Comparison of the Impact of Different Types of nZVI on Lolium westerwoldicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experiment
2.2. Determination of Iron Content in Plants
2.3. Biometric Parameters
2.4. Plant Pigments Analysis
2.5. Determination of Polyphenols
2.6. Determination of Flavonoids Content
2.7. Antioxidant Enzymes Assey
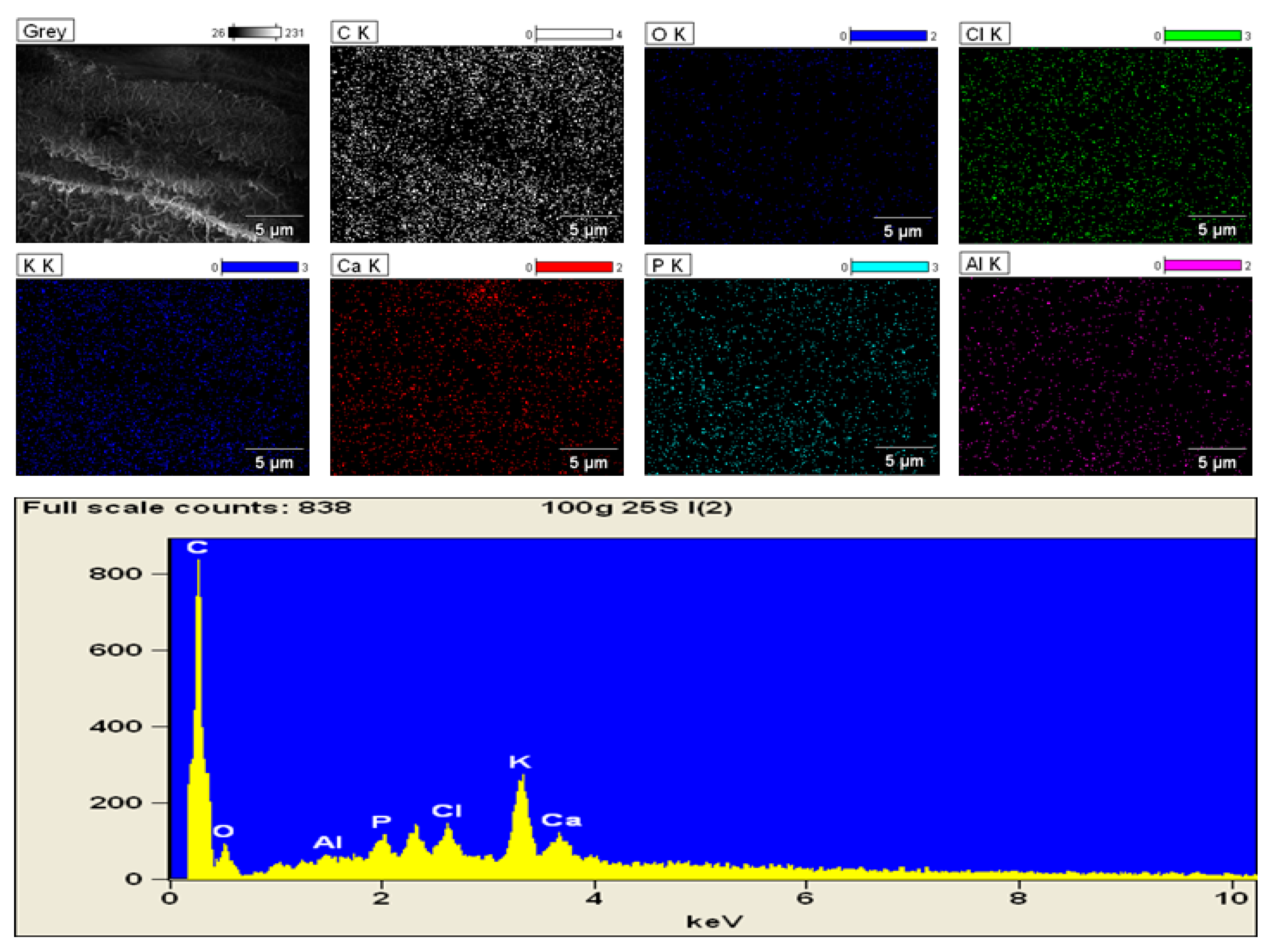
