Abstract
Cattle production is a large source of greenhouse gas (GHG) emissions from the Canadian livestock sector. Efforts to reduce CH4 emissions from enteric fermentation have led to modifications of diet composition for livestock, resulting in a corresponding change in manure properties. We studied the effect of applying manure from cattle fed a barley-based diet with and without the methane inhibitor supplement, 3-nitrooxypropanol (3-NOP), on soil GHG emissions. Three soils common to Alberta, Canada, were used: a Black Chernozem, a Dark Brown Chernozem, and a Gray Luvisol. We compared the supplemented (3-NOPM) and non-supplemented manure (BM) amendments to a composted 3-NOPM (3-NOPC) amendment and a control with no manure amendment (CK). In an 84-day laboratory incubation experiment, 3-NOPM had significantly lower cumulative CO2 emissions compared to BM in both the Black Chernozem and Gray Luvisol. The cumulative N2O emissions were lowest for 3-NOPC and CK and highest for 3-NOPM across all soil types. Cumulative CH4 emissions were only affected by soil type, with a net positive flux from the fine-textured Gray Luvisol and Dark Brown Chernozem and a net negative flux from the coarse-textured Black Chernozem. Cumulative anthropogenic GHG emissions (CO2-equivalent) from soil amended with 3-NOPM were significantly higher than those for both BM and CK amendments in the Black Chernozem, while the cumulative anthropogenic GHG emissions from the 3-NOPC treatment were similar to or significantly lower than those for the BM and CK treatments across all soil types. We conclude that soil GHG emissions resulting from the 3-NOPM amendment are dependent on soil type and 3-NOPM could potentially increase soil GHG emissions compared to BM or CK. Although we show that the composting of 3-NOPM prior to soil application can reduce soil GHG emissions, the composting process also releases GHGs, which should also be considered in assessing the life-cycle of manure application. Our results provide a first look at the potential effect of the next stage in the life cycle of 3-NOP on GHG emissions. Further research related to the effect of soil properties, particularly in field studies, is needed to assess the best management practices related to the use of manure from cattle-fed diets supplemented with 3-NOP as a soil amendment.
1. Introduction
Emissions of greenhouse gases (GHGs) are a growing concern due to the effect of GHGs on the global climate. Cattle production releases 2–7 times more GHGs, mainly in the form of methane (CH4) emissions from enteric fermentation, than the production of any other livestock [1]. Methane has 28 times the greenhouse effect of carbon dioxide (CO2) when evaluated on a 100-year time scale; as such, reducing CH4 emissions is essential for environmental sustainability [2,3]. To reduce the CH4 emissions from livestock production systems, recent research has focused on feeding management practices. such as the use of feed additives and changing the composition of feed to alter microbial compositions and activities during enteric fermentation.
The use of feed additives or changes in feed composition may alter the properties of cattle manure, which could affect soil GHG emissions when the manure is applied to the land [4,5]. While manure from cattle feedlots may pose many environmental problems, such as eutrophication and acidification, using manure as an organic amendment is important to improve soil fertility [1,6,7,8]. Cattle manure amendment increases soil aggregation, water holding capacity, and microbial activities [7]. However, little research has been conducted to understand the effect of manure from cattle fed with additives or different feed compositions on GHG emissions when such manure is applied to the land.
One of the feed additives to help mitigate CH4 emissions from enteric fermentation in ruminant livestock is 3-nitrooxypropanol (3-NOP) [9,10]. The 3-NOP inhibits the activity of methyl coenzyme-M reductase, the enzyme that catalyzes the last step in CH4 production. By substituting a reducible nitrate (NO3-) group in place of a nickel (Ni) ion, 3-NOP inactivates the methyl coenzyme-M reductase by oxidizing its active sites [11]. The use of 3-NOP does not reduce the abundance of bacteria, protozoa, and methanogens, but rather alters the function of specific microorganisms, resulting in a reduction in CH4 formation and a shift in the volatile fatty acid fermentation profile [12]. Earlier research has shown that 3-NOP can reduce enteric CH4 emissions by up to 28–33% [5,13] and increase milk production due to a 2−12% reduction in potential energy loss from CH4 production [4,5,14]. However, it is not clear how the addition of 3-NOP in the feed will affect soil GHG emissions when the cattle manure is applied as an organic amendment to various soil types.
