Root Traits Related with Drought and Phosphorus Tolerance in Common Bean (Phaseolus vulgaris L.)
Abstract
1. Introduction
2. Materials and Methods
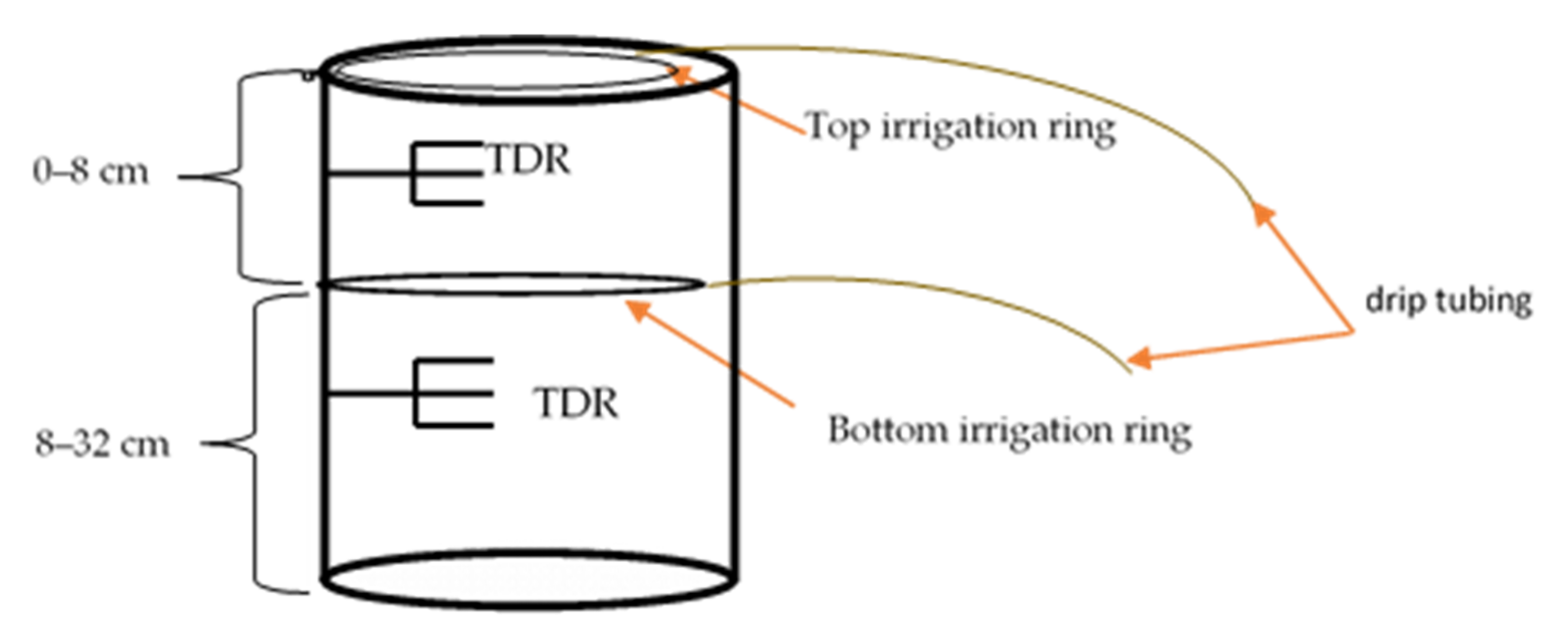
2.1. Experimental Conditions
2.2. Plant Material
2.3. Experimental Design
- (i)
- Control: high water (well-watered) and high phosphorus in both layers;
- (ii)
- Stratified low phosphorus: high phosphorus in the top 0–8 cm, low phosphorus in the bottom 8–32 cm, and adequate water in both layers;
- (iii)
- Stratified low water (drought stress): low water in the top 0–8 cm, adequate water in the bottom 8–36 cm, and high phosphorus in both layers;
- (iv)
- Stratified low water (drought stress) and phosphorus: high phosphorus and low water in the top 0–8 cm and low phosphorus and adequate water in the bottom 8–36 cm layers.