2.8. SEM
2.9. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Biometric Parameters
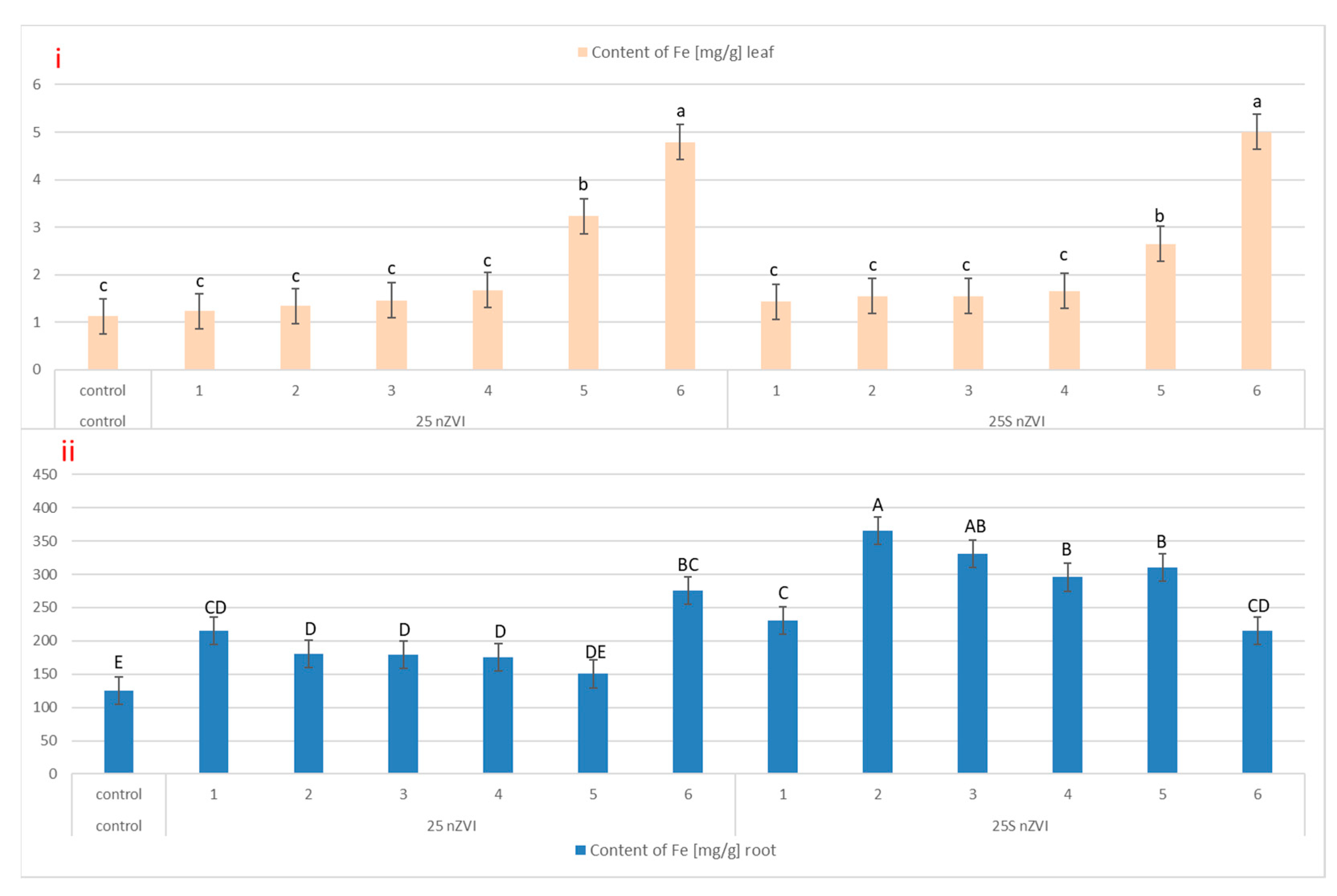
3.2. Iron Content and SEM Imaging
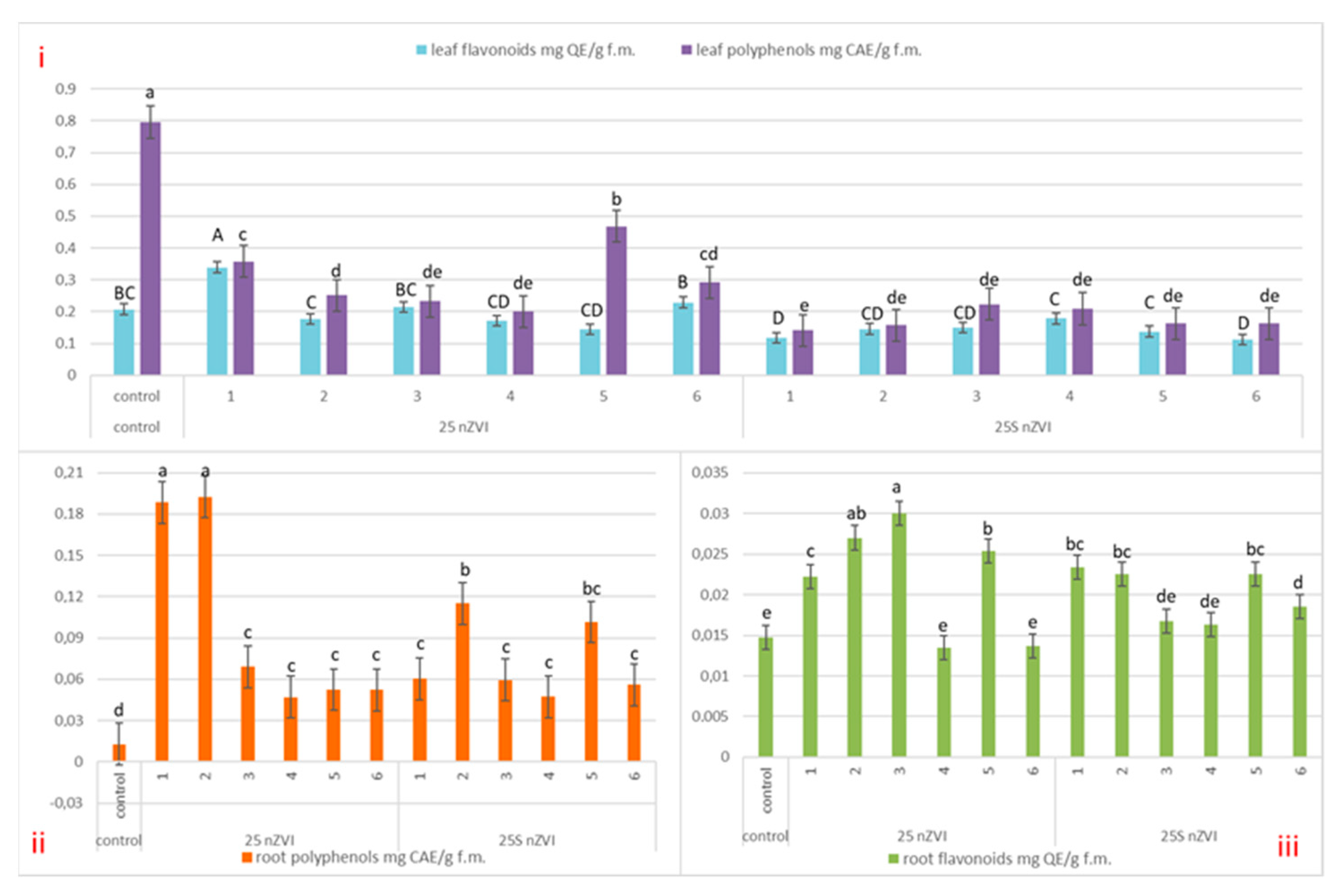
3.3. Non-Enzymatic Cell Metabolites—Content of Polyphenols and Flavonoids
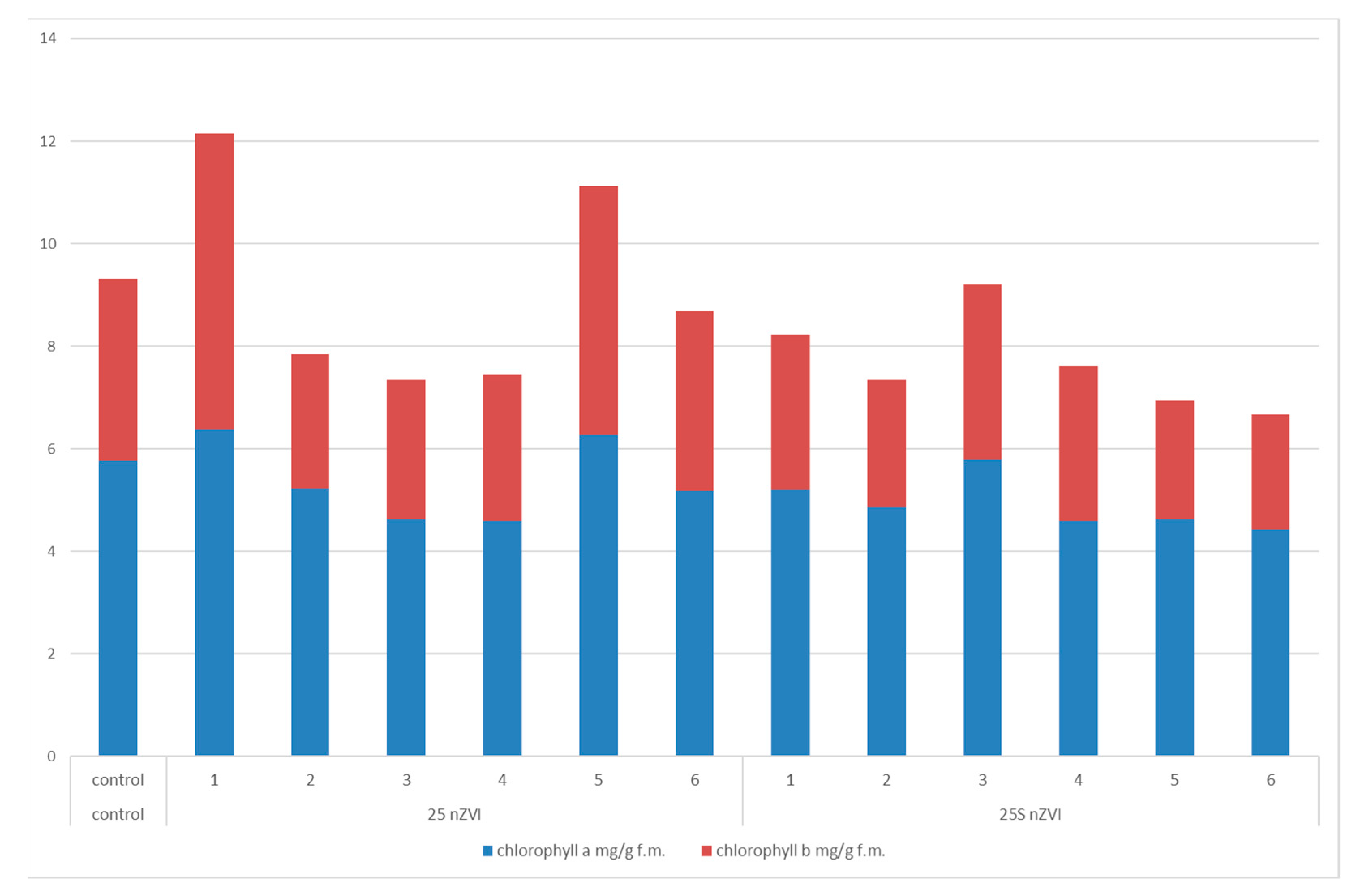
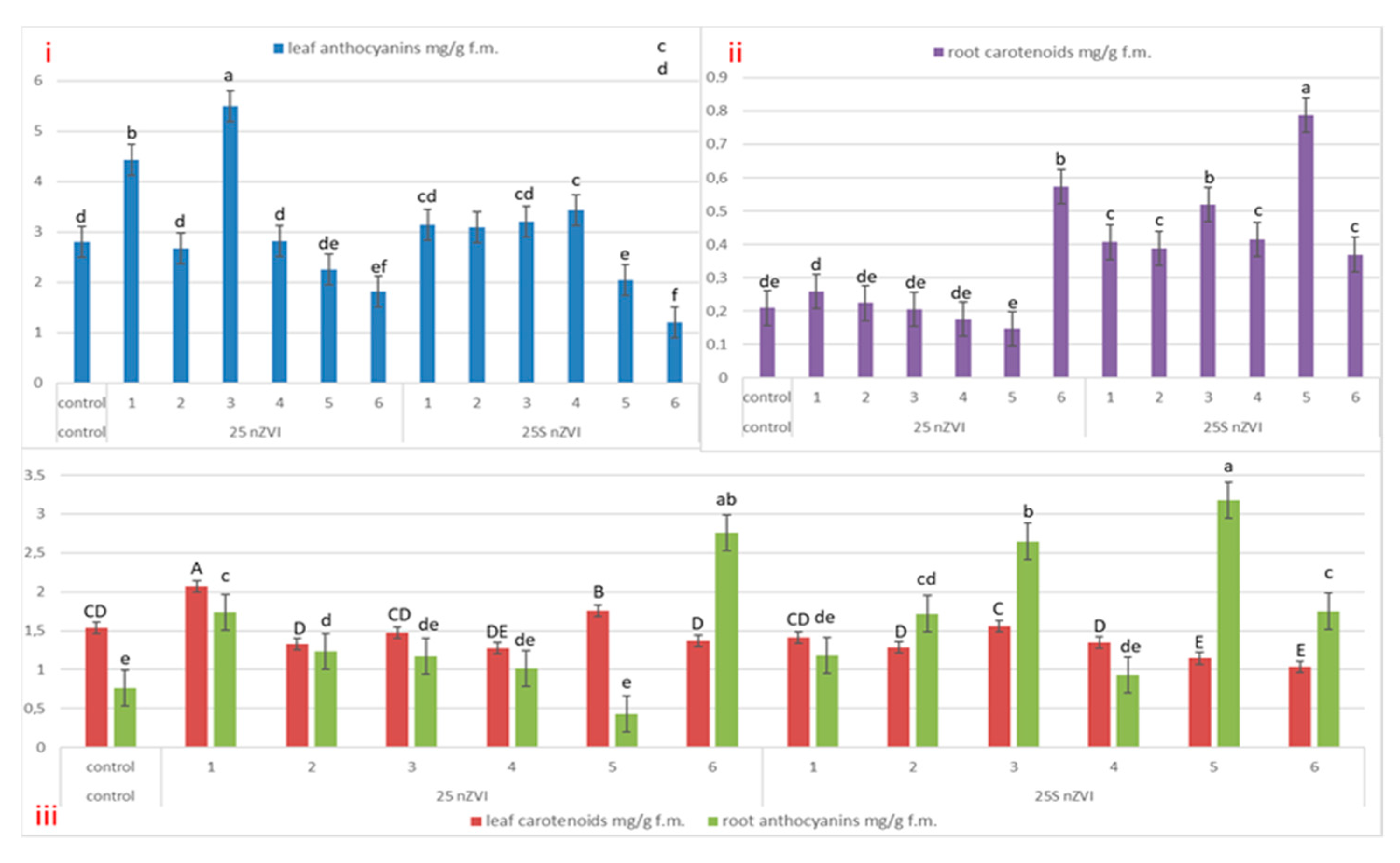
