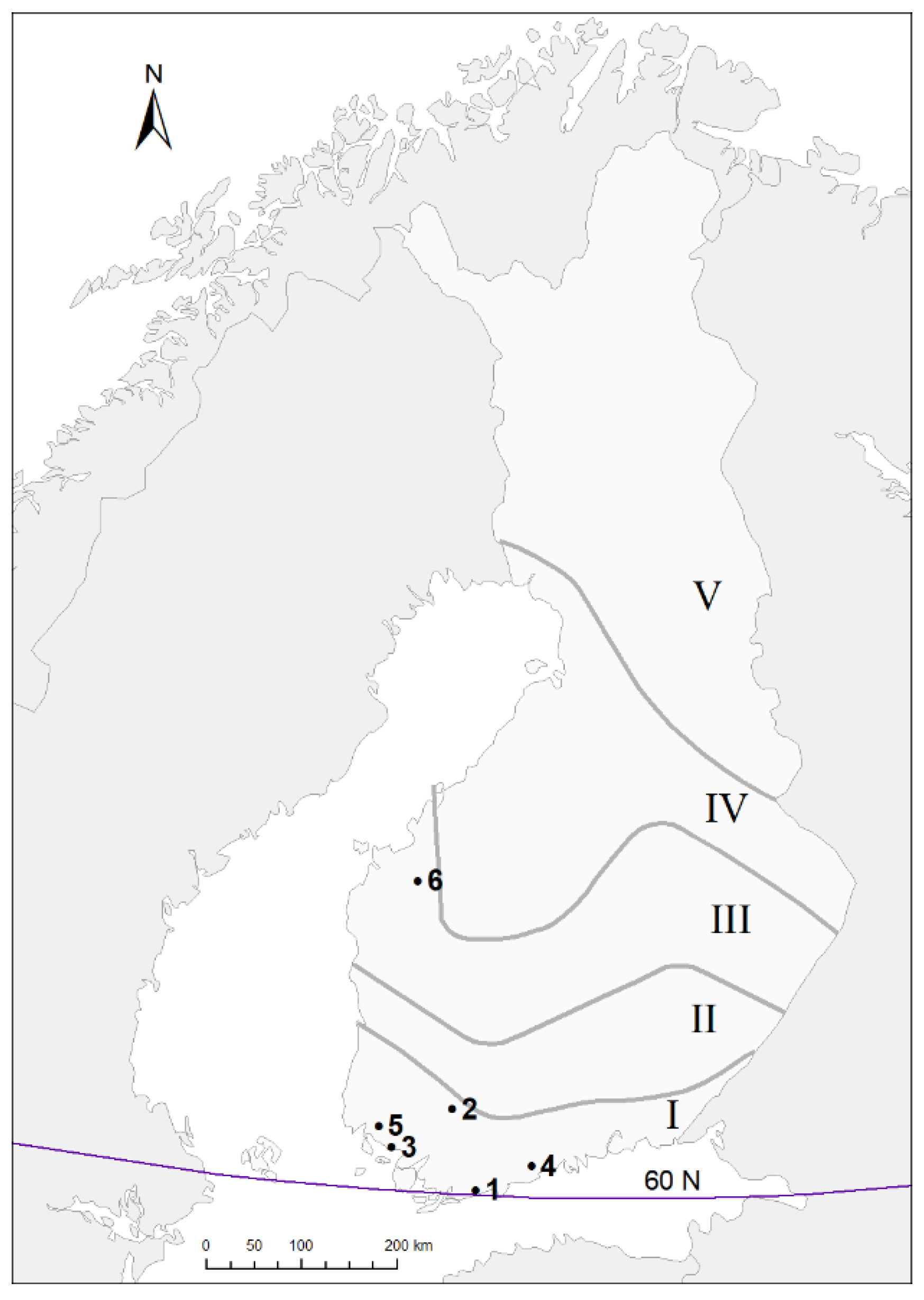
2.1. Data Collection
2.1.1. Weed Trials
2.1.2. Plant Disease Trials
2.1.3. Insect Pest Trials
2.2. Statistical Analysis
2.3. Nitrogen and Phosphorus Yield Loss Estimation
3. Results
3.1. Effect of Weeds on Cereal Yield
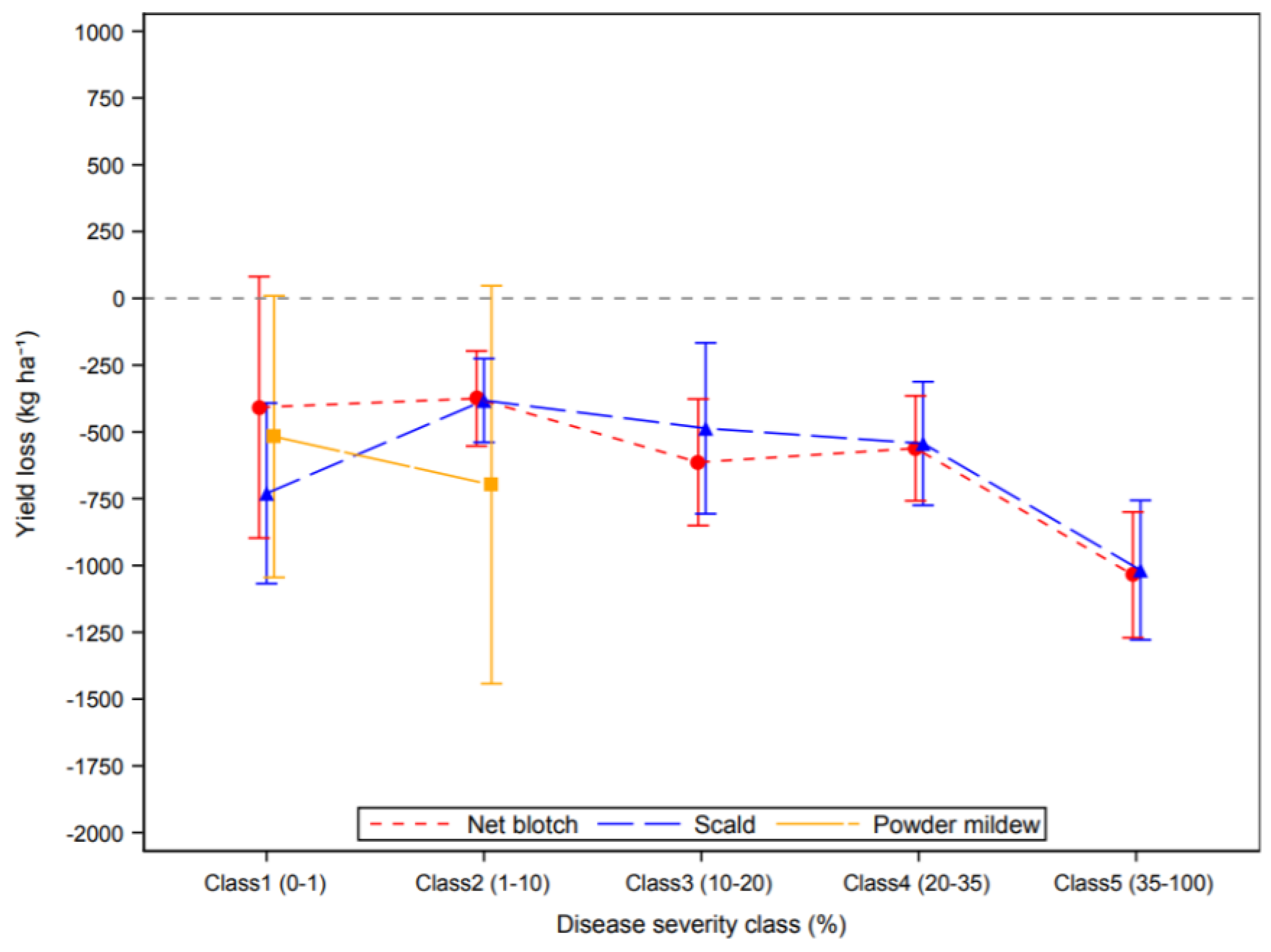
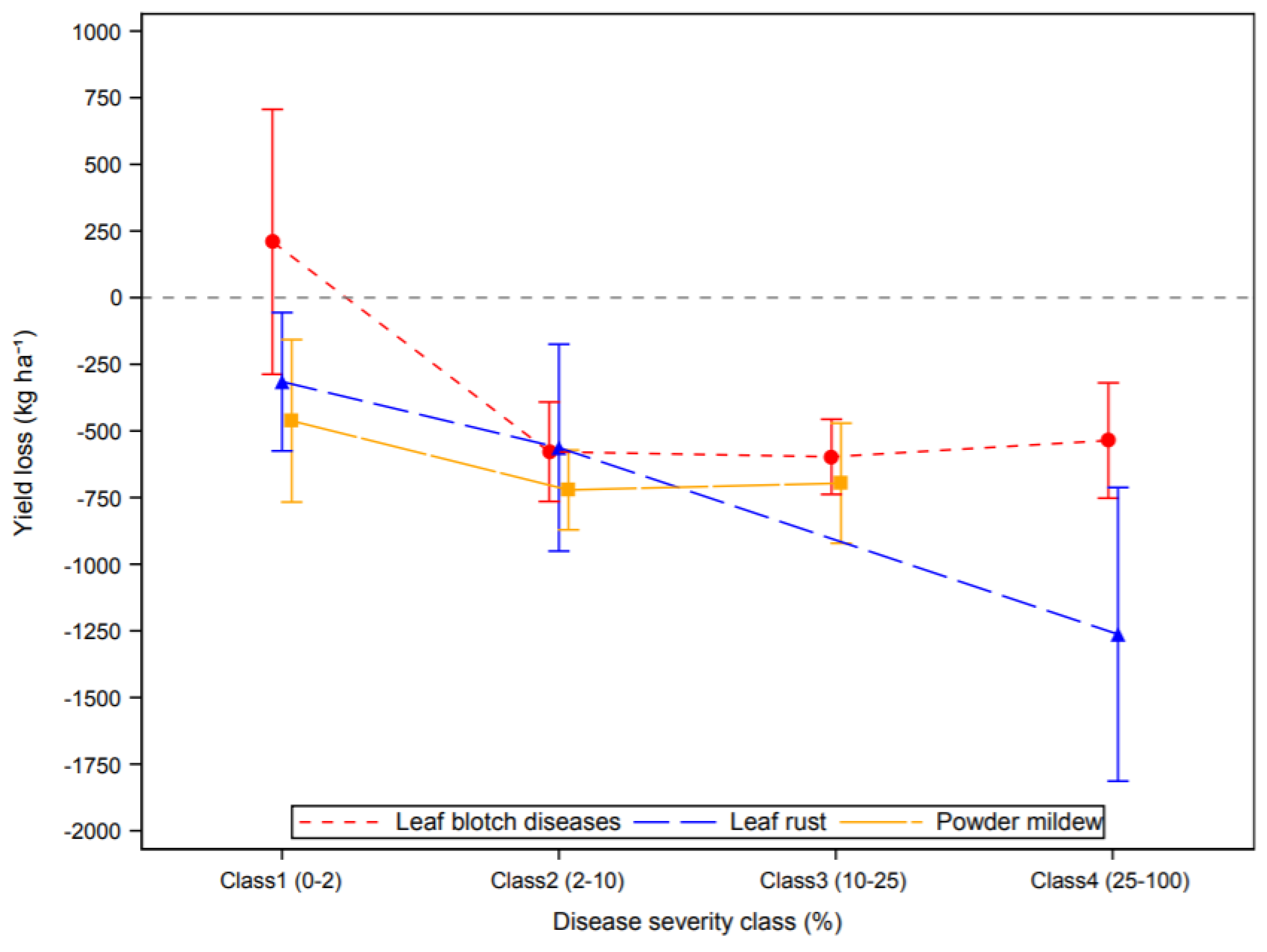
3.2. Effect of Plant Diseases on Cereal Yield
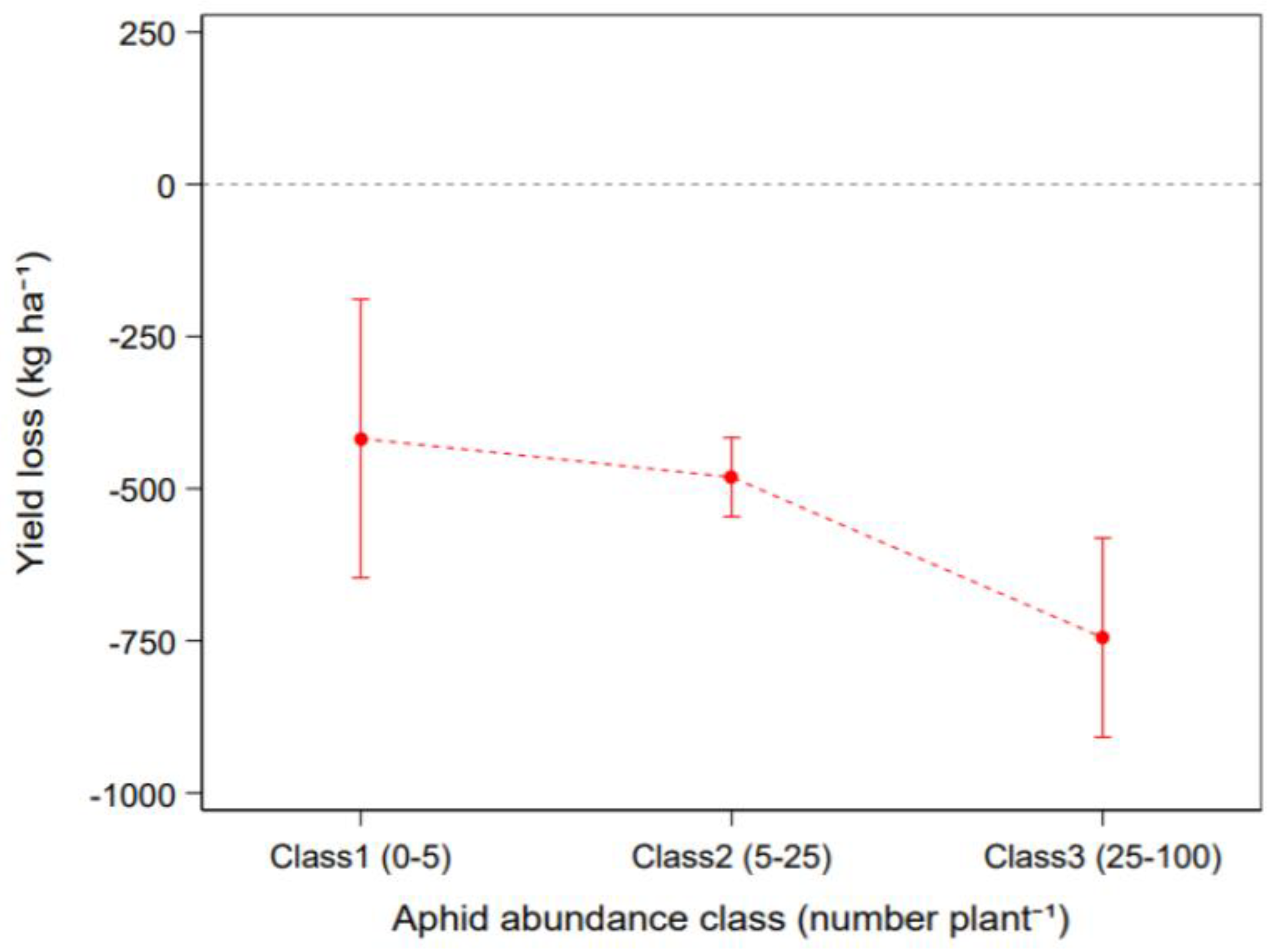
3.3. Effect of Pest Insects on Crop Yield
3.4. Nitrogen and Phosphorus Yield Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peltonen-Sainio, P.; Jauhiainen, L.; Laurila, I.P. Cereal yield trends in northern European conditions: Changes in yield potential and its realization. Field Crop. Res. 2009, 110, 85–90. [Google Scholar] [CrossRef]
- Olesen, J.E.; Bindi, M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002, 16, 239–262. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Palosuo, T.; Ruosteenoja, K.; Jauhiainen, L.; Ojanen, H. Warming autumns at high latitudes of Europe: An opportunity to lose or gain in cereal production? Reg. Environ. Chang. 2018, 18, 1453–1465. [Google Scholar] [CrossRef]
- Coakley, S.M.; Scherm, H.; Chakraborty, S. Climate change and plant disease management. Annu. Rev. Phytopathol. 1999, 37, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Juroszek, P.; von Tiedemann, A. Potential strategies and future requirements for plantdisease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Cammell, M.E.; Knight, J.D. Effects of climatic change on the population dynamics of crop pests. Adv. Ecol. Res. 1992, 22, 117–162. