Assessment of the Interactive Effect of the Use of 1-Methylcyclopropene and Cultivars on the Nutritional Value of Broccoli during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Broccoli Production
2.2. The Broccoli Cultivars
2.3. Harvest and Storage
2.4. Weight Loss
2.5. Laboratory Analysis
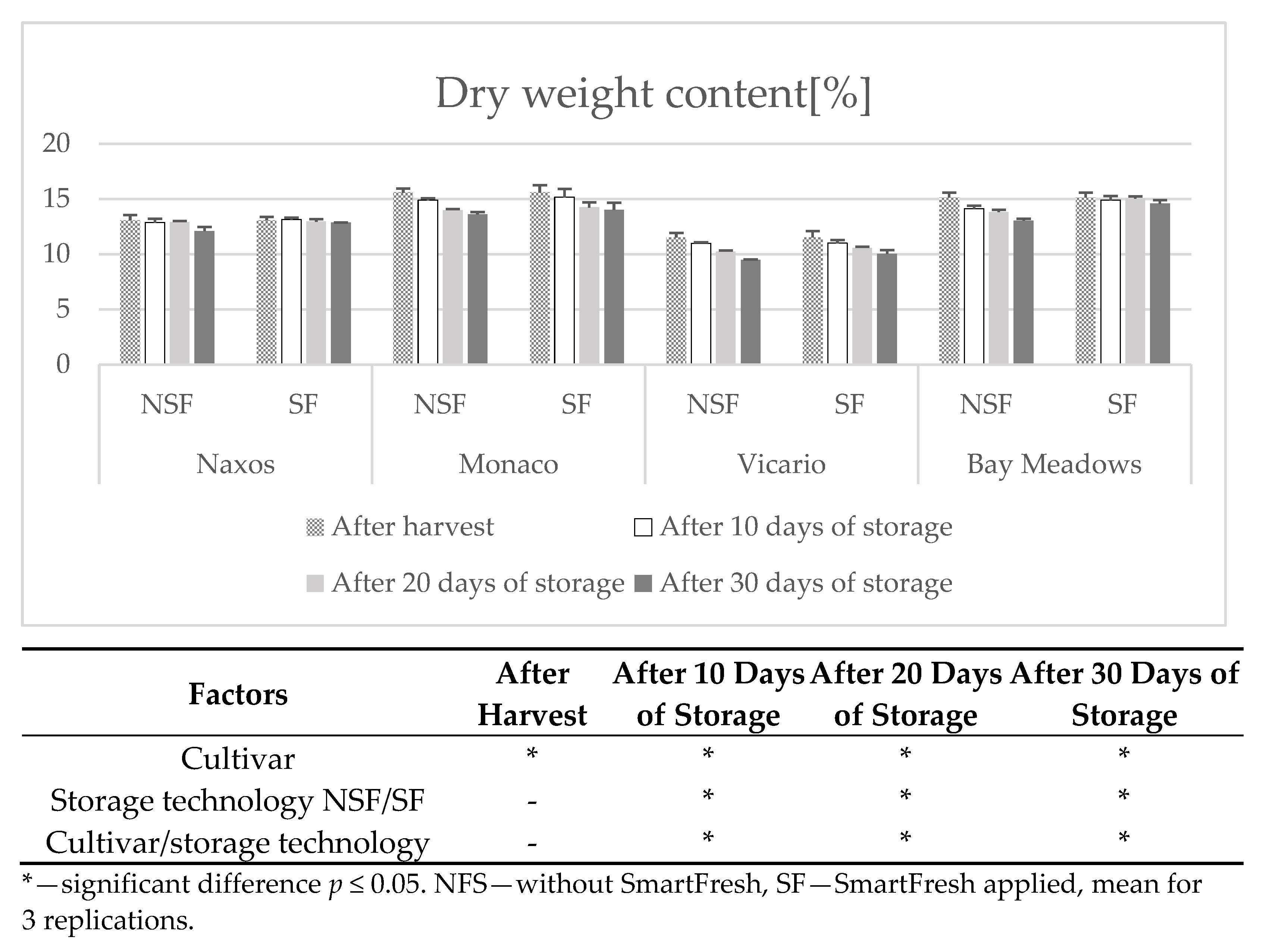
2.6. Determination of Dry Matter
