Multicolor Fluorescence Imaging for the Early Detection of Salt Stress in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Salt-Stress Treatment
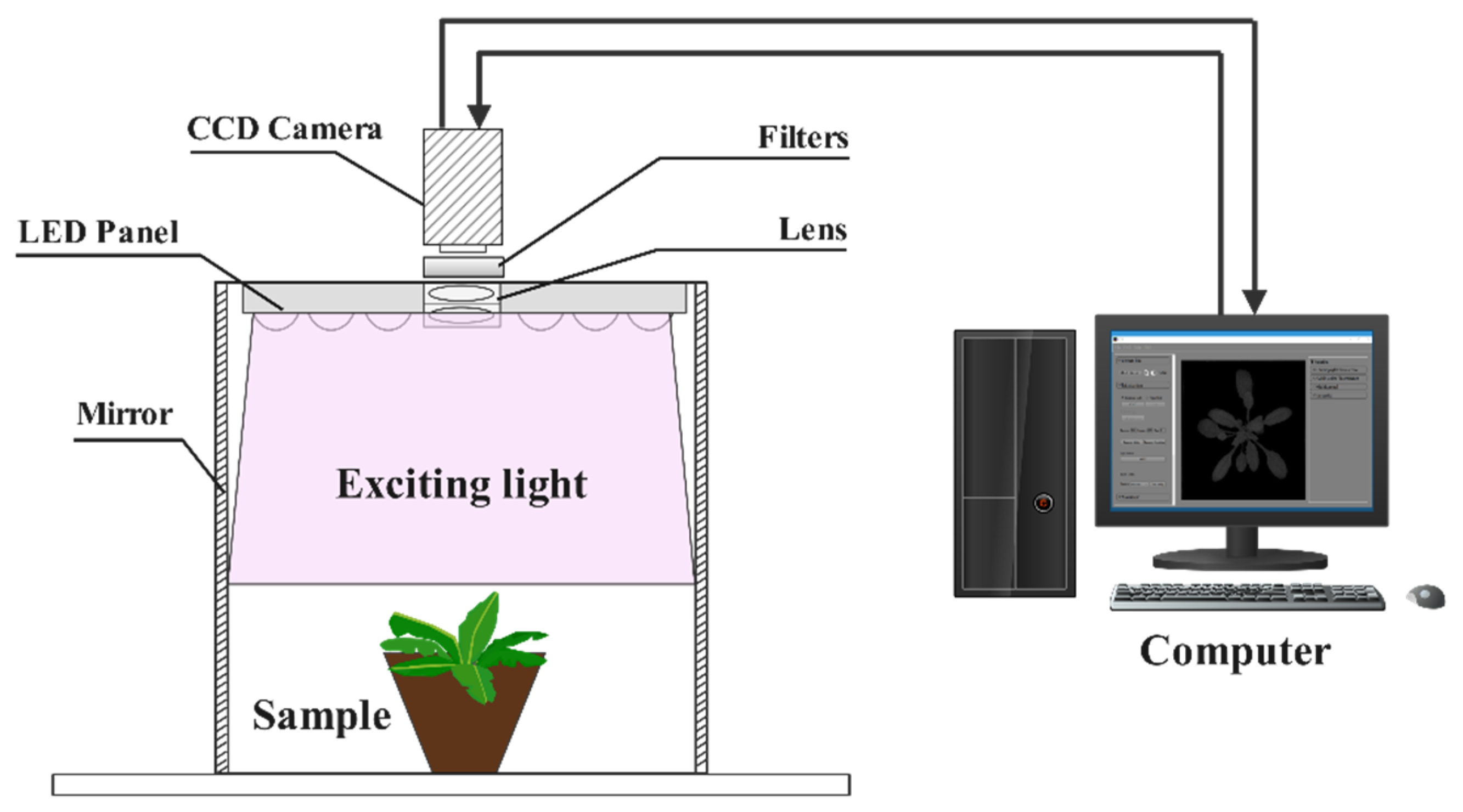
2.3. Multicolor Fluorescence Imaging (MFI) System
2.4. Determination of Leaf Area
2.5. Image Processing and Statistical Analysis
2.6. Construction of a Classification Model Based on PCA and SVM
3. Results and Discussion
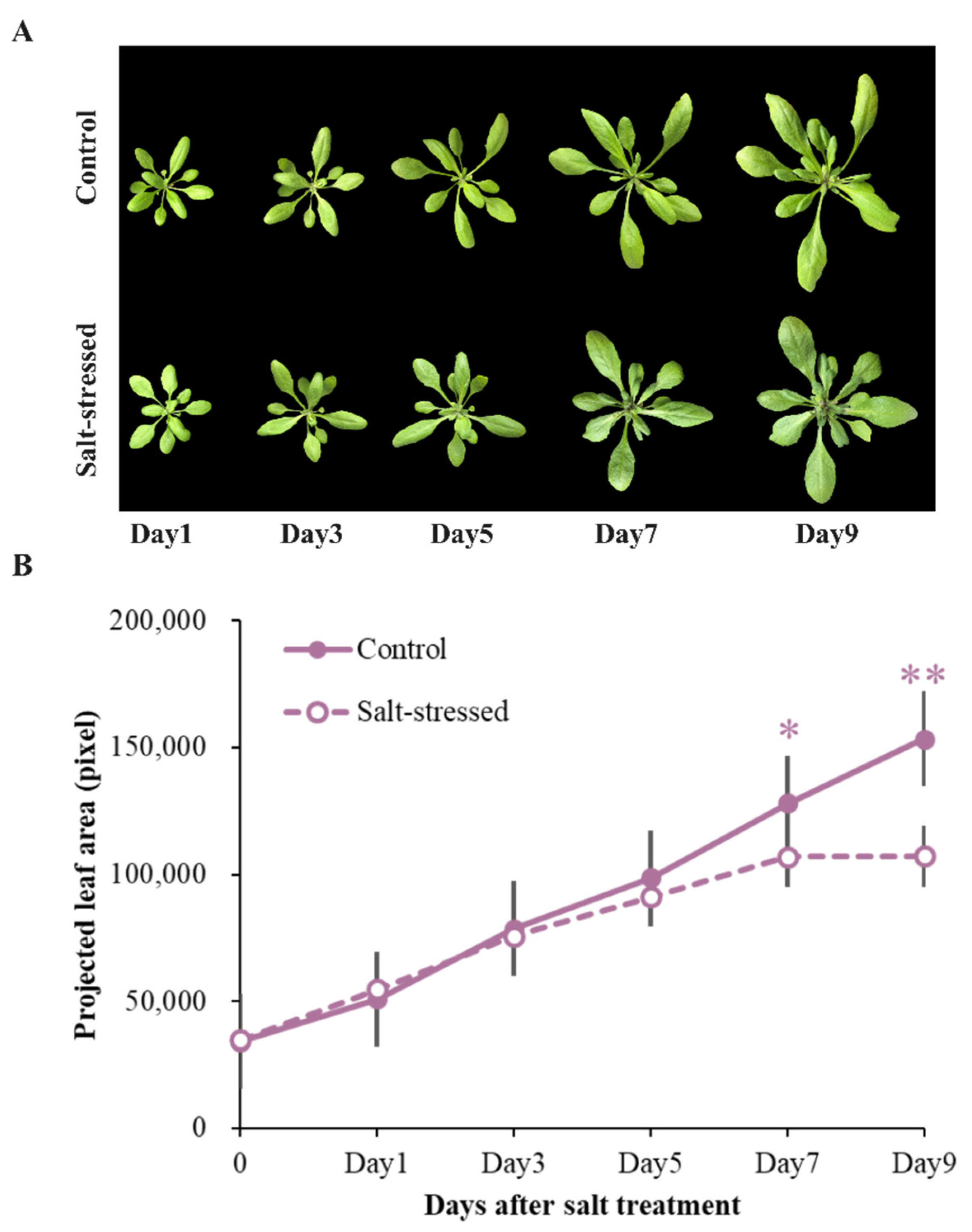
3.1. Salt Stress Affected Growth over Time
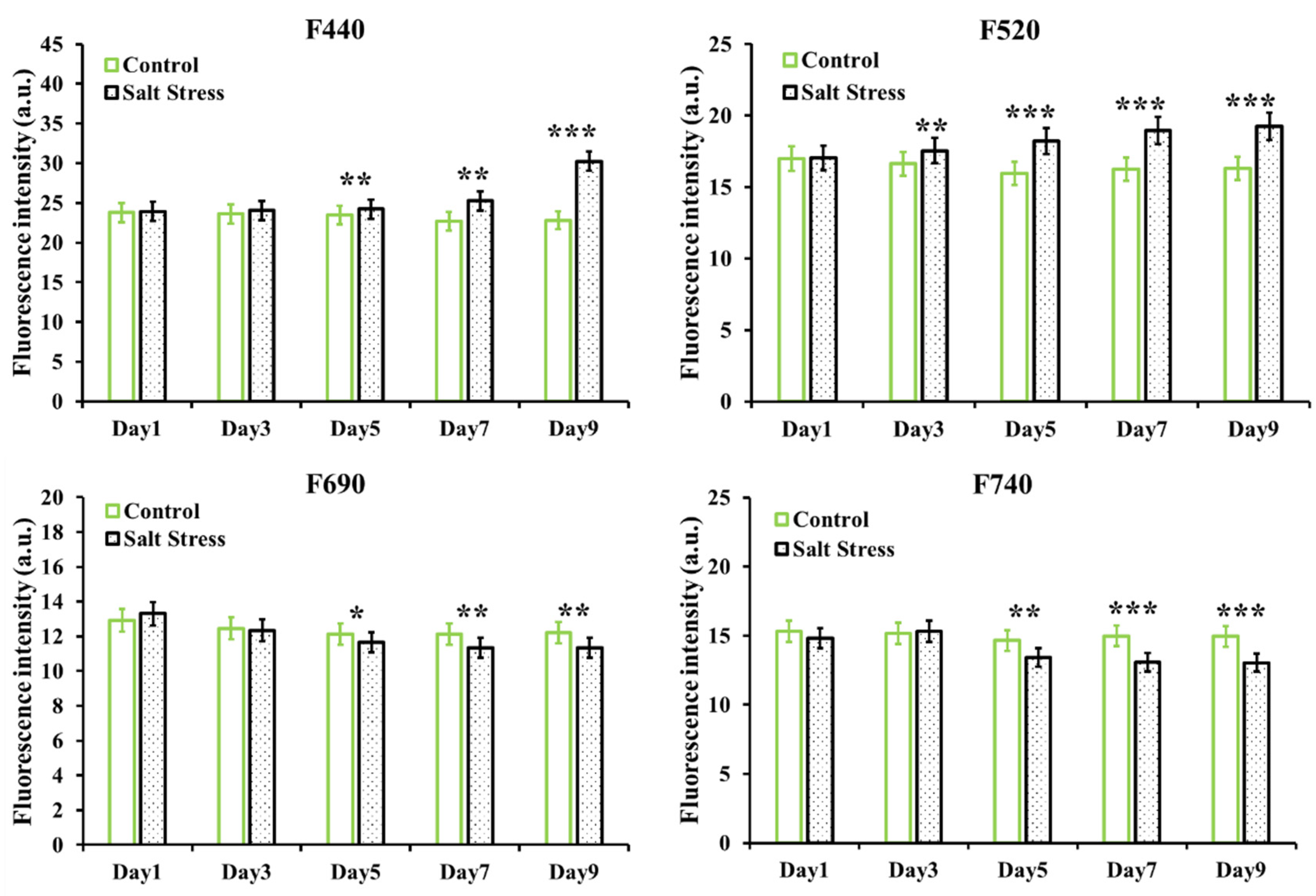
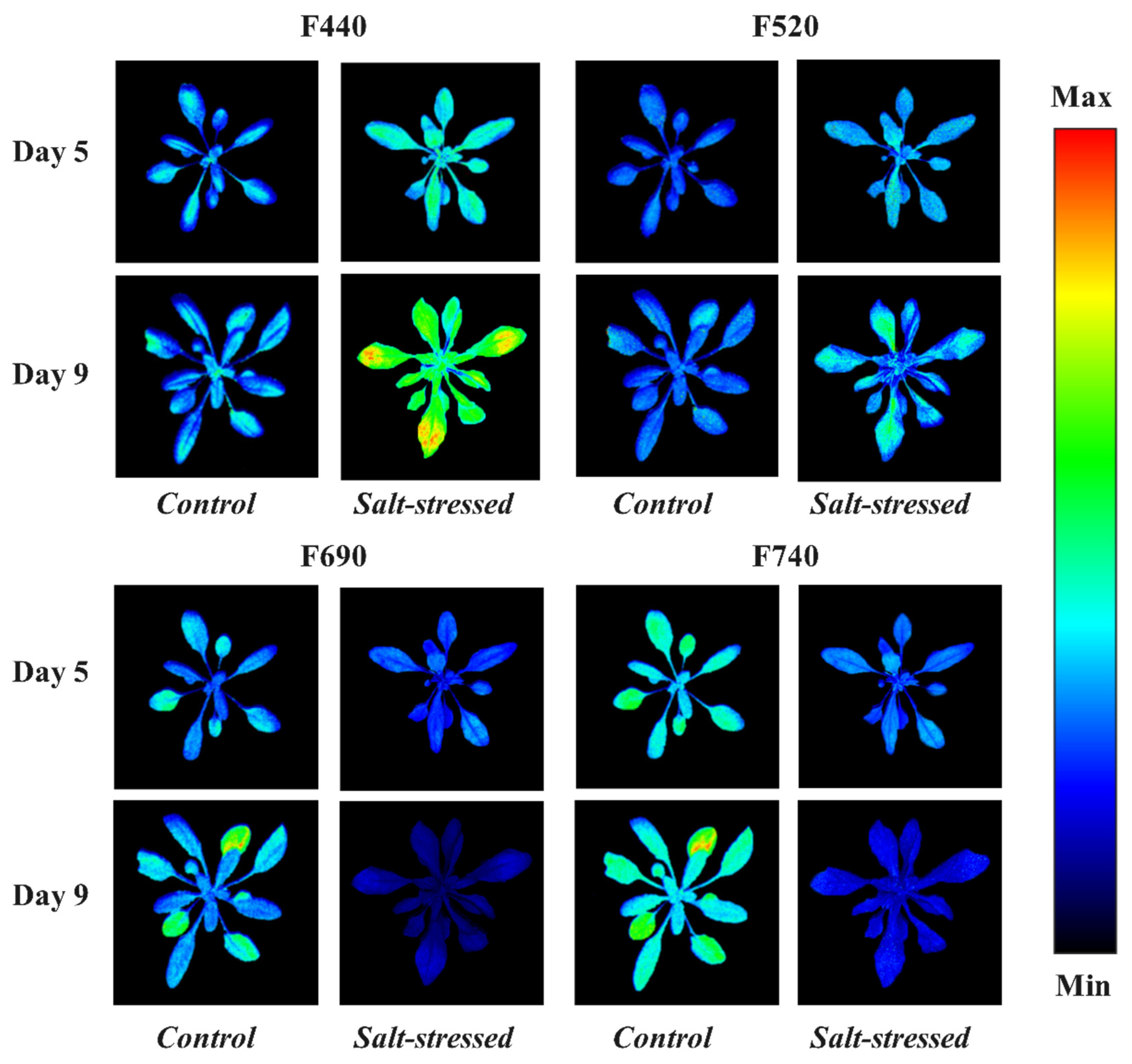
3.2. Effect of Salt Stress on Basic Fluorescence Parameters of Arabidopsis Leaves
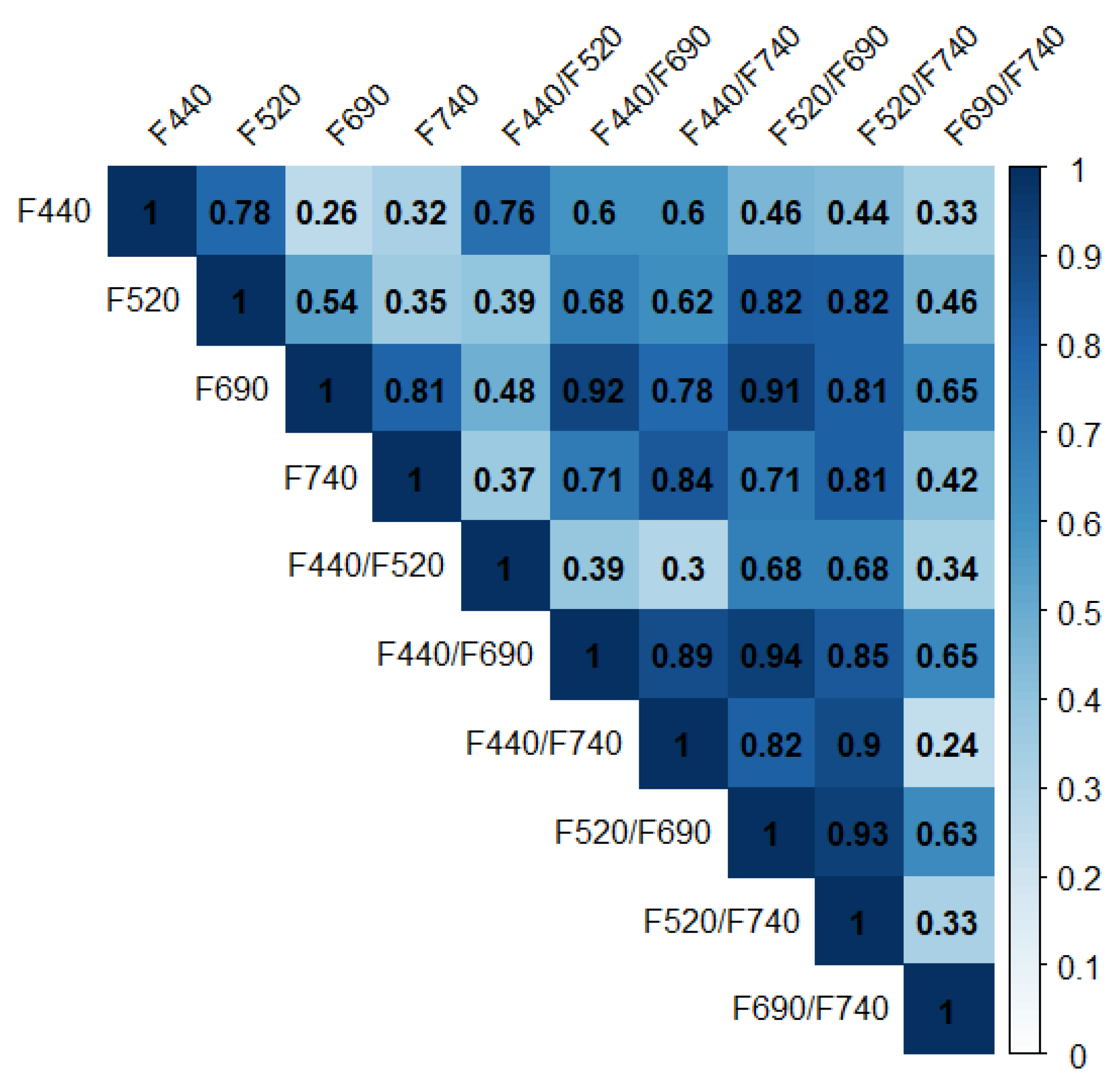
3.3. Correlation Analysis for Multicolor Fluorescence Parameters
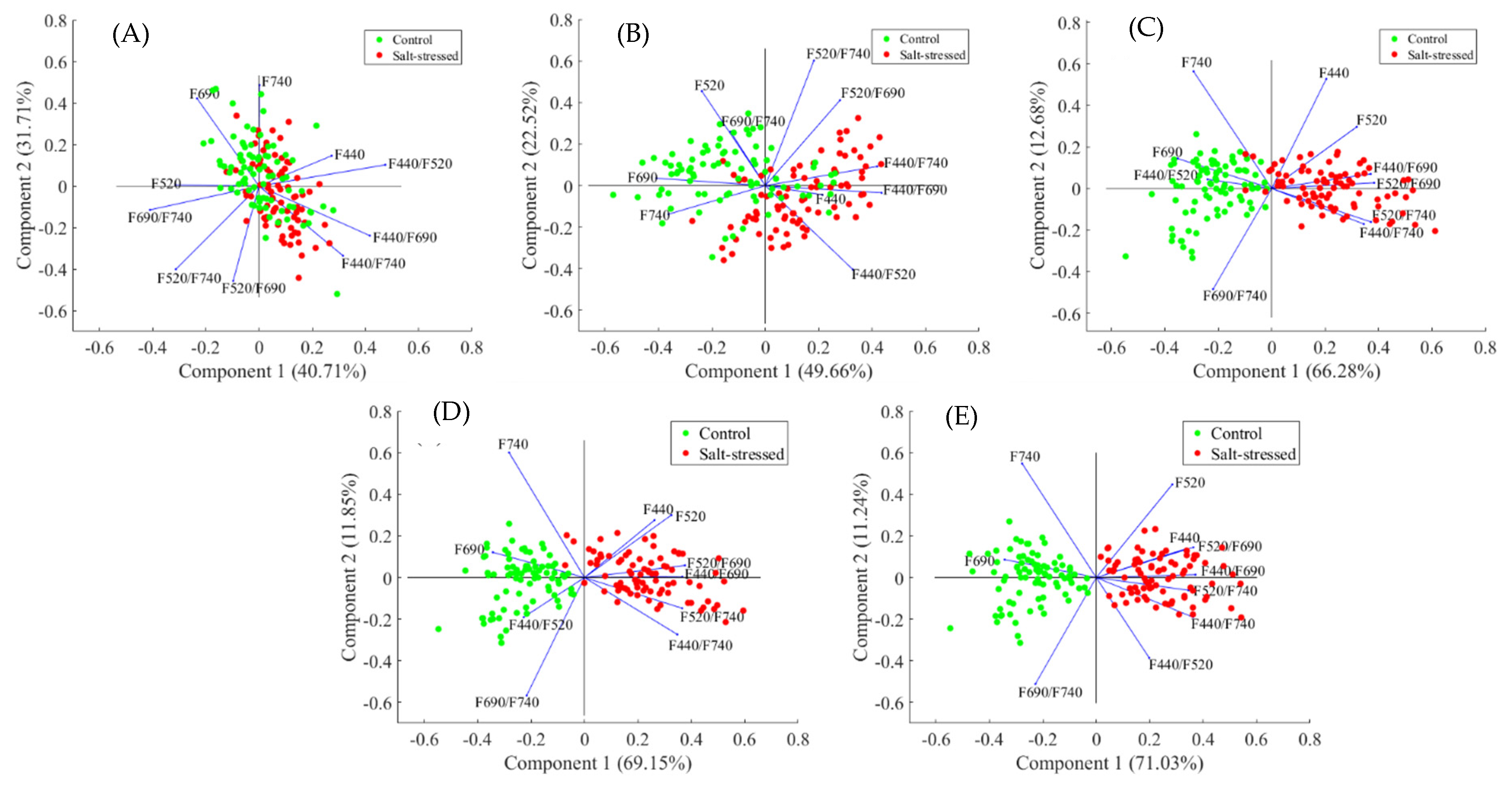
3.4. Principal Component Analysis of Effect of Salt Stress in Arabidopsis
3.5. Classification Model for Salt Stress Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, e701596. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A New Perspective of Phytohormones in Salinity Tolerance: Regulation of Proline Metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Yuan, Y.; Shu, S.; Li, S.; He, L.; Li, H.; Du, N.; Sun, J.; Guo, S. Effects of Exogenous Putrescine on Chlorophyll Fluorescence Imaging and Heat Dissipation Capacity in Cucumber (Cucumis Sativus L.) under Salt Stress. J. Plant Growth Regul. 