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Directeffects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Hakala, K.; Hannukkala, A.O.; Huusela-Veistola, E.; Jalli, M.; Peltonen-Sainio, P. Pests and diseases in a changing climate: A major challenge for Finnish crop production. Agric. Food Sci. 2011, 20, 3–14. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Cousens, R.; Brain, P.; O’Donovan, J.T.; O’Sullivan, A. The use of biolicallu realitic equations to descrobe the effect of weeds density and relative time of emergence on crop yield. Weed Sci. 1987, 35, 720–725. [Google Scholar] [CrossRef]
- Kropff, M.J. Modelling the effect of weeds on crop production. Weed Res. 1988, 28, 465–471. [Google Scholar] [CrossRef]
- Lutman, P.J.W.; Moss, S.R.; Cook, S.; Welham, S.J. A review of the effects of crop agronomy on the management of Alopecurus myosuroides. Weed Res. 2013, 53, 299–313. [Google Scholar] [CrossRef]
- Salonen, J. Yield responses of spring cereals to reduced herbicide doses. Weed Res. 1992, 32, 493–499. [Google Scholar] [CrossRef]
- Milberg, P.; Hallgren, E. Yield loss due to weeds in cereals and its large-scale variability in Sweden. Field Crop. Res. 2004, 86, 199–209. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. The Current and Potential Costs from Diseases of Wheat in Australia; Grains Research and Development Corporation: Kingston, Australia, 2009; p. 21. ISBN 978-1-875477-92-0. [Google Scholar]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian barley industry. Aust. Plant Pathol. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- Bockus, W.W.; Appel, J.A.; Bowden, R.L.; Fritz, A.K.; Gill, B.S.; Martin, T.J.; Sears, R.G.; Seifers, D.L.; Brown-Guedira, G.L.; Eversmeyer, M.G. Success Stories: Breeding for Wheat Disease Resistance in Kansas. Plant Dis. 2001, 85, 453–461. [Google Scholar] [CrossRef]