2.4. Shoot and Root Measurements
2.5. Statistical Analysis
3. Results
3.1. Phene Assessment: Root Traits and Shoot Biomass
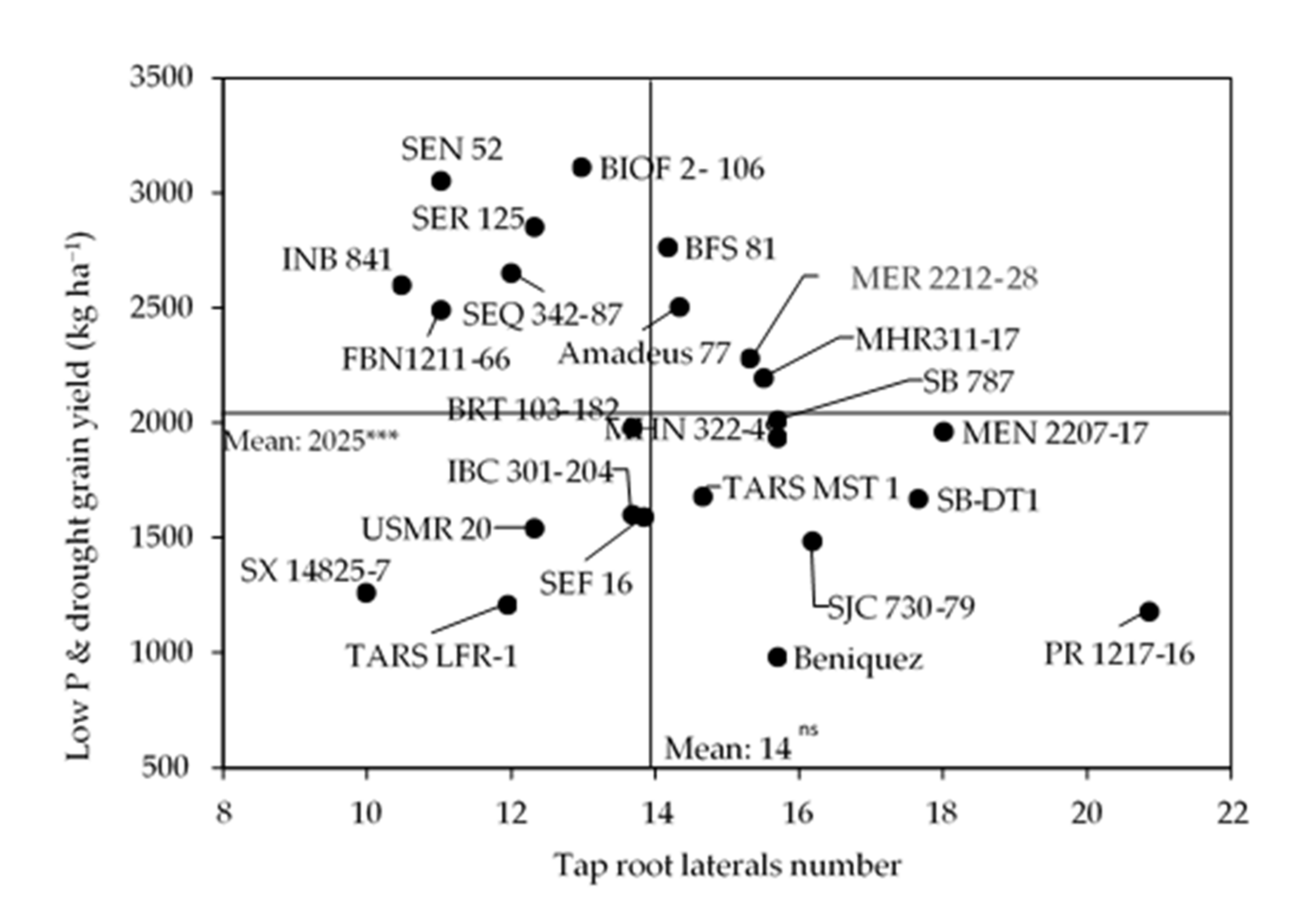
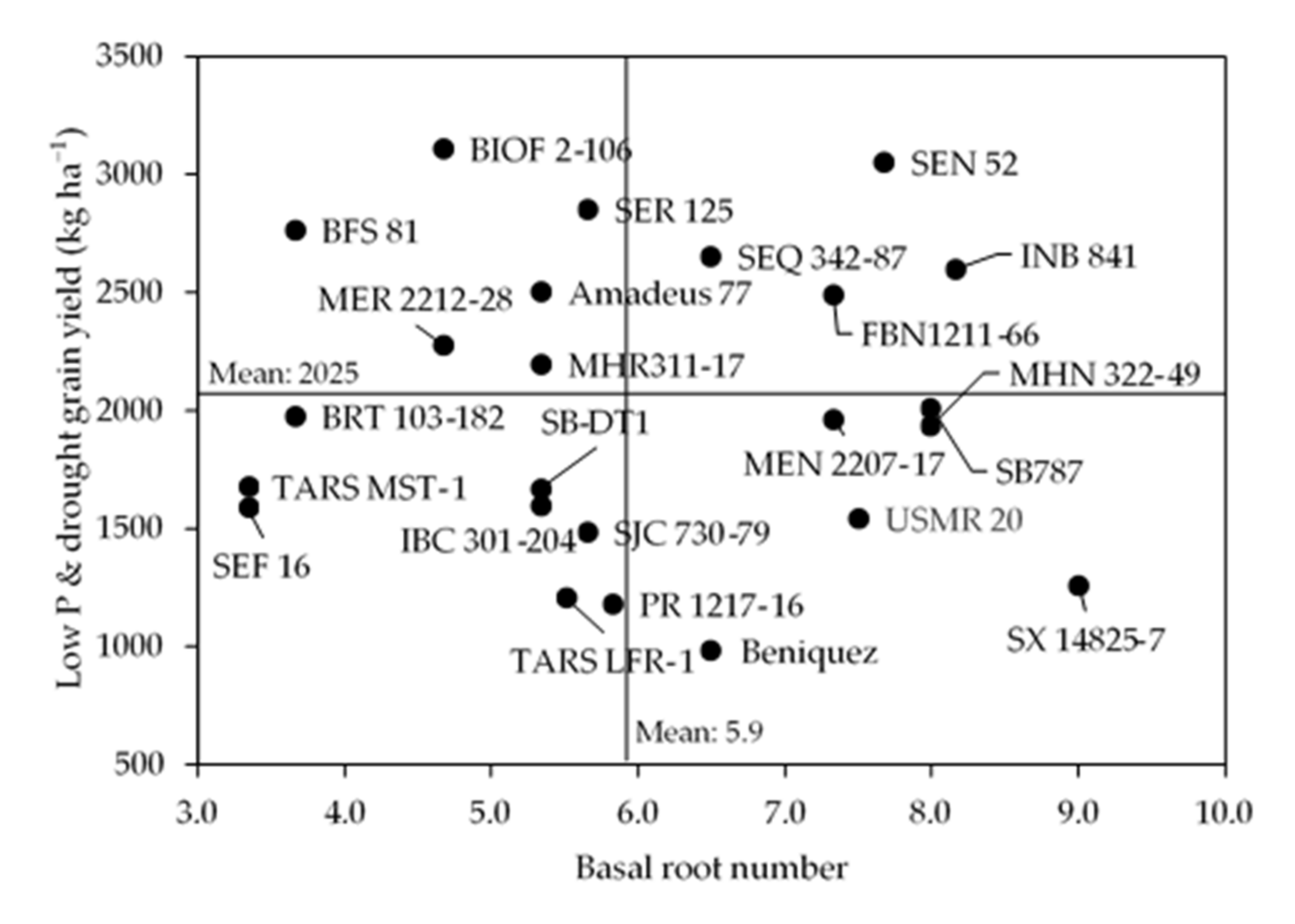
3.2. Primary Root Lateral Branching Effect on Shoot Biomass and Grain Yield