2014, 33, 798–808. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant Growth and Development under Salinity Stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. ISBN 978-1-4020-5578-2. [Google Scholar]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Payne, W.Z.; Kurouski, D. Raman Spectroscopy Enables Phenotyping and Assessment of Nutrition Values of Plants: A Review. Plant Methods 2021, 17, 78. [Google Scholar] [CrossRef]
- Alvarez, J.; Martinez, E.; Diezma, B. Application of Hyperspectral Imaging in the Assessment of Drought and Salt Stress in Magneto-Primed Triticale Seeds. Plants 2021, 10, 835. [Google Scholar] [CrossRef]
- Chan, C.; Nelson, P.R.; Hayes, D.J.; Zhang, Y.-J.; Hall, B. Predicting Water Stress in Wild Blueberry Fields Using Airborne Visible and Near Infrared Imaging Spectroscopy. Remote Sens. 2021, 13, 1425. [Google Scholar] [CrossRef]
- Park, E.; Kim, Y.-S.; Omari, M.K.; Suh, H.-K.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.-K. High-Throughput Phenotyping Approach for the Evaluation of Heat Stress in Korean Ginseng (Panax Ginseng Meyer) Using a Hyperspectral Reflectance Image. Sensors 2021, 21, 5634. [Google Scholar] [CrossRef]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Patra, H.; Bajpai, V.; Das, B.; Kumar, S.; Dhandapani, R.; Vishwakarma, C.; Pal, M.; et al. Application of Thermal Imaging and Hyperspectral Remote Sensing for Crop Water Deficit Stress Monitoring. Geocarto Int. 2021, 36, 481–498. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Zhou, J.; Fu, X.; Ye, H.; Nguyen, H.T. Development of An Automated Phenotyping Platform for Quantifying Soybean Dynamic Responses to Salinity Stress in Greenhouse Environment. Comput. Electron. Agric. 2018, 151, 319–330. [Google Scholar] [CrossRef]
- Al-Rahbi, S.; Al-Mulla, Y.A.; Jayasuriya, H.; Choudri, B. Analysis of True-Color Images from Unmanned Aerial Vehicle to Assess Salinity Stress on Date Palm. JARS 2019, 13, 034514. [Google Scholar] [CrossRef]
- Struthers, R.; Ivanova, A.; Tits, L.; Swennen, R.; Coppin, P. Thermal Infrared Imaging of The Temporal Variability in Stomatal Conductance for Fruit Trees. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 9–17. [Google Scholar] [CrossRef]
- Lee, A.Y.; Kim, S.Y.; Hong, S.J.; Han, Y.; Choi, Y.; Kim, M.; Yun, S.K.; Kim, G. Phenotypic Analysis of Fruit Crops Water Stress Using Infrared Thermal Imaging. J. Biosyst. Eng. 2019, 44, 87–94. [Google Scholar] [CrossRef]