- Jalli, M.; Kaseva, J.; Andersson, B.; Ficke, F.; Nistrup-Jørgensen, L.; Ronis, A.; Kaukoranta, T.; Ørum, J.E.; Djurle, A. Yield increases due to fungicide control of leaf blotch diseases in wheat and barley as a basis for IPM decision-making in the Nordic-Baltic region. Eur. J. Plant Pathol. 2020, 158, 315–333. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.W.; Haley, S.D.; Langham, M.A.C.; Evenson, P.D. Winter wheat responses to bird cherry-oat aphids and barley yellow dwarf virus infection. Crop Sci. 1999, 39, 158–163. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C.; Nutter, F.W., Jr. Quantification of yield losses caused by barley yellow dwarf virus in wheat and oats. Plant Dis. 2002, 86, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, S.P.; King, B.J.; Barr, A.R.; Warner, P.; Logue, S.J.; Langridge, P. Marker-assisted backcross introgression of the Yd2 gene conferring resistance to barley yellow dwarf virus in barley. Plant Breed. 2003, 122, 52–56. [Google Scholar] [CrossRef]
- Helenius, J. Effect of epigeai predators on infestation by the aphid Rhopalosiphum padi and on grain yield of oats in monocrops and mixed intercrops. Entomol. Exp. Appl. 1990, 54, 225–236. [Google Scholar] [CrossRef]
- Hansen, L.M. Establishing control threshold for bird cherry-oat aphid (Rhopalosiphum padi L.) in spring barley (Hordeum vulgare L.) by aphid-days. Crop Prot. 2000, 19, 191–194. [Google Scholar] [CrossRef]