Another way to reduce GHG emissions is by altering livestock manure management practices, such as composting, designed to transform biologically active components in manure into less degradable forms [15]. The composting of manure helps reduce the odor and the volume of manure that needs to be transported for land application [15,16,17,18]. Up to 70% of the mass of fresh manure is water, making the volume to be transported excessively large if fresh manure is used as an organic fertilizer [16]. Composting can even help reduce pathogens, parasites, and weed seeds that come with the manure [15,19,20]. The process of composting manure converts easily degradable C and N into less biologically active forms, and the resulting manure has lower pH, labile organic matter, C/N ratio, and GHG emissions [15]. During the composting process, the rate of mineralization decreases as easily degradable materials are converted to CO2 [15]. In addition, improving aeration during composting can change C and N dynamics and alter GHG emissions [20]. The soil application of composted manure originating from cows fed wheat dried distillers’ grains with solubles has been reported to significantly lower GHG emissions [6]. However, some composted manure from cattle fed with feed additives have a greater risk for N loss due to the additional N that comes with the additives [21]. As far as we know, the effect of composted manure from cattle fed with 3-NOP on soil GHG emissions has not been studied.
Manure is often applied to different soil types to improve sustainable management operations [10]. Given the potential importance of 3-NOP in reducing enteric CH4 emissions, we aimed to (i) understand how manure from cattle fed with 3-NOP-supplemented diets affects soil GHG emissions when used as an amendment; (ii) determine if the resulting GHG emissions could be reduced via composting the 3-NOP-manure prior to soil application; and (iii) assess the effect of soil type on the resulting GHG emissions. By studying the impact of the 3-NOP supplement in cattle diets on GHG emissions when the resulting manure is used as a soil amendment, we hope to provide a first look at the potential effect of the next stage in the life cycle of 3-NOP.
2. Materials and Methods
2.1. Experimental Design and Treatments
The experiment used a completely randomized design (CRD) based on a 3 × 4 factorial experiment with three soil types and four manure treatments. The treatments were replicated four times. Properties of the soils and manure amendments used in the study are presented in Table 1.

Table 1.
Initial chemical properties (means ±SE) of soil and manure samples used in the laboratory incubation experiment (n = 3).
Surface soil samples (0−15 cm) used in this incubation experiment were collected in late spring 2017. Soil types collected include: (i) a Black Chernozem (BLC) from a prairie in Virden, Manitoba, with a sandy loam texture; (ii) a Gray Luvisol (GL) from a cereal-canola field in Beaverlodge, Alberta, with a clay loam texture, and (iii) a Dark Brown Chernozem (DBC) from an alfalfa/grass field in Lethbridge, Alberta, with a clay loam texture.
The manures were generated from a 238-d feeding trial at Agriculture and Agri-Food Canada’s Lethbridge Research and Development Center as reported in Vyas et al. [10]. Soils were amended at 160 Mg ha−1 with (i) stockpiled manure from cattle fed a traditional barley-based diet (BM); manure from cattle fed a barley-based diet supplemented with 3-NOP (at 200 mg kg−1 dry matter for the initial 105-d backgrounding phase and 125 mg kg−1 dry matter for the final 133-d finishing phase) which was (ii) stockpiled (3-NOPM) or (iii) composted (3-NOPC) in open-air windrows following the method described in Larney and Hao [15]; and (iv) a control (CK, soil without manure addition).
2.2. Soil Incubation
After collection from the field, the soils were shipped to the University of Alberta laboratory for incubation. All soil types were air-dried at room temperature, passed through a 2-mm sieve to remove debris and coarse fragments, homogenized and stored at room temperature [22]. Before setting up the incubation experiment, soil samples were dried at 105 °C for 48 h to determine soil water content. The water-filled pore space (WFPS) was then calculated by:
where is the soil water content (g H2O g−1 dry soil), is bulk density (Mg m−3), and is particle density (2.65 Mg m−3) [23,24].