- Feng, X.; Zhan, Y.; Wang, Q.; Yang, X.; Yu, C.; Wang, H.; Tang, Z.; Jiang, D.; Peng, C.; He, Y. Hyperspectral Imaging Combined with Machine Learning as A Tool to Obtain High-Throughput Plant Salt-Stress Phenotyping. Plant J. 2020, 101, 1448–1461. [Google Scholar] [CrossRef]
- Simko, I.; Hayes, R.J.; Furbank, R.T. Non-Destructive Phenotyping of Lettuce Plants in Early Stages of Development with Optical Sensors. Front. Plant Sci. 2016, 7, 1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. Imaging of the Blue, Green, and Red Fluorescence Emission of Plants: An Overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Buschmann, C.; Lichtenthaler, H.K. Principles and Characteristics of Multi-Colour Fluorescence Imaging of Plants. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Samson, G.; Morales, F.; Tremblay, N.; Moya, I. Ultraviolet-Induced Fluorescence for Plant Monitoring: Present State and Prospects. Agronomie 1999, 19, 543–578. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bueno, M.L.; Pineda, M.; Cabeza, F.M.; Barón, M. Multicolor Fluorescence Imaging as a Candidate for Disease Detection in Plant Phenotyping. Front. Plant Sci. 2016, 7, 1790. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Pérez-Bueno, M.L.; Paredes, V.; Barón, M.; Pineda, M.; Pérez-Bueno, M.L.; Paredes, V.; Barón, M. Use of Multicolour Fluorescence Imaging for Diagnosis of Bacterial and Fungal Infection