3.3. Influence of Root Class and Order on Grain Yield
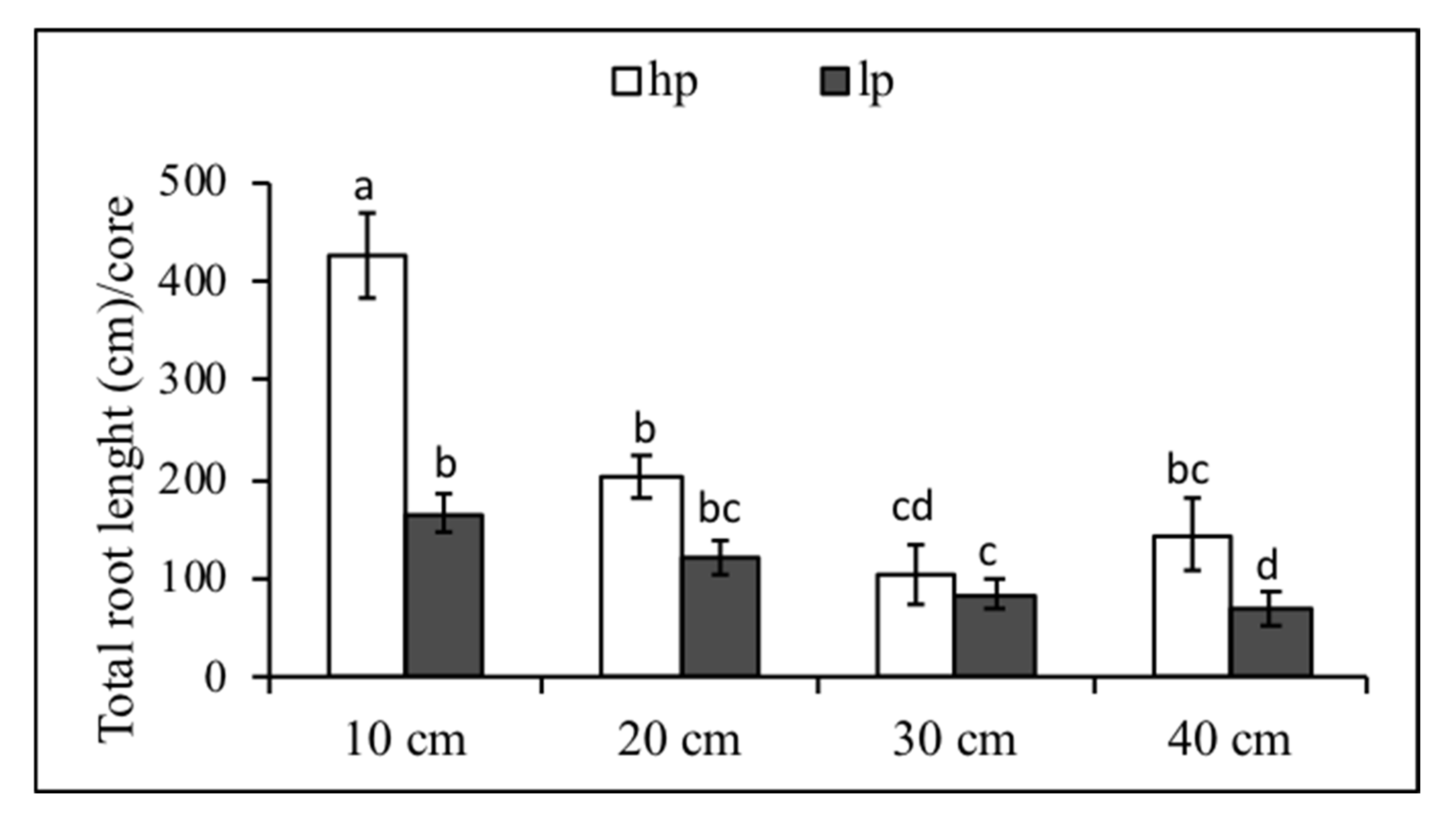
3.4. Root Distribution from Soil Cores
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beebe, S.E.; Rao, I.M.; Devi, M.J.; Polania, J. Common beans, biodiversity, and multiple stresses: Challenges of drought resistance in tropical soils. Crop Pasture Sci. 2014, 65, 667–675. [Google Scholar] [CrossRef]
- The Ecophysiology of Plant-Phosphorus Interactions. In The Ecophysiology of Plantphosphorus Interactions; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; Volume 7, pp. 83–116.