The incubation experiment was conducted in a Forma Diurnal Growth Chamber-Model 3740 (Thermo Fisher Scientific, Waltham, MA, USA) at 25 °C. In setting up the laboratory incubation experiment, 200 g (dry-weight basis) of soil was weighed and placed into one of the 48 (four manure treatments x three soil types x four replications) 1-L Mason jars. The % WFPS of the soil was adjusted to 60% using a 20 mL syringe to distribute deionized water evenly over the surface of the soil, then a piece of aluminum foil with four small pinholes was used to cover the jar to minimize water loss but allow gas exchange [25]. The samples were pre-incubated in the incubation chamber for seven days.
Immediately after the pre-incubation, 16.6 g of manure (dry-weight basis) was mixed into each Mason jar based on a pre-determined treatment distribution, with CK remaining unamended. This amount is comparable to a common rate of field application of 160 Mg ha−1 of the organic amendment, typical for barley forage production [6]. The 60% WFPS was maintained by adjusting the soil water content weekly using a syringe for the first two weeks and was then the soils were adjusted to 80% WFPS for the remainder of the 84-d incubation; the increase in WFPS was made to better understand the response of anaerobic microbial processes [26].
2.3. Gas Sampling
The fluxes of GHGs of CO2, nitrous oxide (N2O), and CH4 were measured on days 1, 4, 7, 14, 21, 35, 38, 42, 49, 63, and 84 of the incubation. During each sampling, gas samples were collected twice by first sealing each Mason jar with a lid containing a butyl rubber stopper. The first gas sample was collected with a 20 mL syringe immediately after the closure of the Mason jar (time 0) and transferred to a pre-evacuated 12 mL exetainer. After 24 h of closure, another gas sample was collected in the same manner, and the butyl rubber stoppers were replaced with the aluminum foil described above to allow air exchange between the Mason jar and the atmosphere to occur. A Varian CP-3800 gas chromatograph (Varian, Palo Alto, CA, USA), equipped with a thermal conductivity detector (TCD), a flame ionization detector (FID), and an electron capture detector (ECD), was used to measure the CO2, CH4, and N2O concentrations, respectively, in the gas samples.
The CO2, N2O, and CH4 fluxes, in units of mg CO2-C kg−1 h−1, μg N2O-N kg−1 h−1, and μg CH4-C kg−1 h−1, respectively, were calculated using the following equation [6]:
where:
= air density at standard state;
= change in concentration (ppbv) between sampling intervals;
= sampling interval (24 h);
= soil mass (200 g);
= headspace volume in the Mason jar;
= incubation temperature (25 °C).
Dissolved N2O was calculated according to Moraghan and Buresh [27] and added to the measured N2O flux to determine the total N2O flux. Total CO2 and CH4 fluxes were determined by measured CO2 and CH4 fluxes only. Cumulative GHG emissions were calculated by summing gas fluxes over the 84 d incubation period. The CO2-eq GHG emissions were calculated using the GWP coefficients of 265 and 28 for N2O and CH4, respectively, over a 100-year time frame based on the amount of mass of a gas is emitted [3].
2.4. Physical and Chemical Analyses
To analyze for total C and N, samples were dried at 70 °C and ground with a mortar and pestle and analyzed using a dry combustion technique with an automated CN analyzer (Carlo Erba, Milan, Italy). Soil pH was analyzed on a suspension using a 1:5 soil weight to deionized water volume ratio with an Orion pH meter (Thermo Fisher Scientific, Waltham, MA, USA). For soils with a pH > 7.2, the inorganic C was removed by treating the soil with 6 M of HCl prior to the determination of SOC content [28,29].
To measure the available N (NO3−-N and NH4+-N), the samples were extracted with 0.5 M K2SO4 solutions at a 1:5 (w:v) soil to extract ratio; the mixture was shaken at 250 rpm on a mechanical shaker for 1 h then filtered using Whatman No. 42 filter paper [30]. Nitrate- and NH4+-N were determined colorimetrically using vanadium (III) in acid and indophenol blue methods, respectively [30,31].
2.5. Statistical Analysis
All the statistical analyses were performed using R v.1.1 (R Core Team, 2020) with statistical significance set at α = 0.05 for all tests. Two-way analysis of variance (ANOVA) was used to analyze the effect of soil type, manure type, and their interaction on cumulative GHG emissions and soil properties in the laboratory incubation experiment [32]. The normality of distribution and the homogeneity of variance of the data were checked with the Shapiro and Bartlett tests. Non-homogeneous data of anthropogenic GHG emissions, CH4 emissions N2O emissions NH4+-N, and C/N ratio were transformed with first-order auto-regression. Cumulative GHG emissions and initial properties were analyzed using a Tukey–Kramer test. Relationships between the initial properties and cumulative GHG emissions were examined using Spearman’s rank correlation.