on Zucchini by Implementing Machine Learning. Funct. Plant Biol. 2017, 44, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis Drought Stress Response Using Kinetic Chlorophyll Fluorescence and Multicolor Fluorescence Imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef]
- Hsiao, S.C.; Chen, S.; Yang, I.C.; Chen, C.T.; Tsai, C.-Y.; Chuang, Y.K.; Wang, F.J.; Chen, Y.L.; Lin, T.S.; Lo, Y.M. Evaluation of Plant Seedling Water Stress Using Dynamic Fluorescence Index with Blue LED-Based Fluorescence Imaging. Comput. Electron. Agric. 2010, 72, 127–133. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing Crop Nitrogen Status with Fluorescence Indicators. A Review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 Mediates Osmotic-Stress-Evoked Ca2+ Increases Vital for Osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Stepien, P.; Johnson, G.N. Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Alvarado, J.M.; Ríos-Moreno, J.G.; Obregón-Biosca, S.A.; Ronquillo-Lomelí, G.; Ventura-Ramos, E.; Trejo-Perea, M. Hybrid Techniques to Predict Solar Radiation Using Support Vector Machine and Search Optimization Algorithms: A Review. Appl. Sci. 2021, 11, 1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, D.; Liu, Y. Automatic Identification Algorithm of the Rice Tiller Period Based on PCA and SVM. IEEE Access 2021, 9, 86843–86854. [Google Scholar] [CrossRef]