- European Comission. Integrated Pest Management (IPM). Available online: https://ec.europa.eu/food/plant/pesticides/sustainable_use_pesticides/ipm_en (accessed on 15 October 2019).
- Official Statistics of Finland (OSF): Used of Pesticides. Helsinki: Natural Resources Institute Finland. Available online: http://statdb.luke.fi/PXWeb/pxweb/en/LUKE/LUKE__02%20Maatalous__04%20Tuotanto__34%20Kasvinsuojeluaineiden%20kaytto%20maataloudessa/02_Kasvinsuojeluainekaytto.px/table/tableViewLayout1/?rxid=dc711a9e-de6d-454b-82c2-74ff79a3a5e0 (accessed on 7 October 2020).
- Farm to Fork Strategy—for a Fair, Healthy and Environmentally-Friendly Food System. Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 18 December 2020).
- World Fertilizer Trends and Outlook to 2018. Available online: http://www.fao.org/3/a-i4324e.pdf (accessed on 21 January 2020).
- Official Statistics of Finland (OSF): Nitrogen and phosphorus balance. Helsinki: Natural Resources Institute Finland. Available online: https://stat.luke.fi/en/indicator/nitrogen-and-phosphorus-balance (accessed on 15 November 2019).
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus: Reconciling changing concepts of soil phosphorus chemistry with agronomic information. In Proceedings of the 18th World Congress of Soil Science, Philadelphia, PA, USA, 9–15 July 2006. [Google Scholar]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.; Andersen, H.; Lauridsen, T.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate Change Effects on Runoff, Catchment Phosphorus Loading and Lake Ecological State, and Potential Adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Tattari, S.; Koskiaho, J.; Kosunen, M.; Lepistö, A.; Linjama, A.; Puustinen, M. Nutrient loads from agricultural and forested areas in Finland from 1981 up to 2010—Can the efficiency of undertaken water protection measures seen? Environ. Monit. Assess. 