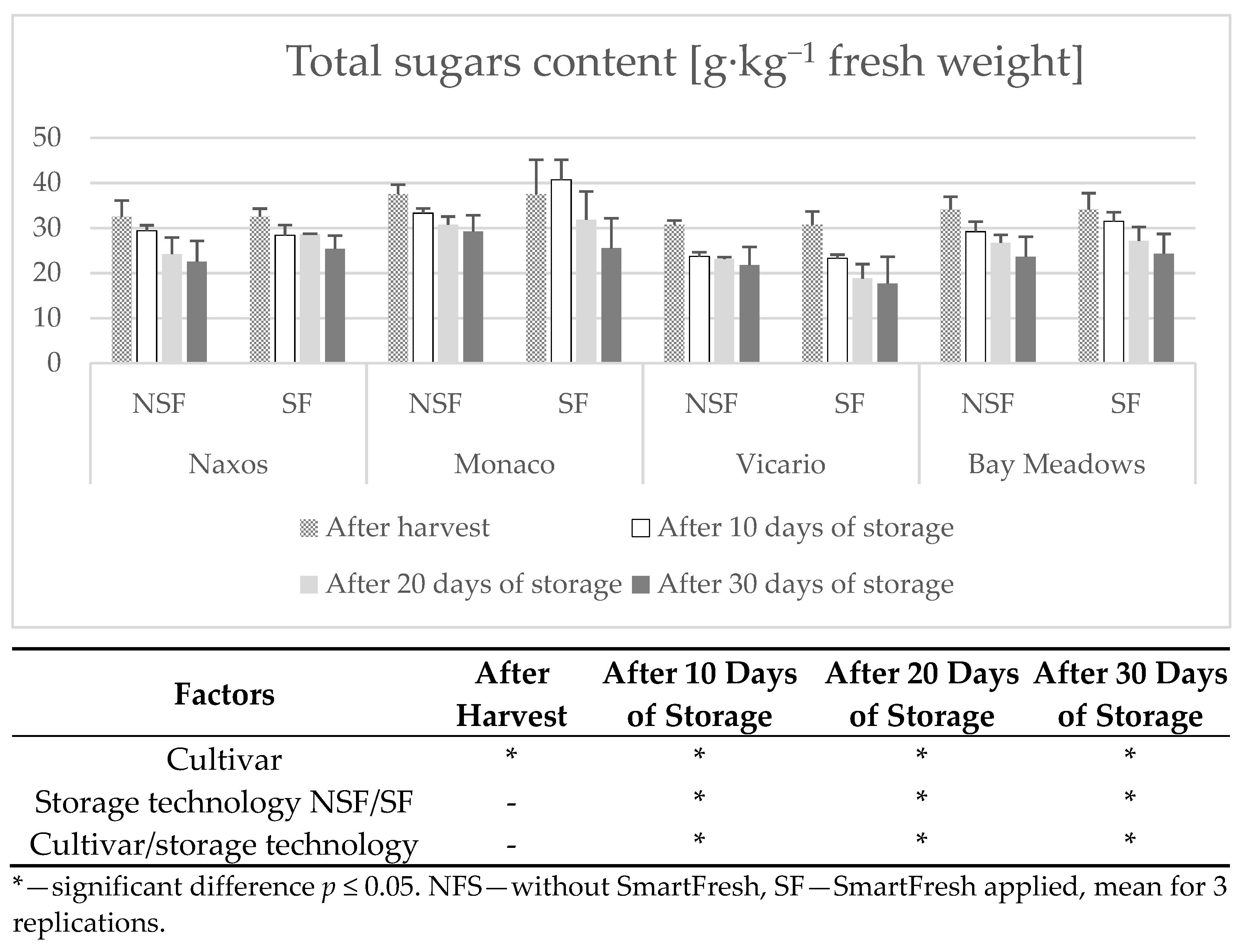
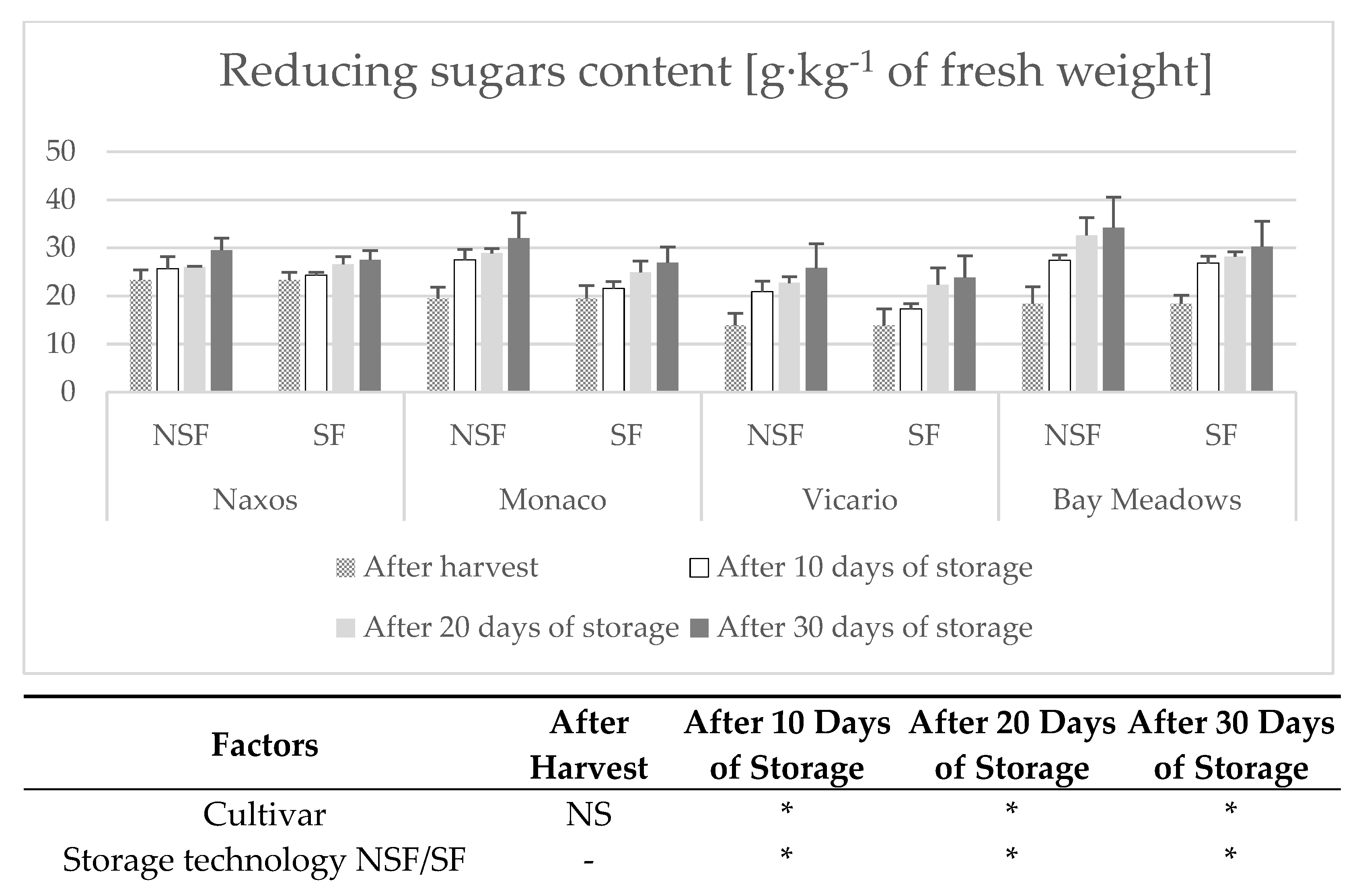
2.7. Determination of Sugars