- Awlia, M.; Nigro, A.; Fajkus, J.; Schmoeckel, S.M.; Negrão, S.; Santelia, D.; Trtílek, M.; Tester, M.; Julkowska, M.M.; Panzarová, K. High-Throughput Non-Destructive Phenotyping of Traits That Contribute to Salinity Tolerance in Arabidopsis Thaliana. Front. Plant Sci. 2016, 7, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stober, F.; Lang, M.; Lichtenthaler, H.K. Blue, Green, and Red Fluorescence Emission Signatures of Green, Etiolated, and White Leaves. Remote Sens. Environ. 1994, 47, 65–71. [Google Scholar] [CrossRef]
- Stober, F.; Lichtenthaler, H.K. Studies on the Localization and Spectral Characteristics of the Fluorescence Emission of Differently Pigmented Wheat Leaves. Bot. Acta 1993, 106, 365–370. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence Emission Spectra of Plant Leaves and Plant Constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Wu, D.; Cui, M.; Hao, Y.; Liu, L.; Zhou, Y.; Wang, W.; Xue, A.; Chingin, K.; Luo, L. In Situ Study of Metabolic Response of Arabidopsis Thaliana Leaves to Salt Stress by Neutral Desorption-Extractive Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 12945–12952. [Google Scholar] [CrossRef]