- Porch, T.G.; Beaver, J.S.; Debouck, D.G.; Jackson, S.A.; Kelly, J.D.; Dempewolf, H. Use of Wild Relatives and Closely Related Species to Adapt Common Bean to Climate Change. Agronomy 2013, 3, 433–461. [Google Scholar] [CrossRef]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop. Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Hanslin, H.M.; Bischoff, A.; Hovstad, K.A. Root growth plasticity to drought in seedlings of perennial grasses. Plant Soil 2019, 440, 551–568. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]
- Strock, C.F.; De La Riva, L.M.; Lynch, J.P. Reduction in Root Secondary Growth as a Strategy for Phosphorus Acquisition. Plant Physiol. 2018, 176, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Bell, T.L.; Pate, J.S. Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant Cell Environ. 2002, 25, 837–850. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- York, L.M.; Nord, E.A.; Lynch, J.P. Integration of root phenes for soil resource acquisition. Front. Plant Sci. 2013, 4, 355. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, R.E.; Nord, E.A.; Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Root cortical burden influences drought tolerance in maize. Ann. Bot. 2013, 112, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root Phenes for Enhanced Soil Exploration and Phosphorus Acquisition: Tools for Future Crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.; Jochua, C.N.; Bucksch, A.; Lynch, J.P. Legume shovelomics: High—Throughput phenotyping of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata subsp, unguiculata) root architecture in the field. Field Crop. Res. 2016, 192, 21–32. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective Use of Water and Increased Dry Matter Partitioned to Grain Contribute to Yield of Common Bean Improved for Drought Resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- Zheng, Z.; Hey, S.; Jubery, T.; Liu, H.; Yang, Y.; Coffey, L.; Miao, C.; Sigmon, B.; Schnable, J.C.; Hochholdinger, F.; et al. Shared Genetic Control of Root System Architecture between Zea mays and Sorghum bicolor. Plant Physiol. 2020, 182, 977–991. [Google Scholar] [CrossRef]
- Liao, H.; Rubio, G.; Yan, X.; Cao, A.; Brown, K.M.; Lynch, J.P. Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 2001, 232, 69–79. [Google Scholar] [CrossRef]
- Miguel, M.A.; Widrig, A.; Vieira, R.F.; Brown, K.M.; Lynch, J.P. Basal root whorl number: A modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Ann. Bot. 2013, 112, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, H.; Postma, J.A.; Lynch, J.P. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann. Bot. 2018, 122, 485–499. [Google Scholar] [CrossRef]
- Yan, X.; Liao, H.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 2004, 265, 17–29. [Google Scholar] [CrossRef]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorusacquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef]
- Ochoa, I.E.; Blair, M.W.; Lynch, J.P. QTL Analysis of adventitious root formation in common bean (Phaseolus vulgaris L.) under contrasting phosphorus availability. Crop Sci. 2006, 46, 1609–1621. [Google Scholar] [CrossRef]
- Walk, T.C.; Jaramillo, R.; Lynch, J.P. Architectural Tradeoffs between Adventitious and Basal Roots for Phosphorus Acquisition. Plant Soil 2006, 279, 347–366. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- WRB-IUSS. World Reference Base for Soil Resources. In World Soil Resources Reports No. 106; WRB-IUSS: Vienna, Austria, 2015. [Google Scholar]