3.4. Plants Pigments—Chlorophyll a and b, Carotenoids and Anthocyanin
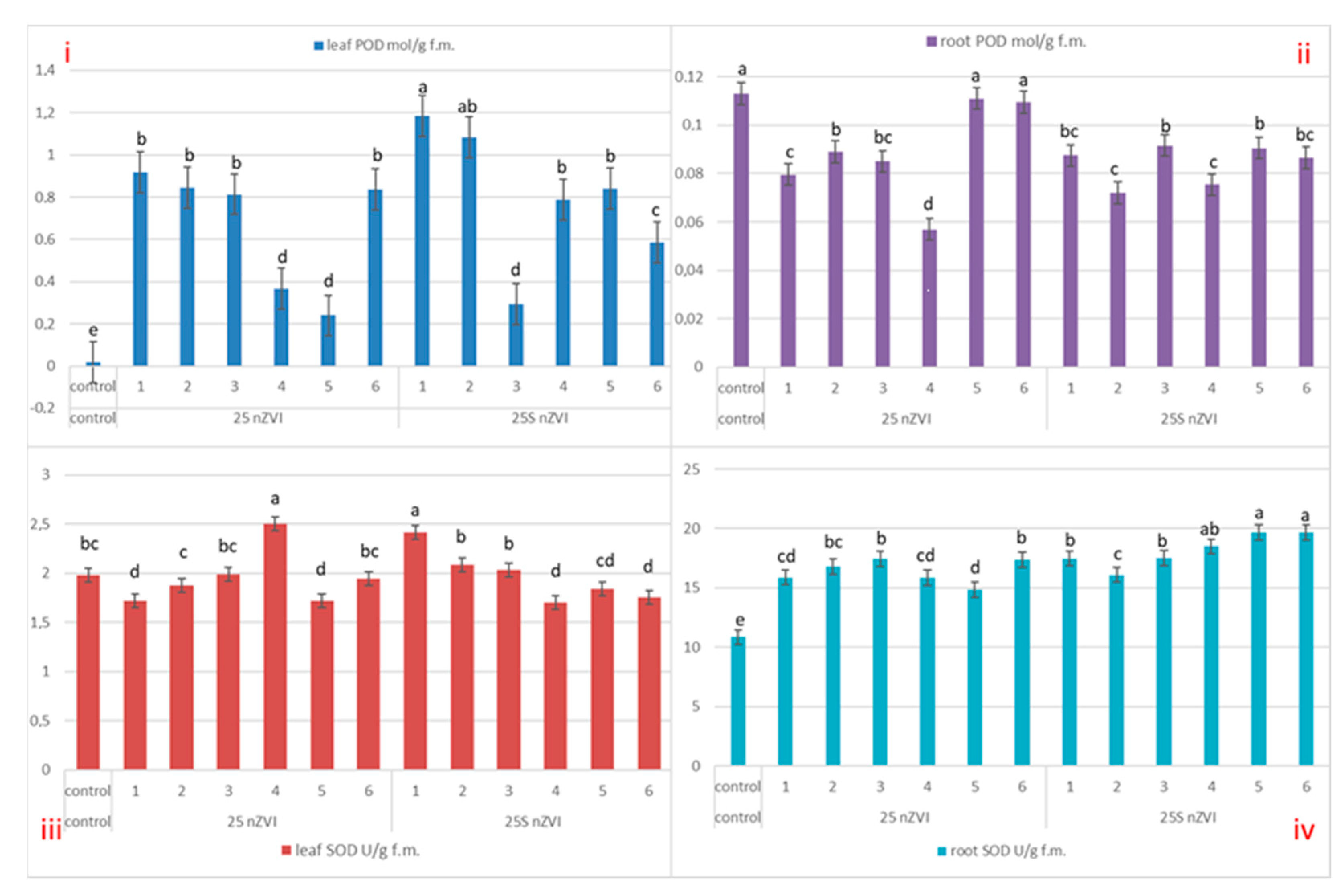
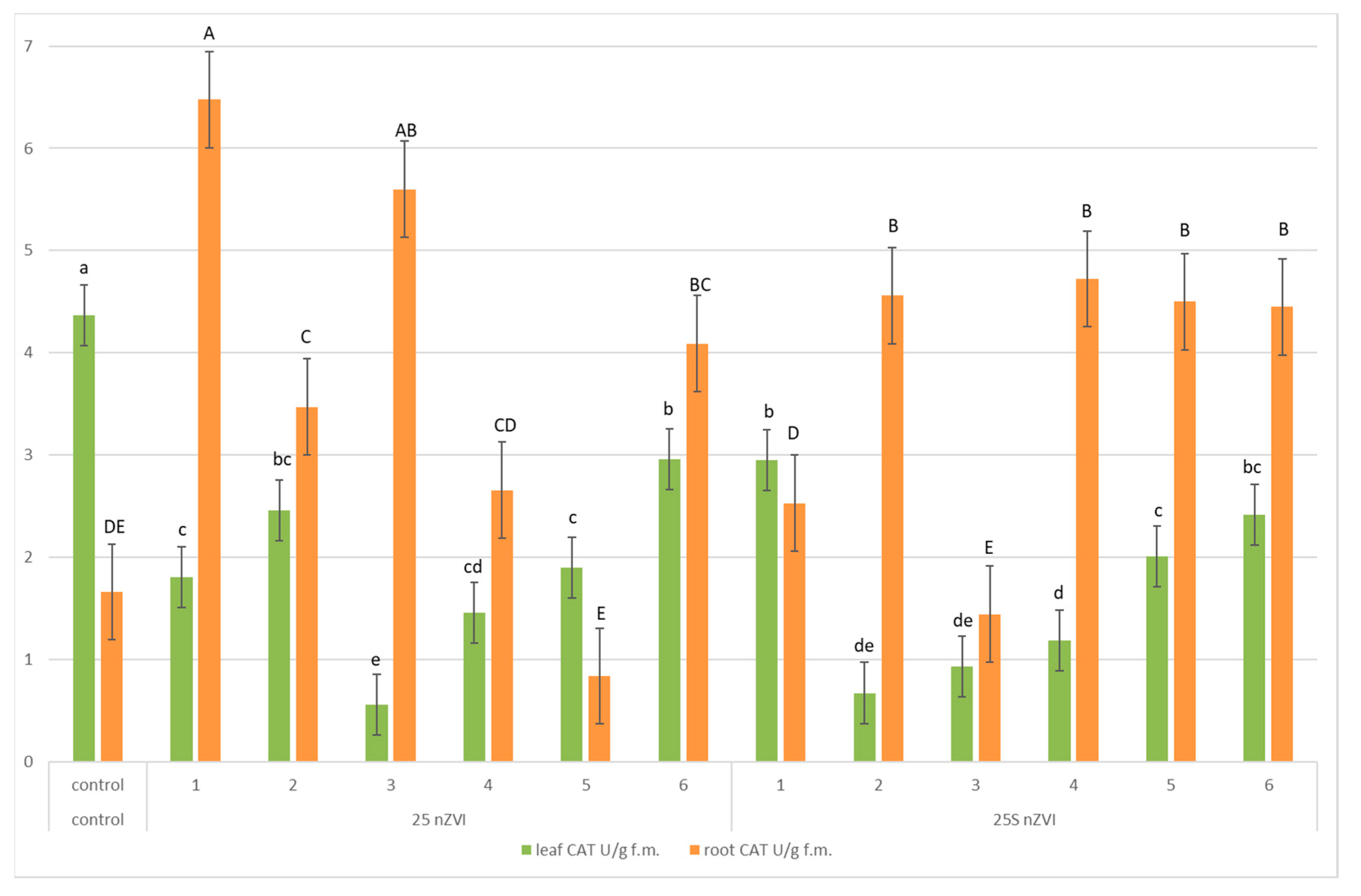
3.5. Enzymes Activities—Pyrogallol Peroxidase (POD), Superoxide Dismutase (SOD) and Catalase (CAT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Fajardo, C.; Gil-Díaz, M.; Costa, G.; Alonso, J.; Guerrero, A.; Nande, M.; Lobo, M.; Martín, M. Residual impact of aged nZVI on heavy metal-polluted soils. Sci. Total Environ. 2015, 535, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, Y.; Kim, E.-J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; An, H.J.; Chang, Y.-S. Exposure of Iron Nanoparticles to Arabidopsis thaliana Enhances Root Elongation by Triggering Cell Wall Loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2010, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2015, 565, 889–901. [Google Scholar] [CrossRef]
- Schmid, D.; Micić, V.; Laumann, S.; Hofmann, T. Measuring the reactivity of commercially available zero-valent iron nanoparticles used for environmental remediation with iopromide. J. Contam. Hydrol. 2015, 181, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Grieger, K.D.; Fjordbøge, A.S.; Hartmann, N.I.B.; Eriksson, E.; Bjerg, P.L.; Baun, A. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off? J. Contam. Hydrol. 2010, 118, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [PubMed]
- Nanoiron s.r.o. Nanoiron, Future Technology. 2018. Available online: https://nanoiron.cz/en/news (accessed on 10 November 2020).