2017, 189, 95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Rajala, A.; Peltonen-Sainio, P.; Jalli, M.; Jauhiainen, L.; Hannukkala, A.; Tenhola-Roininen, T.; Ramsay, L.; Manninen, O. One century of Nordic barley breeding: Nitrogen use efficiency, agronomic traits and genetic diversity. J. Agric. Sci. 2017, 155, 582–598. [Google Scholar] [CrossRef]
- Alam, S.M. Handbook of Plant and Crop Stress, 2nd ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 285–314. ISBN 0-8247-1948-4. [Google Scholar]
- Mela, T.; Haapalainen, M. Hehtaarisatojen ja Tärkeimpien Satoon Vaikuttavien Tekijöiden Kehitys Vuosina 1956-75 ja Ennuste Vuoteen 1985; Kasvinviljelylaitoksen Tiedote Numero 4; Maatalouden Tutkimuskeskus, Tikkurila: Helsinki, Finland, 1976. [Google Scholar]
- Turtola, E.; Salo, T.; Miettinen, A.; Iho, A.; Valkama, E.; Rankinen, K.; Virkajärvi, P.; Tuomisto, J.; Sipilä, A.; Muurinen, S.; et al. Hyötyä Taseista-Ravinnetaseiden Tulkinta Ympäristön ja Viljelyn Hyödyksi; Juvenes Print: Helsinki, Finland, 2017; ISBN 978-952-326-373-4. [Google Scholar]
- Yli-Halla, M.; Nykänen, A.; Siimes, K.; Tuhkanen, H.-R. Ympäristötuen Ehdot ja Maan Helppoliukoisen Fosforin Pitoisuus; Jyväskylän Yliopistopaino: Jyväskylä, Finland, 2001; ISBN 951-729-574-X. [Google Scholar]
- MTTK/MTT Annual Reports of Official Herbicide Testing in Finland in 1965−2012. Available at: Project Management, Pesticide Testing Pentti Ruuttunen, pentti.ruuttunen@luke.fi.