3. Results and Discussion
3.1. CO2 Emissions
There was an initial peak in CO2 emissions in all soils (Figure 1a) from the addition of cattle manure at the beginning of the incubation, likely due to the improved availability of the substrate that stimulated microbial activity and microorganisms from the manure, contributing to an increase in SOM decomposition [7,18,19].
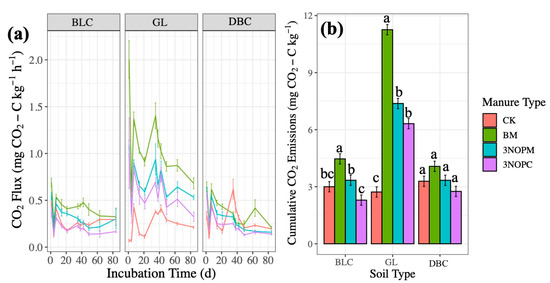
Figure 1.
Effects of manure type on CO2 emissions (a) over time by soil type and (b) cumulatively by soil type in a laboratory incubation experiment. Soil type: BLC, Black Chernozem; GL, Gray Luvisol; DBC, Dark Brown Chernozem. Manure type: CK, control with no amendments; BM, barley-based manure; 3-NOPM, manure from cows fed 3-NOP supplement; 3-NOPC, composted manure from cows fed 3-NOP supplements. Treatments that do not share the same letter are significantly different from each other. Error bars indicate standard errors of the means (n = 4).
On day 35, there was another peak in CO2 emissions, a result of the change from 60 to 80% WFPS (Figure 1a), suggesting that the increased water availability enhanced the microbial activities for a period of time because of the increased availability of easily decomposable material [2,33]. Similar to Hadas and Portnoy [19] and Hao et al. [34], the CO2 emissions declined over time (Figure 1a) due to the decreased organic matter availability and sustained reduction in soil aeration.
The cumulative CO2 emissions were significantly lower in the 3NOPM-amended soils than in the BM-amended soils across Black Chernozem and Gray Luvisol soil types (p < 0.001, Figure 1b), while there was no significant effect of manure type in the Dark Brown Chernozem. Composting (3-NOPC) further significantly reduced cumulative CO2 emissions as compared with the non-composted manure from cattle fed with 3-NOP in the Black Chernozem (Figure 1b).
During the composting process, many hydrophobic nonpolar biomolecules are transformed into hydrophilic, soluble molecules, allowing for greater microbial activity [6,18,20], and the aerobic, rather than anaerobic, decomposition of manure is promoted [20]. The composting process thus results in highly recalcitrant organic matter that is resistant to microbial breakdown [24]. The lower C concentration in 3-NOPC than in 3-NOPM (p < 0.001, Table 1) likely reduced the microbial activity and CO2 emissions upon their application. The manure and soil types interacted to affect the cumulative CO2 emissions for amended treatments (p < 0.001, Table 2).

Table 2.
Results of two-way ANOVAs (p-values) testing the effects of soil type, manure type, and their interactions on the cumulative greenhouse gas (GHG) emissions (n = 4).
The CO2 emission rates were greater in the Gray Luvisol than in Black and Dark Brown Chernozems, and the difference among the soils were greater when BM than when 3-BNOPM and 3-NOPC were applied (p < 0.001, Table 2; Figure 1b). The pH was significantly lower in the Gray Luvisol (3.91, p < 0.001) and was greatest in BM > 3-NOPM > 3-NOPC (p < 0.001) (Table 1). The cumulative CO2 emission rates were highly correlated with pH (p < 0.01, Table 3), consistent with Li et al. [6], suggesting that soil pH played a major role in the interaction effects between manure and soil types on the cumulative CO2 emissions.

Table 3.
Pearson’s correlation coefficients for the correlation among cumulative CO2, N2O, and CH4 emissions and initial soil properties (n = 12).