- Burridge, J.D.; Rangarajan, H.; Lynch, J.P. Comparative phenomics of annual grain legume root architecture. Crop Sci. 2020, 60. [Google Scholar] [CrossRef]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Klaedtke, S.M. Photosynthate remobilization capacity from drought-adapted common bean (Phaseolus vulgaris L.) lines can improve yield potential of interspecific populations within the secondary gene pool. J. Plant Breed. Crop Sci. 2012, 4, 49–61. [Google Scholar] [CrossRef]
- Quilambo, O.A. The vesicular-arbuscular mycorrhizal symbiosis. Afr. J. Biotechnol. 2003, 2, 539–546. [Google Scholar] [CrossRef]
- Song, H. Effects of VAM on host plant in the condition of drought stress and its Mechanisms. Electron. J. Biol. 2005, 1, 44–48. [Google Scholar]
- Beltrano, J.; Ronco, M.G. Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: Effect on growth and cell membrane stability. Braz. J. Plant Physiol. 2008, 20, 29–37. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Kirnak, H.; Tas, I. Mycorrhizal colonisation improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well-watered and water-stressed conditions. Plant Soil 2003, 253, 287–292. [Google Scholar] [CrossRef]
- Shenoy, V.; Kalagudi, G. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Strock, C.F.; Burridge, J.; Massas, A.S.; Beaver, J.; Beebe, S.; Camilo, S.A.; Fourie, D.; Jochua, C.; Miguel, M.; Miklas, P.N.; et al. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crop. Res. 2019, 237, 53–64. [Google Scholar] [CrossRef]
- Asfaw, A.; Ambachew, D.; Shah, T.; Blair, M.W. Trait Associations in Diversity Panels of the Two Common Bean (Phaseolus vulgaris L.) Gene Pools Grown under Well-watered and Water-Stress Conditions. Front. Plant Sci. 2017, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.P.; Schneider, H.M.; Perkins, A.C.; Brown, K.M.; Lynch, J.P. Multiple Integrated Root Phenotypes Are Associated with Improved Drought Tolerance. Plant Physiol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Lynch, J.P.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Nord, E.A.; Shea, K.; Lynch, J.P. Optimizing reproductive phenology in a two-resource world: A dy-namic allocation model of plant growth predicts later reproduction in phosphorus-limited plants. Ann. Bot. 2011, 108, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Rao, S.; Kholova, J.; Krishnamurthy, L.; Kashiwagi, J.; Ratnakumar, P. Root research for drought tolerance in legumes: Quo vadis? J. Food Legumes 2008, 21, 77–85. [Google Scholar]
- Kashiwagi, J.; Krishnamurthy, L.; Crouch, J.; Serraj, R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop. Res. 2006, 95, 171–181. [Google Scholar] [CrossRef]
- Bécel, C.; Vercambre, G.; Pagès, L. Soil penetration resistance, a suitable soil property to account for variations in root elongation and branching. Plant Soil 2011, 353, 169–180. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.-L.; Clément, C.; Montoroi, J.-P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef]



| EC 1: 2.5 | pH (H2O) | pH (KCl) | OM | C/N | Macronutrients | Al | H | Na | CEC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | |||||||||
| mS/cm | % | % | ppm | cmol(+)/kg | cmol(+)/kg | ||||||||
| 0.06 | 6.3 | 5.15 | 0.49 | 14.21 | 0.02 | 3.3 | 0.34 | 0.66 | 0.32 | 0.0 | 0.0 | 0.26 | 1.58 |
| Soil Texture | Ca/Mg | Mg/K | Ca + Mg/K | ||||||||||
| Sand | Silt | clay | Grade | Silt/clay | Coarse sand | Fine sand | |||||||
| % | % | ||||||||||||
| 90.7 | 5.2 | 4.1 | A | 1.27 | 70.8 | 20 | 2.06 | 0.94 | 2.88 | ||||