- Bleiholder, H.; Feller, C.; Hess, M.; Meier, U.; van den Boom, T.; Lancashire, P.D.; Buhr, L.; Hack, H.; Klose, R.; Stauss, R.; et al. Compendium of Growth Stage Identification Keys for Mono- and Dicotyledonous Plants-Extended BBCH Scale, 2nd ed.; A joint publication of BBA BSA IGZ IVA AgrEvo BASF Bayer Novartis ISBN; 1997; ISBN 3-9520749-3-4. [Google Scholar]
- MTT/Luke Annual Reports of Official Pesticide Testing in Finland in 1999−2016. Available at: Project Management, Pesticide Testing Pentti Ruuttunen, pentti.ruuttunen@luke.fi.
- Rajala, A.; Niskanen, M.; Isolahti, M.; Peltonen-Sainio, P. Seed quality effects on seedling emergence, plant stand establishment and grain yield in two-row barley. Agric. Food Sci. 2011, 20, 228–234. [Google Scholar] [CrossRef]
- Khanzada, K.A.; Rajput, M.A.; Shah, G.S.; Lodhi, A.M.; Mehboob, F. Effect of Seed Dressing Fungicides for the Control of Seedborne Mycoflora of Wheat. Asian J. Plant Sci. 2002, 1, 441–444. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stat. Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- Westfall, P.; Tobias, R.D.; Wolfinger, R.D. Multiple Comparisons and Multiple Tests Using SAS, 2nd ed.; SAS Institute Inc.: North Carolina, NC, USA, 2011; ISBN 978-1-60764-783-6. [Google Scholar]
- Vanhat Tuomikirvaennusteet. Available online: https://portal.mtt.fi/portal/page/portal/kasper/pelto/peltopalvelut/tuomikirvaennuste/vanhattuomikirvaennusteet (accessed on 21 April 2020).
- Official Statistics of Finland (OSF): Feed Tables. Available online: https://portal.mtt.fi/portal/page/portal/Rehutaulukot/feed_tables_english (accessed on 26 November 2019).
- Official Statistics of Finland (OSF): Utilised Agricultural Area. Available online: https://stat.luke.fi/en/utilised-agricultural-area (accessed on 21 November 2019).
