The Content of Biologically Active Substances in Crocosmia × crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Saponins
2.2. Determination of Selected Bioactive Compounds Using UPLC
2.3. Identification of Carotenoids
2.4. Total Polyphenolic Content
2.5. ABTS●+ Method
2.6. Statistical Analysis
3. Results and Discussion
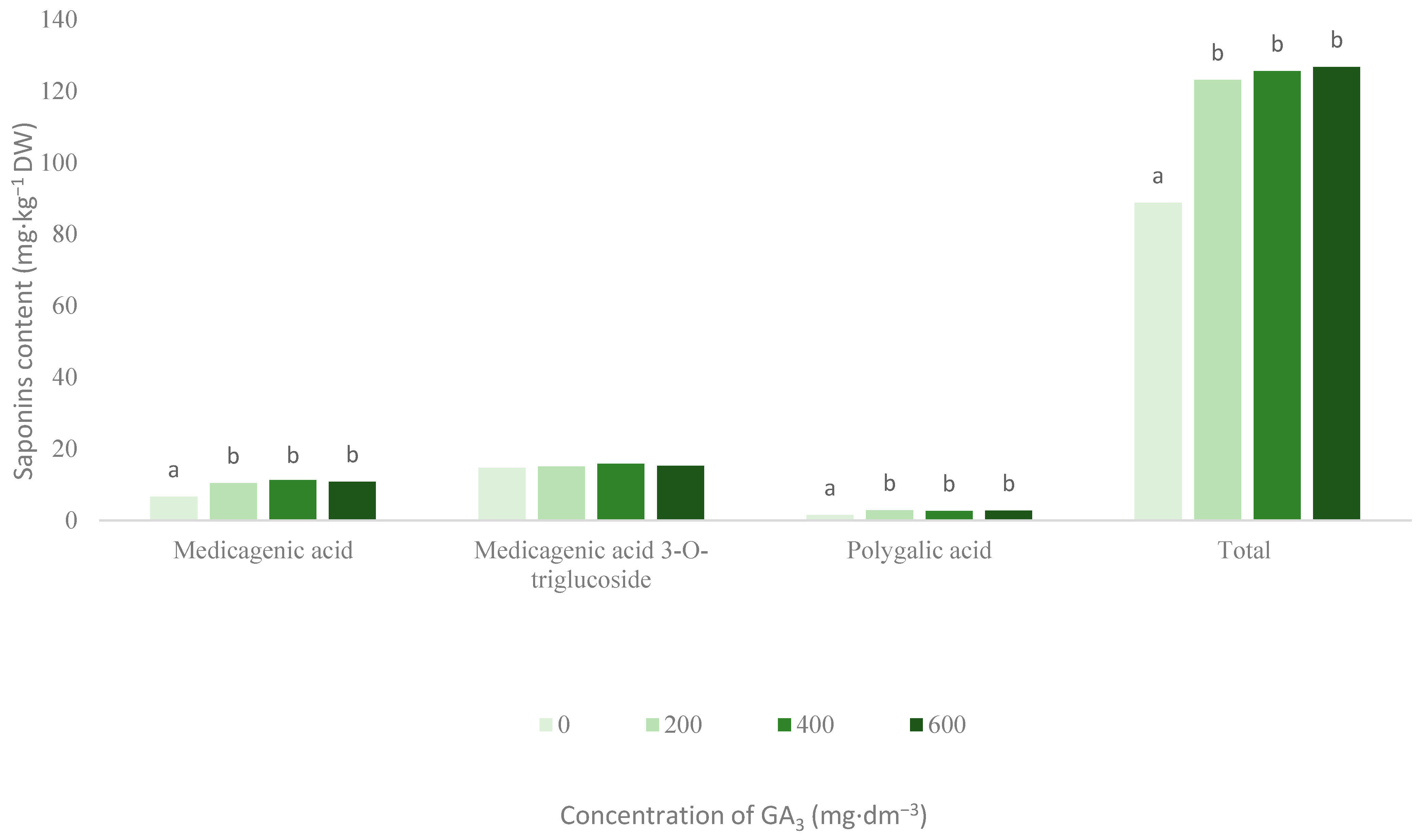
3.1. Saponins
3.2. Phenolic Acids
3.3. Flavonoid Pigments
3.4. Carotenoid Pigments
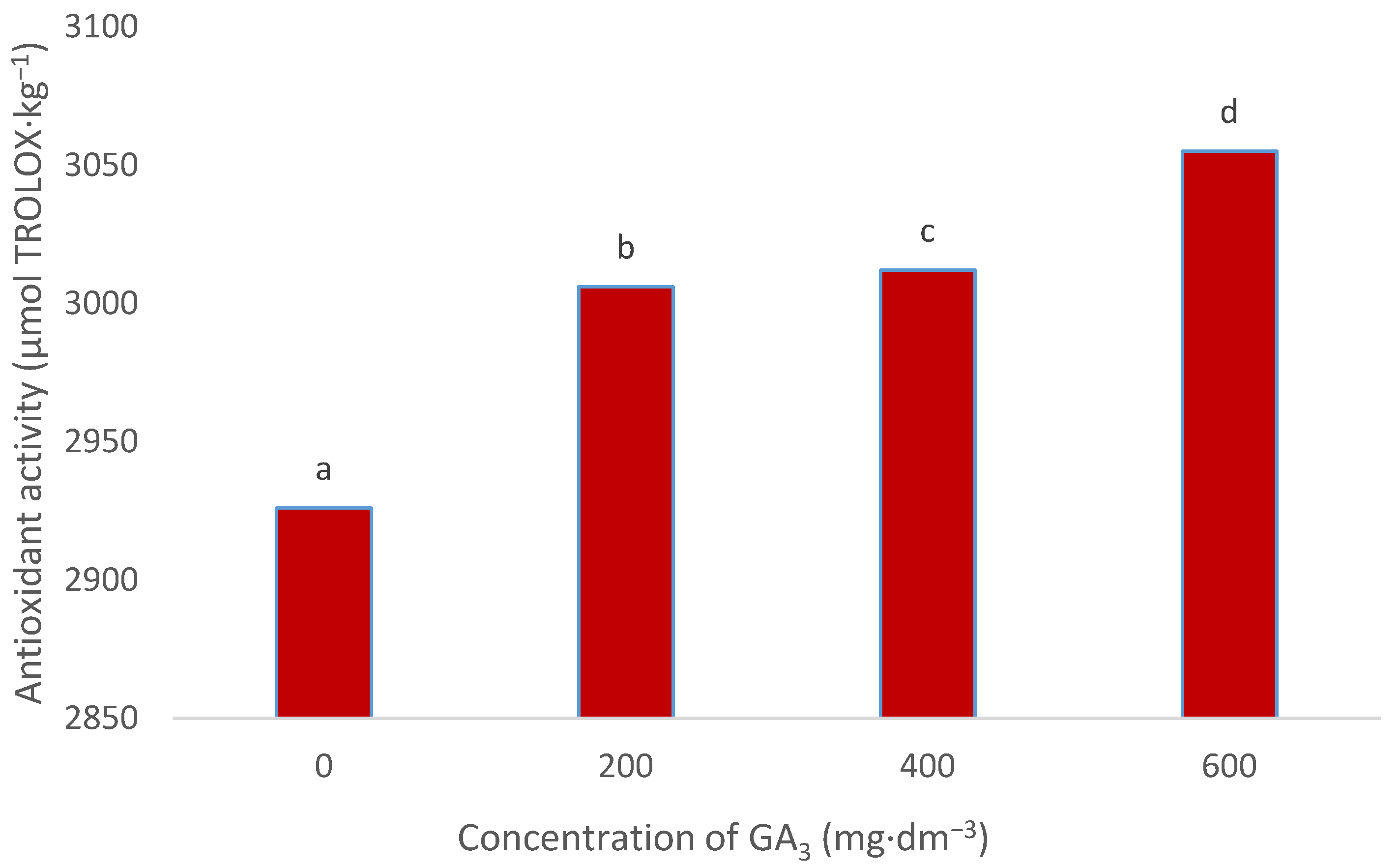
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic determination of plant saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Parus, A. Pharmacological activites of saponins. Post. Fitoter. 2013, 3, 200–204. [Google Scholar]
- Juang, Y.-P.; Liang, P.H. Biological and pharmacological effects of synthetic saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Zehavi, U.; Naim, M.; Polacheck, I.; Evron, R. Structure-biological activity relationships in alfaalfa antimycotic saponins: The relative activity in medicagenic acid and synthetic derivatives thereof against plant pathogenic fungi. J. Phytopathol. 1989, 125, 209–216. [Google Scholar] [CrossRef]
- Tada, H.; Ikeda, Y.; Omoto, T.; Shimomura, K.; Ishimaru, K. Rosmarinic acid and related phenolic in adventitius root cultures of Ociumum basilicum L. Plant Tissue Cult. Lett. 1996, 13, 69–71. [Google Scholar] [CrossRef]