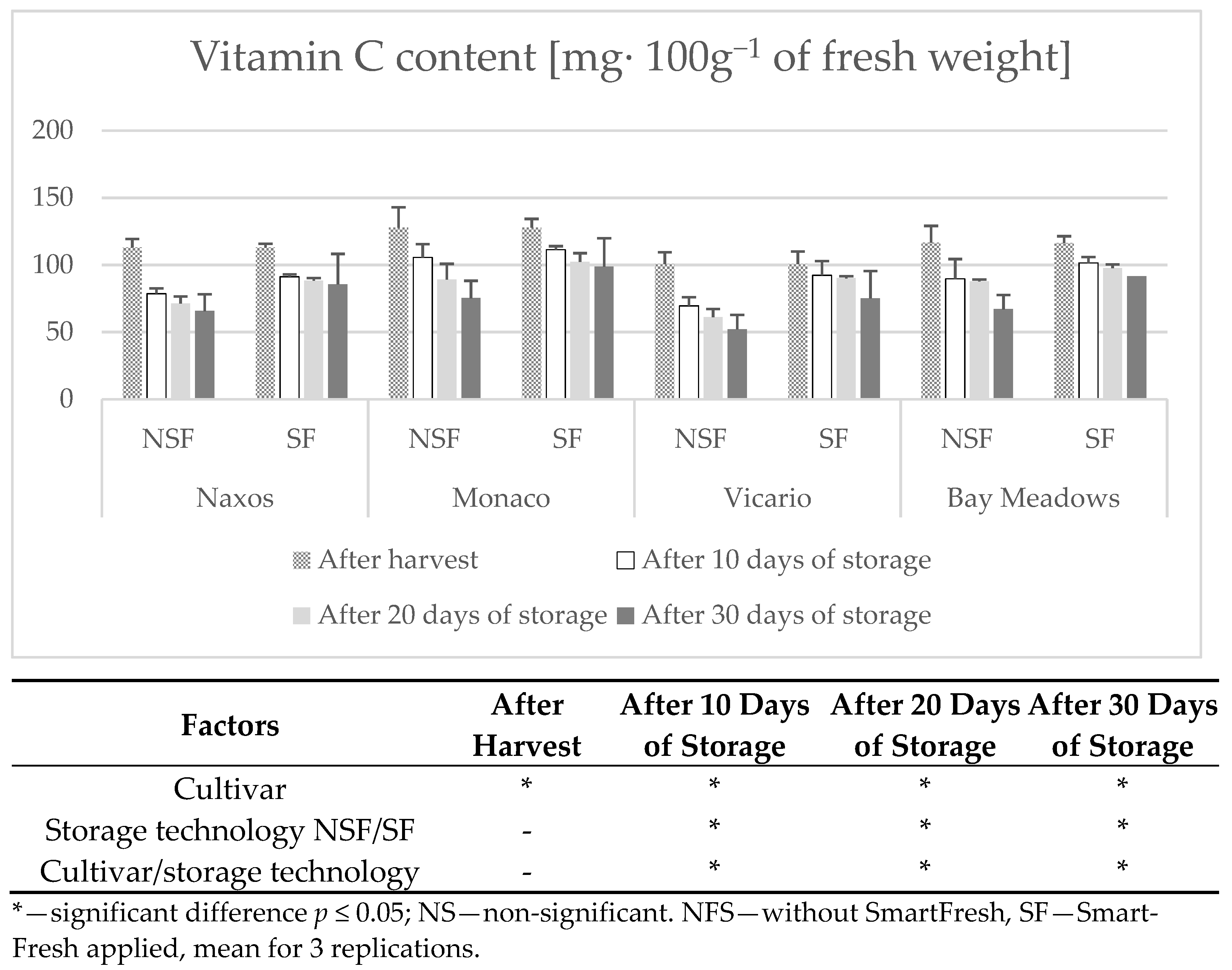
2.8. Determination of Ascorbic Acid
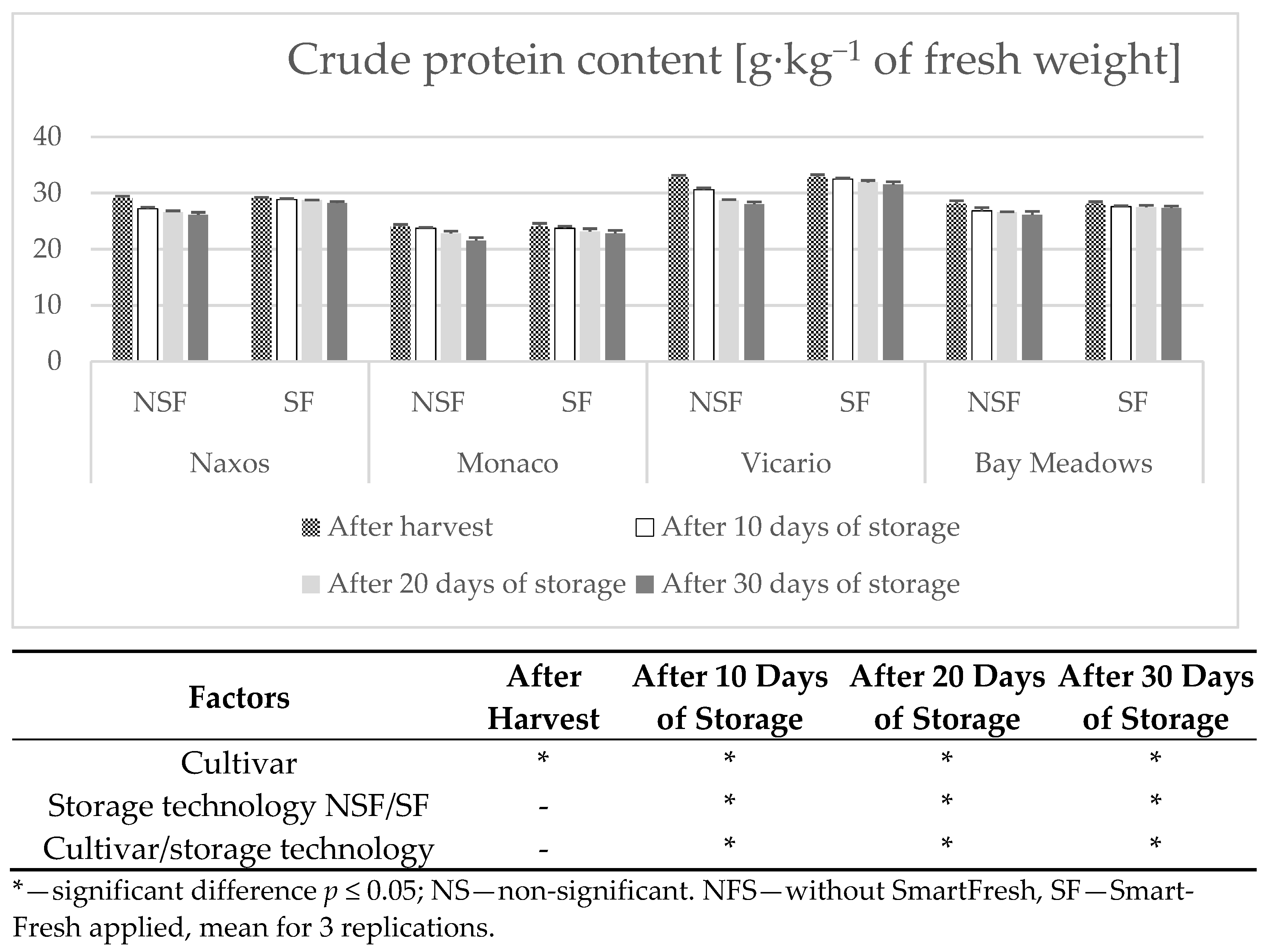