3.2. N2O Emissions
The highest N2O emissions from the different soils and manure types occurred on day one (Figure 2a). The initial N2O emissions in amended treatments likely came from nitrification, which occurs under aerobic conditions when WFPS was maintained at 60% [2,21,35]. The emission of nitrous oxide continued until day 42 for the Gray Luvisol and Dark Brown Chernozem soils (Figure 2a), partly related to the change in the soil water content from 60 to 80% WFPS after 14 days from the initiation of the treatments, allowing denitrification to occur and to contribute to the extended N2O emissions [33].
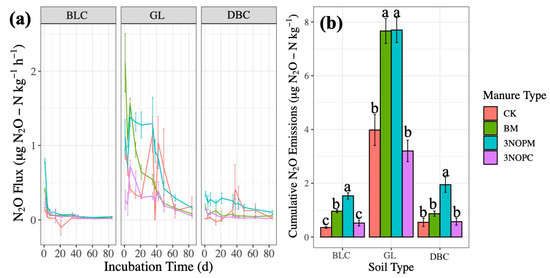
Figure 2.
Effects of manure type on N2O emissions (a) over time by soil type and (b) cumulatively by soil type in a laboratory incubation experiment. Soil type: BLC, Black Chernozem; GL, Gray Luvisol; DBC, Dark Brown Chernozem. Manure type: CK, control with no amendments; BM, barley-based manure; 3-NOPM, manure from cows fed 3-NOP supplement; 3-NOPC, composted manure from cows fed 3-NOP supplements. Treatments that do not share the same letter are significantly different from each other. Error bars indicate standard errors of the means (n = 4).
Under aerobic conditions, N2O emissions would only last for the first a few weeks, as observed by Li et al. [6] and Bhandral et al. [36]. Denitrification occurs under anaerobic conditions (>60% WFPS), when the lack of O2 requires denitrifying microorganisms to utilize NO3−-N instead of O2 as an electron acceptor [2,37,38]. The nitrous oxide emissions then decreased considerably from day 49 until the end of the incubation.
The interaction of soil type and manure type was significant for the N2O emissions (p < 0.001, Table 2) because of the complex interrelationships among soil and manure properties (Table 1). Higher C concentrations (BM and the two Chernozemic soils) can enhance denitrification by directly providing donor electrons and stimulating O2 consumption, and lower levels of NH4+-N (in the Chernozemic soils) reduce microbial assimilatory NO3−-N reduction [24,35].
The nitrous oxide emissions were significantly higher from the 3-NOPM treatment than the other three treatments on Black Chernozem and Dark Brown Chernozem soils, but not on the Gray Luvisol, where the N2O emissions were similar between the 3-NOPM and BM treatments (Figure 2b). There was significantly greater NO3−-N in 3-NOPM and 3-NOPC than in BM (p < 0.001, Table 1); the higher NO3−-N availability increased the N2O emission rates (Table 3) from anaerobic processes [33,34,35].
Soil type also significantly affected the N2O emissions (p < 0.001, Table 2), with the Chernozemic soils having lower cumulative N2O emissions (Figure 2b). The Gray Luvisol soils had significantly higher available N than the Black and Dark Brown Chernozem soils for all treatments (p < 0.01, Table 1), which resulted in greater N2O emissions [2], and N2O emissions were affected by soil pH (p < 0.001). The neutral-basic Black and Dark Brown Chernozem soils started with lower NH4+-N (Table 1), limiting the potential nitrification rates for NO3−-N production.
3.3. CH4 Emissions
Most CH4 emissions occurred before day 35 across all treatments (Figure 3a,b), similar to the ~50% of the total CH4 fluxes occurring during the first 28 days of a laboratory incubation experiment reported by Hao et al. [34]. Methane emissions increased when the total C increases in our study (p < 0.01, Table 3), as organic materials with a high C/N ratio are rich in labile C and methanogenic potential and organic matter content are positively related [34,39]. Methane emissions are produced when organic matter is mineralized in anaerobic environments with a low redox potential [2,39].