- Slavin, J.; Marquart, L.; Jakoby, D.I. Comsumption of whole-grain food and decreased risk of cancer: Proposed mechanisms. Cereal Foods World 2000, 2, 54–58. [Google Scholar]
- Jeszka, M.; Flaczek, E.; Kobus-Cisowska, J.; Dziedzic, K. Phenolics—Characteristic and significance in food technology. Nauka Przyr. Technol. 2010, 4, 1–13. [Google Scholar]
- Abdeen, S.M.; Mathew, T.C.; Dashti, H.M.; Astars, S. Protective of green tea on intestinal ischemia-reperfusion injury. Nutrition 2011, 27, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Westo, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, S.; Jurzysta, M. Antyfungial Gaeumannomyces graminis var. tritici activity of various glycosides of medicagenic acid. Acta Agrob. 2005, 58, 71–80. [Google Scholar]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Post. Fitoter. 2013, 1, 48–53. [Google Scholar]
- Cuvelier, M.E.; Richard, H.; Berse, T.C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, J.P.D. Phenolic antioxidant. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Shaig, M.; Hussain, H.; Lee, J.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Simkin, A.J.; Moreau, H.; Kuntz, M.; Pagny, G.; Lin, C.; Tanksley, S.; McCarthy, J. An investigation of carotenoids biosynthesisin Coffea canephora and Coffea arabica. J. Plant Physiol. 2008, 165, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Gryszczyńska, A.; Gryszczyńska, B.; Opala, B. Carotenoids. Natural sources, biosynthesis, influence on human body. Post. Fitoter. 