2.9. Determination of Crude Protein
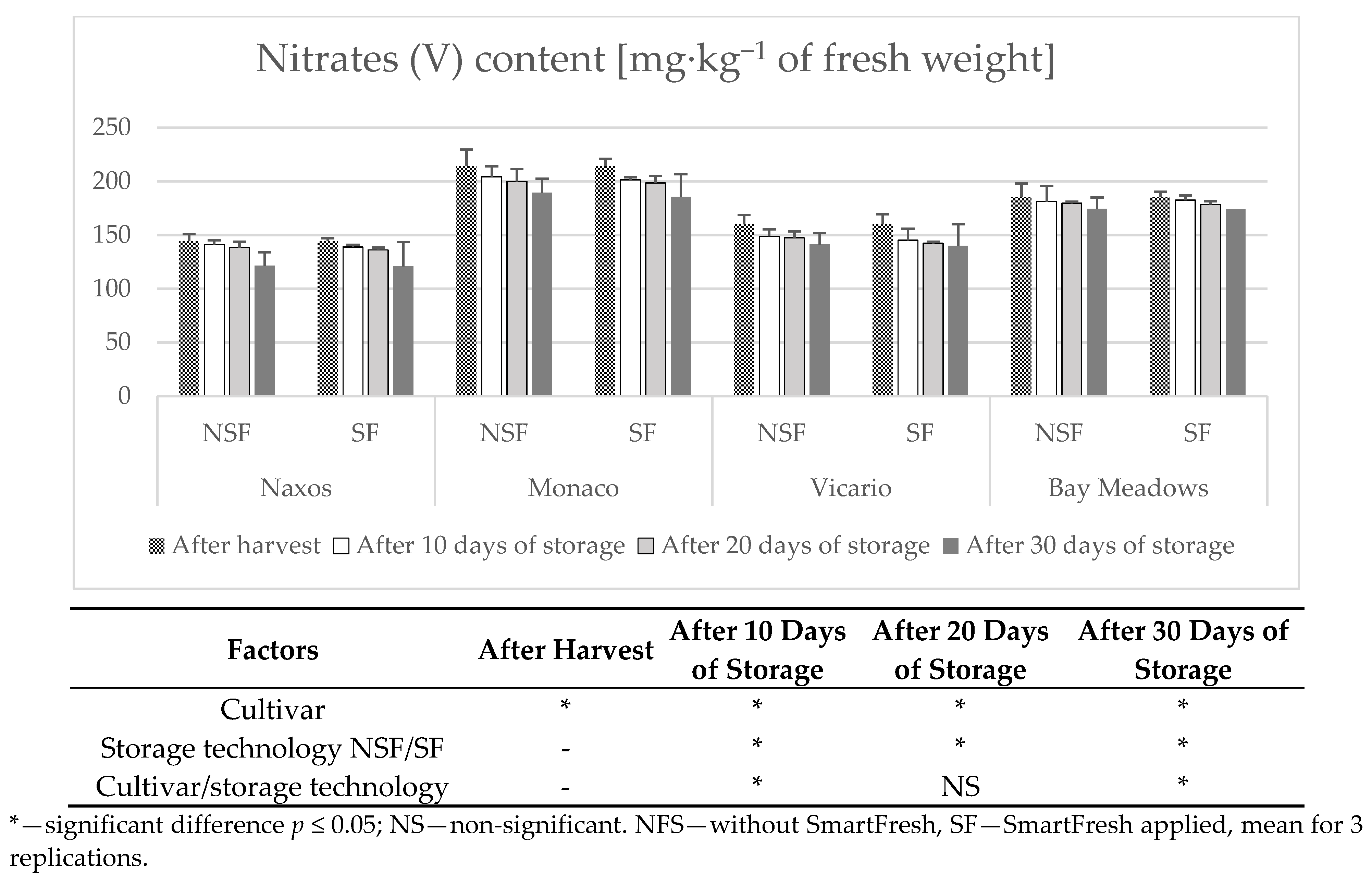
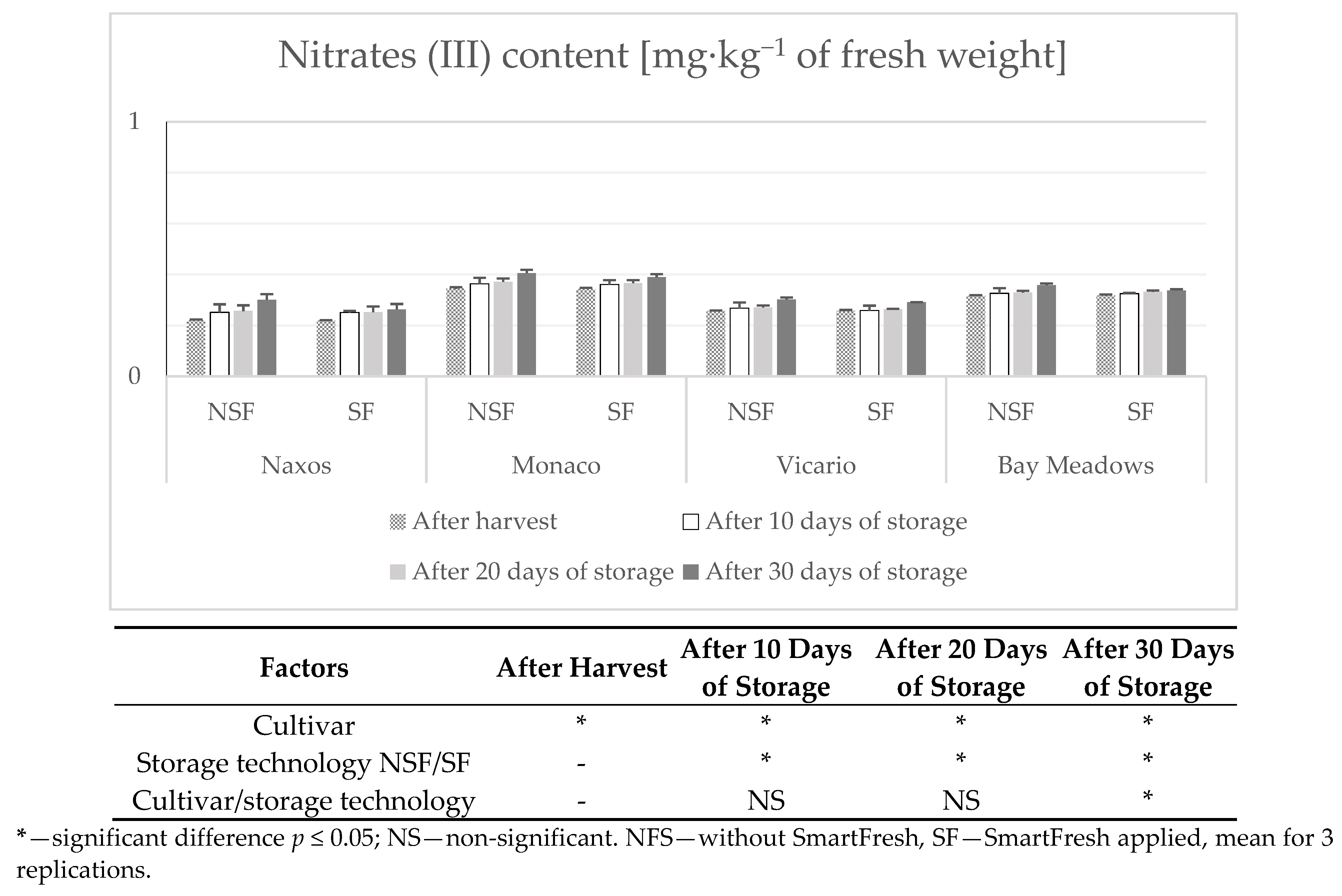