- Salonen, J.; Hyvönen, T.; Jalli, H. Composition of weed flora in spring cereals in Finland—A fourth survey. Agric. Food Sci. 2011, 20, 245–261. [Google Scholar] [CrossRef]
- Leather, S.R.; Lehti, J.P. Field studies on the factors affecting the population dynamics of the bird cherry-oat aphid, Rhopalosiphum Padi (L.) in Finland. Ann. Agric. Fenn. 1982, 21, 20–31. [Google Scholar]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosystems 2002, 46, 237–256. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Delgado, J.A. Principles for managing nitrogen leaching. J. Soil Water Conserv. 2002, 57, 485–498. [Google Scholar]
- Uusitalo, R.; Närvänen, A.; Kaseva, A.; Launto-Tiuttu, A.; Heikkinen, J.; Joki-Hieskala, P.; Rasa, K.; Salo, T. Conversion of dissolved phosphorus in runoff by ferric sulfate to a form less available to algae: Field performance and cost assessment. AMBIO 2015, 44, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, N.; Saad, J.K.; Van Laethem, C.; Machet, J.M.; Maucorps, J.; Mary, B. Nitrate leaching in intensive agriculture in Northern France: Effect of farming practices, soils and crop rotations. Agric. Ecosyst. Environ. 2005, 111, 292–310. [Google Scholar] [CrossRef]
- Bell, A.R.; Nalewaja, J.D. Competition of Wild Oat in Wheat and Barley. Weed Sci. 1968, 16, 505–508. [Google Scholar] [CrossRef]
- Bertholdsson, N.-O. Early vigour and allelopathy—two useful traits forenhanced barley and wheat competitiveness against weeds. Weed Res. 2005, 45, 94–102. [Google Scholar] [CrossRef]
- Hakala, K.; Jauhiainen, L.; Himanen, S.J.; Rötter, R.; Salo, T.; Kahiluoto, H. Sensitivity of barley varieties to weather in Finland. J. Agric. Sci. 2012, 150, 145–160. [Google Scholar] [CrossRef]
- Korres, N.E.; Froud-Williams, R.J. Effects of winter wheat cultivars and seed rate on the biological characteristics of naturally occurring weed flora. Weed Res. 2002, 42, 417–428. [Google Scholar] [CrossRef]
- Das, T.K.; Yaduraju, N.T. Effects of missing-row sowing supplemented with row spacing and nitrogen on weed competition and growth and yield of wheat. Crop Pasture Sci. 2011, 62, 48–57. [Google Scholar] [CrossRef]
- Qasem, J.R. Nutrient accumulation by weeds and their associated vegetable crops. J. Hortic. Sci. 1991, 67, 189–195. [Google Scholar] [CrossRef]
- Andreasen, C.; Litz, A.-S.; Streibig, J.C. Growth response of six weed species and spring barley (Hordeum vulgare) to increasing levels of nitrogen and phosphorus. Weed Res. 2006, 46, 503–512. [Google Scholar] [CrossRef]
- Lim, L.G.; Gaunt, R.E. The effect of powdery mildew (Erysiphe graminis f. sp. hordei) and leaf rust (Puccinia hordei) on spring barley in New Zealand. I. Epidemic development, green leaf area and yield. Plant Pathol. 1986, 35, 44–53. [Google Scholar] [CrossRef]
- Jayasena, K.W.; Van Burgel, A.; Tanaka, K.; Majewski, J.; Loughman, R. Yield reduction in barley in relation to spot-type net blotch. Australas. Plant Pathol. 2007, 36, 429–433. [Google Scholar] [CrossRef]
- Kieckhefer, R.W.; Kantack, B.H. Yield Losses in Winter Grains Caused by Cereal Aphids (Homoptera: Aphididae) in South Dakota. J. Econ. Entomol. 1988, 81, 317–321. [Google Scholar] [CrossRef]
- Official Statistics of Finland (OSF): Yield of the Main Crop. Available online: http://statdb.luke.fi/PXWeb/pxweb/en/LUKE/LUKE__02%20Maatalous__04%20Tuotanto__14%20Satotilasto/01_Viljelykasvien_sato.px/table/tableViewLayout1/?rxid=69c91bf6-f37a-4386-97bb-3c510b782811 (accessed on 21 April 2020).
- OECD-FAO Agricultural Outlook 2019-2028. Available online: https://stats.oecd.org/viewhtml.aspx?datasetcode=HIGH_AGLINK_2019&lang=en (accessed on 19 August 2020).
- Bingham, I.; Karley, A.; White, P.; Thomas, W.; Russell, J. Analysis of improvements in nitrogen use efficiency associated with 75 years of spring barley breeding. Eur. J. Agron. 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Delin, S.; Nyberg, A.; Lindén, B.; Ferm, M.; Torstensson, G.; Lerenius, C.; Gruvaeus, I. Impact of crop protection on nitrogen utilisation and losses in winter wheat production. Eur. J. Agron. 2008, 28, 361–370. [Google Scholar] [CrossRef]
- Huusela-Veistola, E.; Jalli, M.; Ylivainio, K.; Turtola, E.; Lemola, R.; Ruuttunen, P. Kasvintuhoojilla Merkitystä Ravinnekuormituksessa- Verkkolaikkutartunta Vähentää Ohran Ravinteiden Ottoa. Available online: https://journal.fi/smst/article/view/75820/37204 (accessed on 10 February 2020).