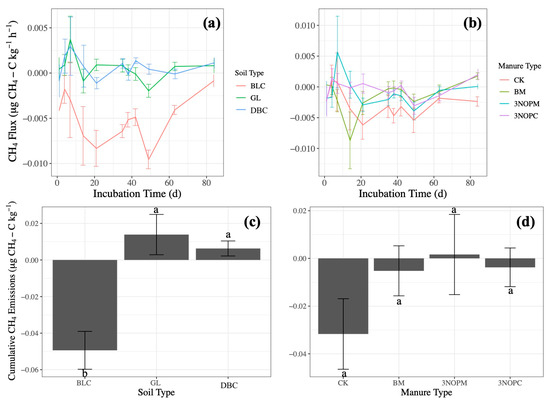
Figure 3.
Effects of (a) soil type on CH4 emissions over time, (b) manure type on CH4 emissions over time, (c) soil type on cumulative CH4 emissions, and (d) manure type on cumulative CH4 emissions in a laboratory incubation experiment. Soil type: BLC, Black Chernozem; GL, Gray Luvisol; DBC, Dark Brown Chernozem. Manure type: CK, control with no amendments; BM, barley-based manure; 3-NOPM, manure from cows fed 3-NOP supplement; 3-NOPC, composted manure from cows fed 3-NOP supplements. Treatments that do not share the same letter are significantly different from each other. Error bars indicate standard errors of the means (n = 4).
The addition of manure can temporarily create an anaerobic zone in the soil, enhancing the initial CH4 production [8,34]. After day 21, most of the CH4 emissions were negative (Figure 3a,b), indicating that the manure and soil had become CH4 sinks, with the sum of CH4 production by methanogenic bacteria and consumption by methanotrophic bacteria being negative [27,40,41]. The CH4 emissions may decline as nutrients from the manure amendment become depleted [37].
Only soil type had a significant impact on CH4 emissions (p < 0.001, Table 2). The Black Chernozem had negative CH4 emissions under all amendment types, indicating that the Black Chernozem served as a CH4 sink regardless of manure application (p < 0.001, Figure 3c). The Black Chernozem had the lowest C/N (p = 0.001, Table 1), and the low C availability might limit microbial production of CH4 [22,36,40]. The finer soil texture of the Dark Brown Chernozem and Gray Luvisol (clay loam) than the Black Chernozem (sandy loam) might have caused the higher CH4 emissions due to anaerobic conditions created in the finer-textured soils [39].
3.4. Total GHG Emissions
The impact of N2O emissions is magnitudes greater than CH4 on the cumulative anthropogenic GHG emissions (N2O and CH4 as CO2-equivalents) (Figure 2b and Figure3c,d). Positive and negative fluxes up until day 35 from Black and Dark Brown Chernozemic soils and positive GHG emissions up until day 60 from the Gray Luvisolic soil (Figure 4a) indicate prolonged microbial respiration. Substantially greater cumulative total GHG emissions from the Gray Luvisol highlight the importance of considering different soil types and their potential interaction with manure amendments (Figure 4b). The cumulative anthropogenic GHG emissions from the Black Chernozemic soil amended with 3-NOPM were significantly higher than for both BM and CK amendments, while the GHG emissions from 3-NOPC amendments were similar to or significantly lower than for BM and CK amendments across all soil types (Figure 4b). Relative to 3-NOPM, 3-NOPC significantly reduced total GHG emissions across all soil types, likely due to the composting process converting easily degradable C and N into less biologically active forms [24,38].
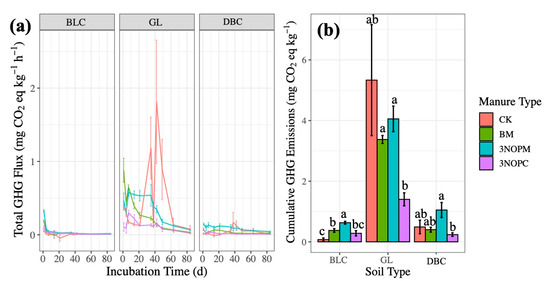
Figure 4.
Effects of manure type on anthropogenic GHG emissions (N2O and CH4 as CO2-equivalents) (a) over time by soil type and (b) cumulatively by soil type in a laboratory incubation experiment. Soil type: BLC, Black Chernozem; GL, Gray Luvisol; DBC, Dark Brown Chernozem. Manure type: CK, control with no amendments; BM, barley-based manure; 3-NOPM, manure from cows fed 3-NOP supplement; 3-NOPC, composted manure from cows fed 3-NOP supplements. Treatments that do not share the same letter are significantly different from each other. Error bars indicate standard errors of the means (n = 4).