- Yastreb, T.O.; Kolupaev, Y.E.; Lugovaya, A.A.; Dmitriev, A.P. Content of osmolytes and flavonoids under salt stress in Arabidopsis thaliana plants defective in jasmonate signaling. Prikl. Biokhim. Mikrobiol. 2016, 52, 223–229. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Miehé, J.A. Fluorescence Imaging as a Diagnostic Tool for Plant Stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Hák, R.; Lichtenthaler, H.K.; Rinderle, U. Decrease of the Chlorophyll Fluorescence Ratio F690/F730 during Greening and Development of Leaves. Radiat. Environ. Biophys. 1990, 29, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Multi-Colour Fluorescence Imaging of Photosynthetic Activityand Plant Stress. Photosynthetica 2021, 59, 364–380. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The Role of Chlorophyll Fluorescence in the Detection of Stress Conditions in Plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Solti, Á. F690-F740 Is More Suitable than F690/F740 for Mapping the Regeneration of Cd-Induced Chlorosis in Poplar Leaves by Fluorescence Imaging. Acta Biol. Szeged. 2008, 52, 191–194. [Google Scholar]
- Lichtenthaler, H.K.; Langsdorf, G.; Buschmann, C. Multicolor Fluorescence Images and Fluorescence Ratio Images of Green Apples at Harvest and during Storage. Isr. J. Plant Sci. 2012, 60, 97–106. [Google Scholar] [CrossRef]