2011, 2, 127–143. [Google Scholar]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D. The flowering and nutritional status of Gladiolus hybridus cv. Black Velvet following a cytokinin treatment. J. Elem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D. The flowering and nutritional status of Gladiolus hybridus ‘Black Velvet’ following gibberellin treatment. Hort. Sci. 2018, 45, 205–210. [Google Scholar] [CrossRef]
- Okoli, C.O.; Akah, P.A.; Okoli, A.S. Potentials of leaves of Aspilia africana (Compositae) in wound care: An experimental evaluation. BMC Complement. Altern. Med. 2007, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, J.; Ju, Y.; Zhang, H.; Liu, M.; Li, X. Effects of two saponins extracted from the Polygonatum zanlanscianense Pamp on the human leukemia (HL-60) cells. Biol. Pharm. Bul. 2001, 24, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in phenylpropanoid and trichothecene production by Fusarium culmorum and sensu stricto via exposure to flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 1–14. [Google Scholar] [CrossRef]
- Martyniuk, S.; Jurzysta, M.; Wróblewska, B. Influence of powdered aerial parts of various Medicago species on the growth of Gaeumannomyces graminis and Cephalosporium gramineum. Bul. Polish Acad. Sci. Biol. 1999, 47, 163–165. [Google Scholar]