- Bullock, D.G. Crop rotation. Crit. Rev. Plant Sci. 1992, 11, 309–326. [Google Scholar] [CrossRef]
- Bailey, K.L.; Gossen, B.D.; Lafond, G.P.; Watson, P.R.; Derksen, D.A. Effect of tillage and crop rotation on root and foliar diseases of wheat and pea in Saskatchewan from 1991 to 1998: Univariate and multivariate analyses. Can. J. Plant Sci. 2001, 81, 789–803. [Google Scholar] [CrossRef]
- Mamolos, A.P.; Kalburtji, K.L. Significance of Allelopathy in Crop Rotation. J. Crop Prod. 2001, 4, 197–218. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Henriksen, K.E. Application of pathogen surveys, disease nurseries and varietal resistance characteristics in an IPM approach for the control of wheat yellow rust. Eur. J. Plant Pathol. 2008, 121, 377–385. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Bi, J.-L.; Yang, X.-Q.; Huang, Y.; Zhao, X.; Hu, Y.; Cai, Q.-N. Constitutive and induced activities of defense-related enzymes in aphid-resistant and aphid-susceptible cultivars of wheat. J. Chem. Ecol. 2009, 35, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Andrew, I.K.S.; Storkey, J.; Sparkes, D.L. A review of the potential for competitive cereal cultivars as a tool in integrated weed management. Weed Res. 2015, 55, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A. Plant protection in spring cereal production with reduced tillage. II. Pests and beneficial insects. Crop Prot. 1999, 18, 651–657. [Google Scholar] [CrossRef]
- Bárberi, P.; Lo Cascio, B. Long-term tillage and crop rotation effects on weed seedbank size and composition. Weed Res. 2000, 41, 325–340. [Google Scholar] [CrossRef]
- Murphy, S.D.; Clements, D.R.; Belaoussoff, S.; Kevan, P.G.; Swanton, C.J. Promotion of weed species diversity and reduction of weed seedbanks with conservation tillage and crop rotation. Weed Sci. 2006, 54, 69–77. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Schroeder, K.L.; Schillinger, W.F. Soilborne Pathogens of Cereals in an Irrigated Cropping System: Effects of Tillage, Residue Management, and Crop Rotation. Plant Dis. 2010, 94, 61–68. [Google Scholar] [CrossRef]

| Weed Species | Median, Plants m−2 (Min–Max) | Median, g m−2 (Min–Max) |
|---|---|---|
| Stellaria media (L.) | 10 (0–999) | 0.60 (0–156.8) |
| Chenopodium album (L.) | 9 (0–1282) | 0.80 (0–159.7) |
| Viola arvensis (L.) | 6 (0–510) | 0.20 (0–48.0) |
| Galeopsis species (L.) | 6 (0–714) | 1.10 (0–109.4) |
| Barley | Yield Loss kg ha−1 | N-Yield Loss kg ha−1 | P-Yield Loss kg P ha−1 | N-Yield Loss Mkg N Production Area−1 * | P-Yield Loss Mkg P Production Area−1 * |
|---|---|---|---|---|---|
| no seed treatment ** | 300 | 4.8 | 0.9 | 2.42 | 0.46 |
| no herbicide treatment | 200 | 3.2 | 0.6 | 1.61 | 0.31 |
| no fungicide treatment | 500 | 8.1 | 1.5 | 4.04 | 0.77 |
| Wheat | |||||
| no seed treatment *** | 300 | 5.5 | 0.9 | 1.11 | 0.19 |
| no herbicide treatment | 200 | 3.7 | 0.6 | 0.73 | 0.13 |
| no fungicide treatment | 500 | 9.2 | 1.6 | 1.84 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauppi, K.; Rajala, A.; Huusela, E.; Kaseva, J.; Ruuttunen, P.; Jalli, H.; Alakukku, L.; Jalli, M. Impact of Pests on Cereal Grain and Nutrient Yield in Boreal Growing Conditions. Agronomy 2021, 11, 592. https://doi.org/10.3390/agronomy11030592
Kauppi K, Rajala A, Huusela E, Kaseva J, Ruuttunen P, Jalli H, Alakukku L, Jalli M. Impact of Pests on Cereal Grain and Nutrient Yield in Boreal Growing Conditions. Agronomy. 2021; 11(3):592. https://doi.org/10.3390/agronomy11030592
Chicago/Turabian StyleKauppi, Katja, Ari Rajala, Erja Huusela, Janne Kaseva, Pentti Ruuttunen, Heikki Jalli, Laura Alakukku, and Marja Jalli. 2021. "Impact of Pests on Cereal Grain and Nutrient Yield in Boreal Growing Conditions" Agronomy 11, no. 3: 592. https://doi.org/10.3390/agronomy11030592
APA StyleKauppi, K., Rajala, A., Huusela, E., Kaseva, J., Ruuttunen, P., Jalli, H., Alakukku, L., & Jalli, M. (2021). Impact of Pests on Cereal Grain and Nutrient Yield in Boreal Growing Conditions. Agronomy, 11(3), 592. https://doi.org/10.3390/agronomy11030592