- Wulfsohn, D. Sampling Techniques for Plants and Soil. Landbauforsch. Völk. 2010, 340, 3–30. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Meng, C.C.; Jalil, A.M.M.; Ismail, A. Phenolic and Theobromine Contents of Commercial Dark, Milk and White Chocolates on the Malaysian Market. Molecules 2009, 14, 200–209. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnat, A. Levels of principal flavonoids in flowers and leaves of Crataegus-Monogyna Jacq and Crataegus-Laevigata (Poiret) Dc (Rosaceae). Pharm Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Mielcarz-Skalska, L.; Smolińska, B. Response of Lepidium sativum to soil contamination with zinc in molecular and nanoparticle form. World Sci. News. 2018, 114, 55–67. [Google Scholar]
- Roth, E.F.; Gilbert, H.S. The pyrogallol assay for superoxide dismutase: Absence of a glutathione artifact. Anal. Biochem. 1984, 137, 50–53. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Cheng, W.; Yan, X.; Tsang, P.E.; Zhao, D. Higher concentrations of nanoscale zero-valent iron (nZVI) in soil induced rice chlorosis due to inhibited active iron transportation. Environ. Pollut. 2016, 210, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gurung, A.; Deng, Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci. Total Environ. 2013, 443, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Marusenko, Y.; Shipp, J.; Hamilton, G.A.; Morgan, J.L.; Keebaugh, M.; Hill, H.; Dutta, A.; Zhuo, X.; Upadhyay, N.; Hutchings, J.; et al. Bioavailability of nanoparticulate hematite to Arabidopsis thaliana. Environ. Pollut. 2013, 174, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, D.; Komárek, M. Comparative effects of nanoscale zero-valent iron (nZVI) and Fe2O3nanoparticles on root hydraulic conductivity of Solanum lycopersicum L. Environ. Exp. Bot. 2016, 131, 128–136. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; González, A.; Alonso, J.; Lobo, M. Evaluation of the stability of a nanoremediation strategy using barley plants. J. Environ. Manag. 2016, 165, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yirsaw, B.D.; Megharaj, M.; Chen, Z.; Naidu, R. Environmental application and ecological significance of nano-zero valent iron. J. Environ. Sci. 2016, 44, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr(VI) from aqueous solutions. Adv. Powder Technol. 2017, 28, 122–130. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Megharaj, M. Characterization of Iron–Polyphenol Nanoparticles Synthesized by Three Plant Extracts and Their Fenton Oxidation of Azo Dye. ACS Sustain. Chem. Eng. 2014, 2, 1022–1025. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Content |
|---|---|
| Corg | 35.74% |
| N total | 0.071 mg/g |
| SO42− | 168 mg/L |
| Mg | 54 mg/L |
| K | 3.8 mg/g |
| Ca | 292.5 mg/L |
| Mn | 37.5 µg/g |
| P total | 24.4 mg/L |
| Cl− | <1.9 g/L |
| pH | 6.0 |
| Variant (g/kg of Soil) | Mass of Biomass (g) | Shoot Mass (g) | Root Mass (g) | |
|---|---|---|---|---|