- Martyniuk, S.; Biały, Z.; Jurzysta, M. Inhibitory effect of Medicago arabica and Medicago murex constituents on the growth and pathogenicity of Gaeumannomyces graminis var. tritici. In Modern Fungicides and Antifungal Compounds; Dehne, H.-W., Gis, I.U., Kuck, K.H., Russell, P.E., Russel, H., Eds.; AgroConcept GmbH: Bonn, Germany, 2002; pp. 395–400. [Google Scholar]
- Gruiz, K. Fungitoxic activity of saponins: Practical use and fundamental principles. In Saponin Used in Traditional and Modern Medicine; Waller, G.R., Yamasaki, K., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 527–534. [Google Scholar]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitami, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Fischer, C.; Speth, V.; Fleig-Eberenz, S.; Neuhaus, G. Induction of zygotic polyembryos in wheat: Influence of auxin polar transport. Plant Cell 1997, 9, 1767–1780. [Google Scholar] [CrossRef]
- Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutr. J. 2011, 4, 189–198. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecki, M. Effect of rhizome soaking in a mixture of BA and GA3 on the earliness of flowering and quality of the yield of flowers and leaves in the calla lily (Zantedeschia Spreng.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 3–12. [Google Scholar]
- Janowska, B. Effect of growth regulators on flower and leaf yield of the calla lily (Zantedeschia Spreng.). Hort. Sci. 2013, 40, 78–82. [Google Scholar] [CrossRef]