| Salt-Stressed Time | Training Sample Number | Testing Sample Number | Accuracy (%) | ||
|---|---|---|---|---|---|
| Control | Salt-Stressed | Overall | |||
| Day 1 | 500 | 68 | 58.82 | 61.76 | 60.29 |
| Day 3 | 68 | 70.59 | 76.47 | 73.53 | |
| Day 5 | 68 | 94.12 | 91.18 | 92.65 | |
| Day 7 | 68 | 97.06 | 94.12 | 95.59 | |
| Day 9 | 68 | 100 | 97.06 | 98.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Xie, L.; Wu, M.; Yang, B.; Ishimwe, C.; Ye, D.; Weng, H. Multicolor Fluorescence Imaging for the Early Detection of Salt Stress in Arabidopsis. Agronomy 2021, 11, 2577. https://doi.org/10.3390/agronomy11122577
Tian Y, Xie L, Wu M, Yang B, Ishimwe C, Ye D, Weng H. Multicolor Fluorescence Imaging for the Early Detection of Salt Stress in Arabidopsis. Agronomy. 2021; 11(12):2577. https://doi.org/10.3390/agronomy11122577
Chicago/Turabian StyleTian, Ya, Limin Xie, Mingyang Wu, Biyun Yang, Captoline Ishimwe, Dapeng Ye, and Haiyong Weng. 2021. "Multicolor Fluorescence Imaging for the Early Detection of Salt Stress in Arabidopsis" Agronomy 11, no. 12: 2577. https://doi.org/10.3390/agronomy11122577
APA StyleTian, Y., Xie, L., Wu, M., Yang, B., Ishimwe, C., Ye, D., & Weng, H. (2021). Multicolor Fluorescence Imaging for the Early Detection of Salt Stress in Arabidopsis. Agronomy, 11(12), 2577. https://doi.org/10.3390/agronomy11122577