| Control | 20.93 ± 5.84 (b) | 16.53 ± 3.53 (a) | 11.74 ± 4.13 (d) | |
| 25 nZVI | 0.5 g | 41.23 ± 13.66 (a) | 30.10 ± 8.84 (a) | 24.77 ± 6.53 (a) |
| 1 g | 36.5 ± 7.40 (a) | 28.90 ± 6.01 (ab) | 22.83 ± 3.49 (a) | |
| 5 g | 37.1 ± 7.17 (a) | 26.27 ± 8.39 (b) | 17.23 ± 1.08 (bc) | |
| 10 g | 38.37 ± 10.44 (a) | 26.70 ± 6.04 (ab) | 18.77 ± 1.53 (b) | |
| 50 g | 40.55 ± 10.46 (a) | 28.83 ± 4.04 (ab) | 14.59 ± 3.64 (c) | |
| 100 g | 37.05 ± 9.66 (a) | 26.72 ± 6.20 (b) | 15.57 ± 4.33 (c) | |
| Control | 20.93 ± 5.84 (B) | 16.53 ± 3.53 (AB) | 11.74 ± 4.13 (B) | |
| 25S nZVI | 0.5 g | 25.27 ± 2.73 (AB) | 19.27 ± 2.88 (A) | 13.33 ± 6.45 (AB) |
| 1 g | 19.6 ± 8.97(B) | 15.63 ± 3.03 (B) | 12.97 ± 1.39 (B) | |
| 5 g | 17.23 ± 3.32(B) | 13.53 ± 2.21 (B) | 5.70 ± 1.32 (C) | |
| 10 g | 19.92 ± 8.86 (B) | 15.37 ± 6.83 (B) | 15.22 ± 3.96 (AB) | |
| 50 g | 21.43 ± 7.14 (B) | 16.67 ± 2.59 (AB) | 15.77 ± 2.68 (A) | |
| 100 g | 30.02 ± 9.44 (A) | 20.58 ± 2.31 (A) | 13.43 ± 2.45 (AB) |
| Variant (g/kg of Soil) | Root Length (cm) | Shoot Length (cm) | Plant Length (cm) | |
|---|---|---|---|---|
| Control | 10.83 ± 3.18 (e) | 14.90 ± 1.35 (cd) | 22.40 ± 1.65 (d) | |
| 25 nZVI | 0.5 g | 19.1 ± 0.69 (a) | 18.70 ± 1.39 (a) | 34.47 ± 7.85 (a) |
| 1 g | 12.5 ± 3.06 (d) | 17.43 ± 0.7 (b) | 26.60 ± 1.04 (c) | |
| 5 g | 17.73 ± 0.40 (b) | 15.47 ± 1.79 (c) | 29.87 ± 7.16 (b) | |
| 10 g | 19.70 ± 2.25 (a) | 13.93 ± 0.75 (d) | 30.30 ± 7.27 (b) | |
| 50 g | 15.87 ± 1.96 (c) | 14.47 ± 1.79 (d) | 27.00 ± 5.53 (c) | |
| 100 g | 13.57 ± 0.12 (d) | 13.60 ± 1.56 (d) | 23.83 ± 2.32 (d) | |
| Control | 10.83 ± 3.18 (B) | 14.90 ± 1.35 (AB) | 22.40 ± 1.65 (B) | |
| 25S nZVI | 0.5 g | 13.2 ± 0.35 (A) | 15.60 ± 1.56 (A) | 25.47 ± 4.99 (A) |
| 1 g | 11.57 ± 1.85 (AB) | 14.63 ± 1.50 (B) | 22.87± 5.43 (AB) | |
| 5 g | 11.60 ± 1.04 (AB) | 13.10 ± 1.91 (C) | 21.37 ± 2.83 (B) | |
| 10 g | 10.47 ± 2.54 (B) | 14.53 ± 1.79 (B) | 21.67 ± 1.44 (B) | |
| 50 g | 12.53 ± 0.06 (A) | 14.40 ± 0.17 (B) | 23.60 ± 5.89 (AB) | |
| 100 g | 12.73 ± 1.33 (A) | 15.37 ± 2.83 (A) | 24.77 ± 1.93 (A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarz-Skalska, L.; Smolińska, B.; Szynkowska-Jóźwik, M. Comparison of the Impact of Different Types of nZVI on Lolium westerwoldicum. Agronomy 2021, 11, 467. https://doi.org/10.3390/agronomy11030467
Mielcarz-Skalska L, Smolińska B, Szynkowska-Jóźwik M. Comparison of the Impact of Different Types of nZVI on Lolium westerwoldicum. Agronomy. 2021; 11(3):467. https://doi.org/10.3390/agronomy11030467
Chicago/Turabian StyleMielcarz-Skalska, Lidia, Beata Smolińska, and Małgorzata Szynkowska-Jóźwik. 2021. "Comparison of the Impact of Different Types of nZVI on Lolium westerwoldicum" Agronomy 11, no. 3: 467. https://doi.org/10.3390/agronomy11030467
APA StyleMielcarz-Skalska, L., Smolińska, B., & Szynkowska-Jóźwik, M. (2021). Comparison of the Impact of Different Types of nZVI on Lolium westerwoldicum. Agronomy, 11(3), 467. https://doi.org/10.3390/agronomy11030467