- Janowska, B. Effect of benzyladenine on flower and leaf yield of Calla lily (Zantedeschia Spreng.). Bul. J. Agric. Sci. 2014, 20, 633–637. [Google Scholar]
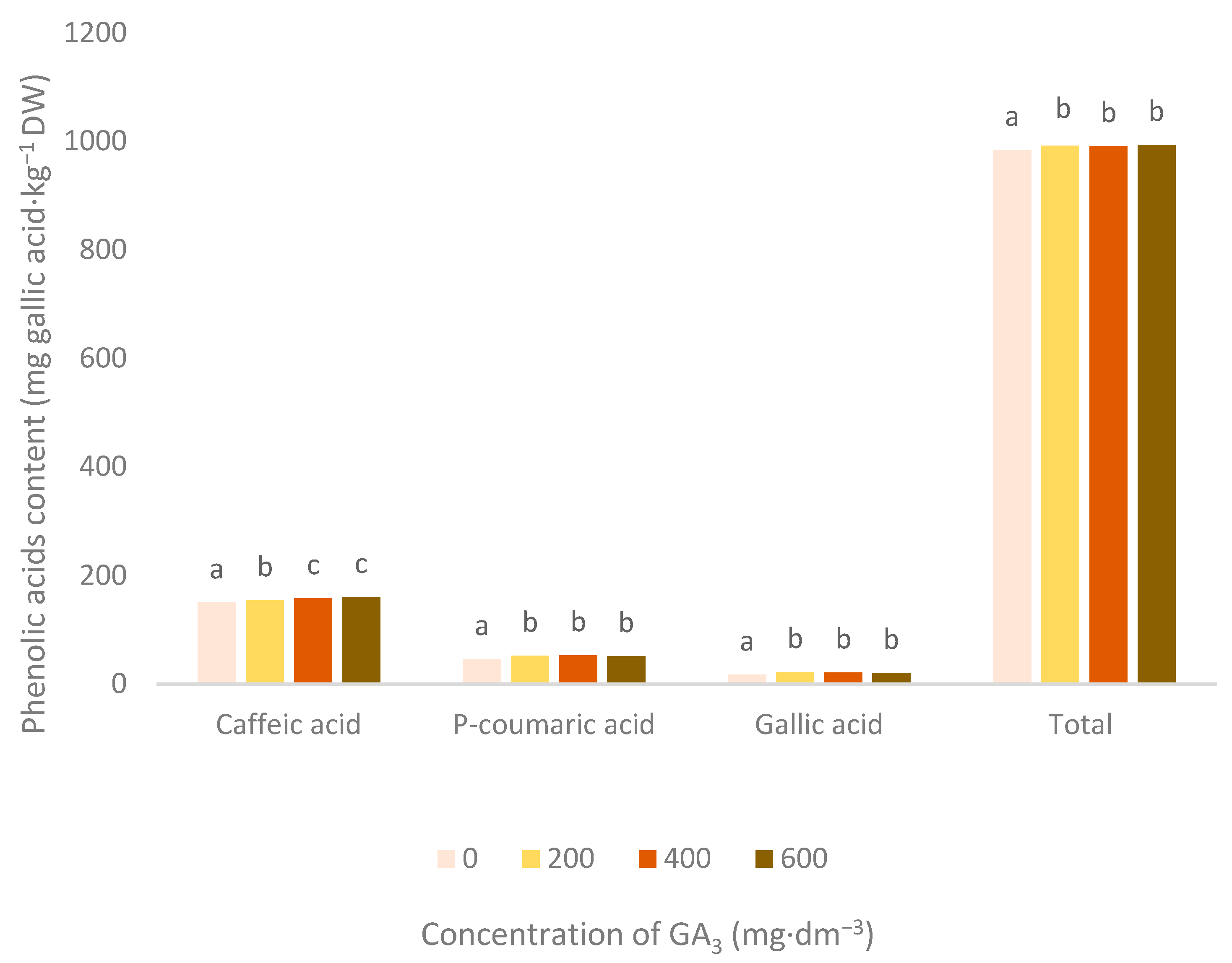
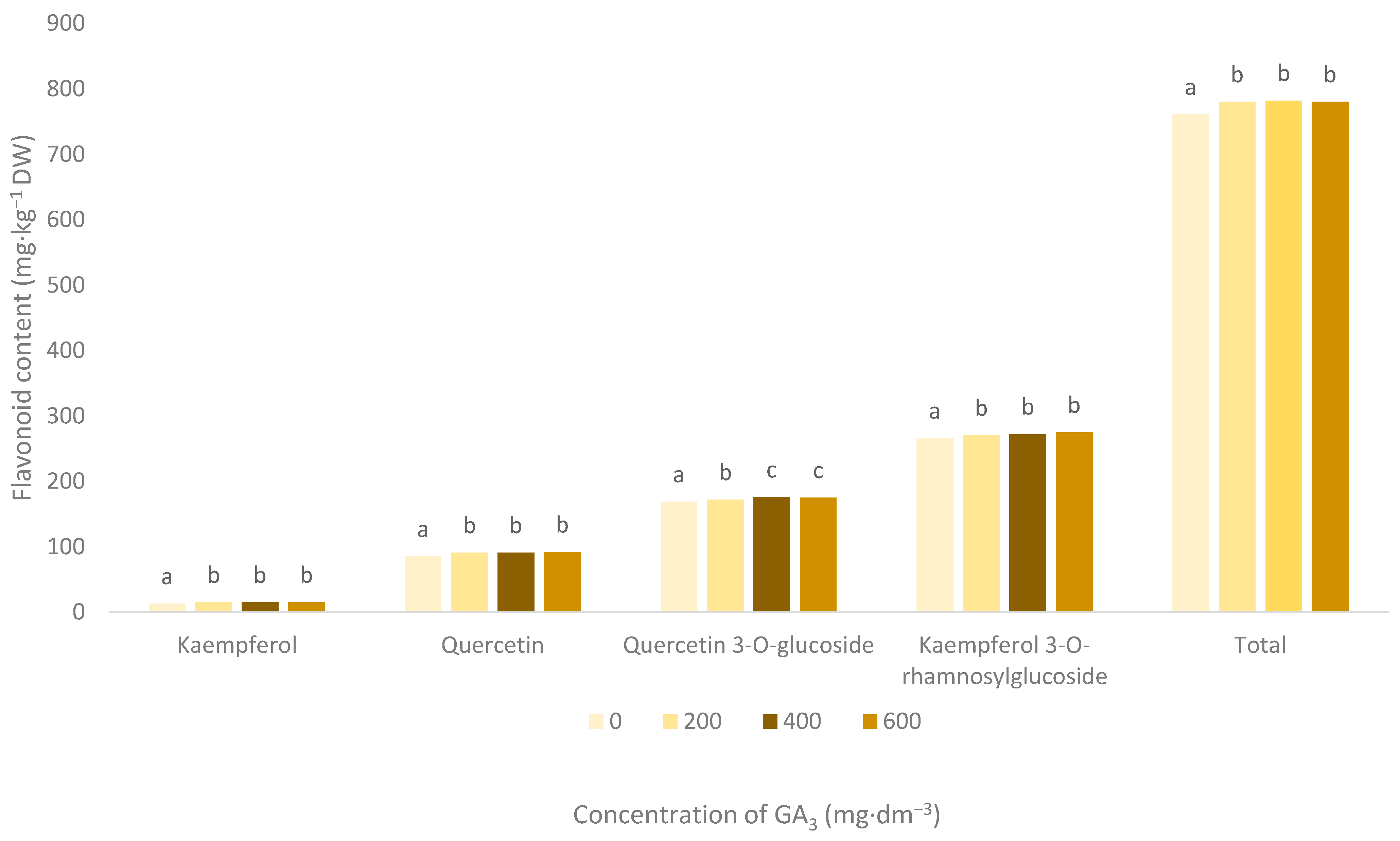

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowska, B.; Andrzejak, R.; Szwajkowska-Michałek, L.; Stuper-Szablewska, K. The Content of Biologically Active Substances in Crocosmia × crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3. Agronomy 2021, 11, 553. https://doi.org/10.3390/agronomy11030553
Janowska B, Andrzejak R, Szwajkowska-Michałek L, Stuper-Szablewska K. The Content of Biologically Active Substances in Crocosmia × crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3. Agronomy. 2021; 11(3):553. https://doi.org/10.3390/agronomy11030553
Chicago/Turabian StyleJanowska, Beata, Roman Andrzejak, Lidia Szwajkowska-Michałek, and Kinga Stuper-Szablewska. 2021. "The Content of Biologically Active Substances in Crocosmia × crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3" Agronomy 11, no. 3: 553. https://doi.org/10.3390/agronomy11030553
APA StyleJanowska, B., Andrzejak, R., Szwajkowska-Michałek, L., & Stuper-Szablewska, K. (2021). The Content of Biologically Active Substances in Crocosmia × crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3. Agronomy, 11(3), 553. https://doi.org/10.3390/agronomy11030553