4. Conclusions
Our results show that both cattle diet and manure management approaches affect GHG emissions from soils amended with manure. In this first look at the potential effect of the next stage in the life cycle of 3-NOP on GHG emissions, we found that GHG emissions resulting from soil amended with 3-NOPM are dependent on soil type (e.g., texture). For the coarse-textured Black Chernozemic soil (which had the lowest total GHG emissions across all soil types), the 3-NOPM amendment resulted in greater cumulative anthropogenic GHG emissions compared to both BM and CK. The composting of 3-NOPM prior to amendment reduced GHG emissions across all soil types. However, we caution that the composting process also releases GHGs which can be similar or even greater in magnitude than those released following soil amendment.
Further research related to the effect of soil properties, as well as field studies, are needed to provide farmers with best management practices related to the use of manure as a soil amendment from cattle fed a diet supplemented with 3-NOP. In particular, we recommend caution in applying the results of this incubation experiment to the field, which would have different conditions (e.g., growth of plants). Nonetheless, this research provides an important contribution by showing that the GHG emissions resulting from soil amended with manure from cattle fed a 3-NOP-supplemented diet potentially can be mitigated by applying the manure to certain types of soil (e.g., fine-textured soil) or by composting the manure prior to application.
Author Contributions
Conceptualization and methodology: X.H. and S.X.C.; investigation: T.L.W.; writing and editing: T.L.W., C.D.G., X.H., K.A.B. and S.X.C.; resources and supervision, X.H. and S.X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Agriculture and Agri-Food Canada (AAFC), Alberta Livestock and Meat Agency (ALMA), Natural Science and Engineering Research Council of Canada (NSERC), and the University of Alberta.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Vries, M.; de Boer, I.J.M. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- IPCC. Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; ISBN 92-9169-113-5. [Google Scholar]
- Lopes, J.C.; de Matos, L.F.; Harper, M.T.; Giallongo, F.; Oh, J.; Gruen, D.; Ono, S.; Kindermann, M.; Duval, S.; Hristov, A.N. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 2016, 99, 5335–5344. [Google Scholar] [CrossRef] [PubMed]
- Haisan, J.; Sun, Y.; Guan, L.L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Li, C.; Hao, X. Nitrous oxide and carbon dioxide emissions from soils amended with compost and manure from cattle fed diets containing wheat dried distillers’ grains with solubles. Can. J. Soil Sci. 2016, 97, 522–531. [Google Scholar] [CrossRef]
- Reeve, J.R.; Endelman, J.B.; Miller, B.E.; Hole, D.J. Residual effects of compost on soil quality and dryland wheat yield sixteen years after compost application. Soil Sci. Soc. Am. J. 2012, 76, 278. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Webster, C.P.; Powlwn, D.S. Long-term effects of nitrogen on methane oxidation in soil of the wheat experiment. Soil Sci. 1993, 25, 1307–1315. [Google Scholar]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef]
- Vyas, D.; Alemu, A.W.; McGinn, S.M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets. J. Anim. Sci. 2018, 96, 2923–2938. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.R.; Duval, S.; Rümbeli, R.; Stemmler, R.T.; Thauer, R.K.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Romero-Perez, A.; Okine, E.K.; McGinn, S.M.; Guan, L.L.; Oba, M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. J. Anim. Sci. 2014, 92, 4682–4693. [Google Scholar] [CrossRef]
- Jayasundara, S.; Ranga Niroshan Appuhamy, J.A.D.; Kebreab, E.; Wagner-Riddle, C. Methane and nitrous oxide emissions from Canadian dairy farms and mitigation options: An updated review. Can. J. Anim. Sci. 2016, 96, 306–331. [Google Scholar] [CrossRef]