| Water content of soil | |||||||||||||
| Field Capacyty | Permanent wilting point | ||||||||||||
| % | % | ||||||||||||
| 85–90 | 50–60% | ||||||||||||
| Cultivar | Seed Color | Seed Size | Growth Habit | Drought Reaction | P Stress Reaction |
|---|---|---|---|---|---|
| Amadeus 77 | Cream | S | Sensitive | ||
| Beniquez | Cream | S | Tolerant | Tolerant | |
| BFS 81 | Red | S | IIA | Tolerant | |
| BIOF 2-106 | Red | S | Tolerant | Tolerant | |
| BRT 103-182 | Red | S | Tolerant | Tolerant | |
| FBN 1211-66 | Red | S | IIA | Tolerant | Tolerant |
| IBC 301-204 | Red | S | Tolerant | ||
| INB 841 | Brown | S | IIA | Tolerant | Tolerant |
| MEN 2207-17 | Black | S | Tolerant | ||
| MER 2212-28 | Red | S | Tolerant | Tolerant | |
| MHN 322-49 | Red | S | IIA | Tolerant | |
| MHR 311-17 | Red | S | Sensitive | Tolerant | |
| PR 1217-16 | Red | S | Sensitive | Sensitive | |
| SB 787 | Black | S | Tolerant | ||
| SB-DT1 | Black | S | Tolerant | ||
| SEF 16 | Red | S | Tolerant | Tolerant | |
| SEN 52 | Black | S | IIA | Tolerant | |
| SEQ 342-87 | Red | S | Tolerant | ||
| SER 125 | Red | S | IIB | Tolerant | |
| SJC 730-79 | Red | S | Sensitive | ||
| SX 14825-7-1 | Cream | S | Tolerant | ||
| TARS LFR-1 | Red | S | Sensitive | ||
| TARS MST-1 | Red | S | Sensitive | ||
| USMR 20 | Cream | S | Tolerant | Tolerant |
| SB | TRB | tRB | TRL | SRL | TRLB | TD | BRN | BRWN | ARN | |
|---|---|---|---|---|---|---|---|---|---|---|
| TRB | −0.06 ** | |||||||||
| tRB | −0.40 *** | 0.80 *** | ||||||||
| TRL | 0.78 *** | 0.94 *** | 0.66 ** | |||||||
| SRL | −0.40 ** | 0.63 | 1.00 *** | 0.66 | ||||||
| TRLB | 0.46 * | 0.78 * | −0.74 | −0.45 | −0.74 * | |||||
| TD | 0.45 | 0.19 | 0.22 | −0.21 | 0.29 | −0.29 ** | ||||
| BRN | 0.15 | 0.81 ** | 0.39 | 0.55 | −0.70 ** | −0.57 | −0.70 | |||
| BRWN | 0.36 | 0.48 * | 0.78 ** | 0.06 | 0.09 | −0.30 | −0.19 | 0.90 *** | ||
| ARN | 0.45 | 0.67 | 0.46 | 0.15 | 0.57 | 0.70 | 0.81 | −0.591 | −0.75 * | |
| GY | 0.26 *** | 0.46 * | −0.45 ** | 0.66 | −0.66 ** | 0.12 ** | 0.47 | 0.148 | 0.16 | 0.39 |
| Basal Root Biomass Score (1–8) | Tap Root Biomass Score (1–8) | Total Root Biomass Score (1–8) | Taproot Length Score (1–8) | Total Tap Root Length Score (1–8) | Shoot Biomass Score (1–8) |
|---|---|---|---|---|---|
| 1A | 1A | 1A | 1A | 1A | 8H |
| 2B | 2B | 4D | 3C | 4D | 6F |
| 3C | 4D | 3C | 4D | 3C | 5E |
| 4D | 7G | 6F | 7G | 6F | 4D |
| 5E | 5E | 5E | 5E | 5E | 7G |
| 6F | 3C | 2B | 2B | 2B | 2B |
| 7G | 8H | 8H | 8H | 8H | 3C |
| 8H | 6F | 7G | 6F | 7G | 1A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilo, S.; Odindo, A.O.; Kondwakwenda, A.; Sibiya, J. Root Traits Related with Drought and Phosphorus Tolerance in Common Bean (Phaseolus vulgaris L.). Agronomy 2021, 11, 552. https://doi.org/10.3390/agronomy11030552
Camilo S, Odindo AO, Kondwakwenda A, Sibiya J. Root Traits Related with Drought and Phosphorus Tolerance in Common Bean (Phaseolus vulgaris L.). Agronomy. 2021; 11(3):552. https://doi.org/10.3390/agronomy11030552
Chicago/Turabian StyleCamilo, Samuel, Alfred O. Odindo, Aleck Kondwakwenda, and Julia Sibiya. 2021. "Root Traits Related with Drought and Phosphorus Tolerance in Common Bean (Phaseolus vulgaris L.)" Agronomy 11, no. 3: 552. https://doi.org/10.3390/agronomy11030552
APA StyleCamilo, S., Odindo, A. O., Kondwakwenda, A., & Sibiya, J. (2021). Root Traits Related with Drought and Phosphorus Tolerance in Common Bean (Phaseolus vulgaris L.). Agronomy, 11(3), 552. https://doi.org/10.3390/agronomy11030552