- Romero-Perez, A.; Okine, E.K.; McGinn, S.M.; Guan, L.L.; Oba, M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Sustained reduction in methane production from long-term addition of 3-nitrooxypropanol to a beef cattle diet. J. Anim. Sci. 2015, 93, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Larney, F.J.; Hao, X. A review of composting as a management alternative for beef cattle feedlot manure in southern Alberta, Canada. Bioresour. Technol. 2007, 98, 3221–3227. [Google Scholar] [CrossRef]
- Schlegel, A.J. Effect of composted manure on soil chemical properties and nitrogen use by grain sorghum. J. Prod. Agric. 1992, 5, 153–157. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Ketterings, Q.M.; Vermeylen, F.; Godwin, G.S.; Czymmek, K.J. Soil properties under nitrogen- vs. phosphorus-based manure and compost management of corn. Soil Sci. Soc. Am. J. 2016, 80, 185–195. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Hadas, A.; Portnoy, R. Nitrogen and carbon mineralization rates of composted manures incubated in soil. J. Environ. Qual. 1994, 23, 1184–1189. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C.; Larney, F.J.; Travis, G.R. Greenhouse gas emissions during cattle feedlot manure composting. J. Environ. Qual. 2001, 30, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124159556. [Google Scholar]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lang, M.; Li, C.; Thomas, B.W.; Hao, X. Nutrient leaching from soil amended with manure and compost from cattle fed diets containing wheat dried distillers’ grains with solubles. Water Air Soil Pollut. 2016, 227, 393. [Google Scholar] [CrossRef]
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Microbial activity in response to water-filled pore space of variably eroded southern Piedmont soils. Appl. Soil Ecol. 1999, 11, 91–101. [Google Scholar] [CrossRef]
- Murphy, D.; Recous, S.; Stockdale, E.; Fillery, I.R.; Jensen, L.; Hatch, D.; Goulding, K.W. Gross nitrogen fluxes in soi: Theory, measurement and application of 15N pool dilution techniques. Adv. Agron. 2003, 79, 69–118. [Google Scholar]
- Gregorich, E.; Rochette, P.; VandenBygaart, A.; Angers, D. Greenhouse gas contributions of agricultural soils and potential mitigation practices in Eastern Canada. Soil Tillage Res. 2005, 83, 53–72. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Buresh, R. Correction for dissolved nitrous oxide in nitrogen studies. Soil Sci. Soc. Am. J. 1977, 41, 1201–1202. [Google Scholar] [CrossRef]
- Ramnarine, R.; Voroney, R.P.; Wagner-Riddle, C.; Dunfield, K.E. Carbonate removal by acid fumigation for measuring the δ13C of soil organic carbon. Can. J. Soil Sci. 2011, 91, 247–250. [Google Scholar] [CrossRef]
- Premrov, A.; Cummins, T.; Byrne, K.A. Bulk-density modelling using optimal power-transformation of measured physical and chemical soil parameters. Geoderma 2018, 314, 205–220. [Google Scholar] [CrossRef]
- Norman, A.G.; Bremner, J.M. Inorganic Forms of Nitrogen. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1965; pp. 672–676. ISBN 978-0-89118-204-7. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Logan, M. Biostatistical Design and Analysis Using R; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Paustian, K.; Parton, W.J.; Persson, J. Modeling soil organic matter in organic-amended and nitrogen-fertilized long-term plots. Soil Sci. Soc. Am. J. 1992, 56, 476–488. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C.; Larney, F.J. Carbon, nitrogen balances and greenhouse gas emission during cattle feedlot manure composting. J. Environ. Qual. 2004, 33, 37–44. [Google Scholar] [CrossRef]
- Bremner, J.M.; Shaw, K. Denitrification in soil. II. Factors affecting denitrification. J. Agric. Sci. 1958, 51, 40–52. [Google Scholar] [CrossRef]
- Datta, M.; Jha, P.; Arumbaka, S. Effects of nitrate supplementation on nutrition, performance and methane mitigation in ruminants: A review. Int. J. Livest. Res. 2017, 7, 19–29. [Google Scholar] [CrossRef]
- Bhandral, R.; Bolan, N.S.; Saggar, S.; Hedley, M.J. Nitrogen transformation and nitrous oxide emissions from various types of farm effluents. Nutr. Cycl. Agroecosyst. 2007, 79, 193–208. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Mor, S.; De Visscher, A.; Ravindra, K.; Dahiya, R.P.; Chandra, A.; Van Cleemput, O. Induction of enhanced methane oxidation in compost: Temperature and moisture response. Waste Manag. 2006, 26, 381–388. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).