2.10. Determination of Nitrates (V) and Nitrates (III)
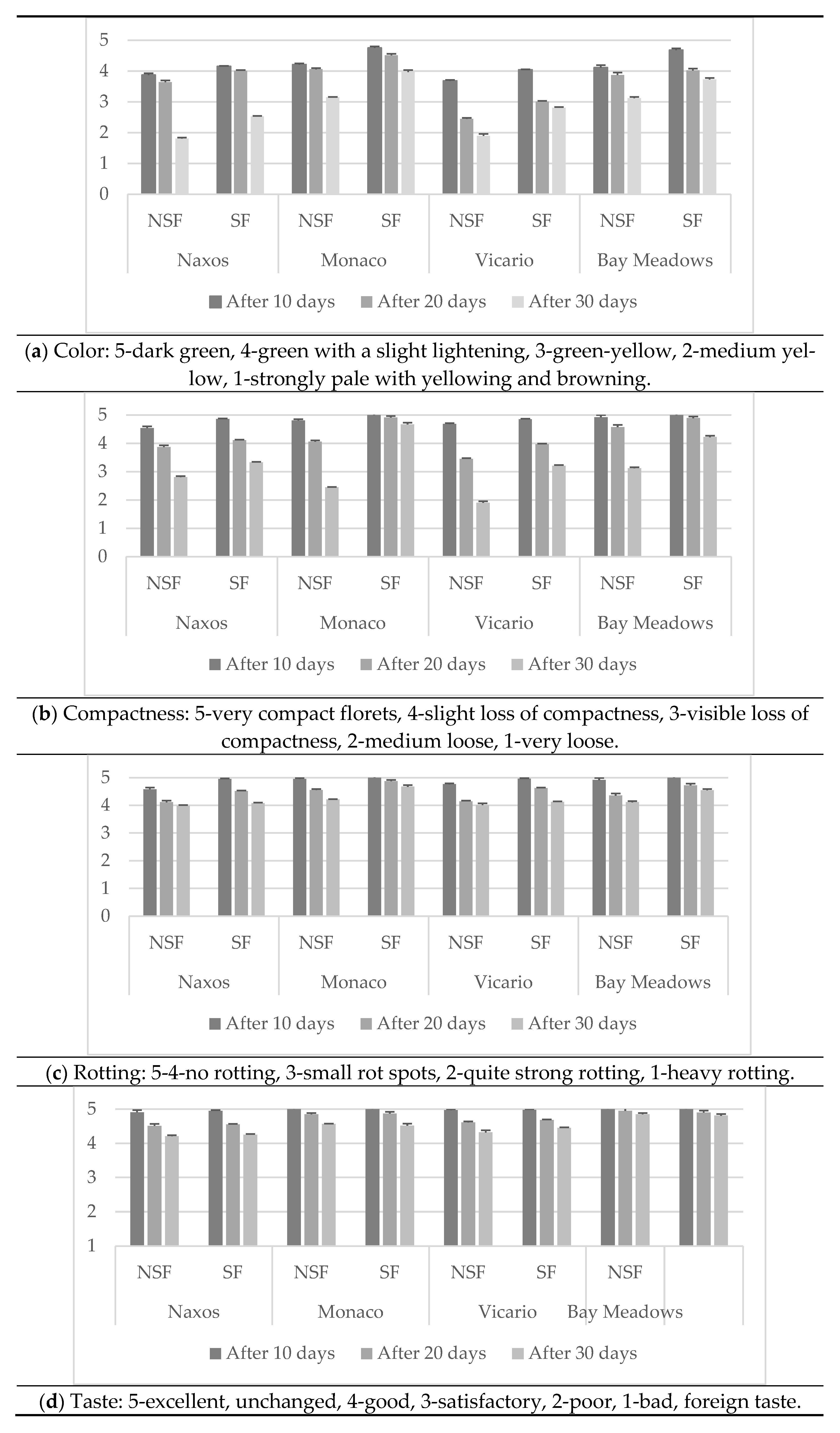
2.11. Organoleptic Assessment
2.12. Statistical Analysis
3. Results
Weight Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, A. Fruit maturity ripening and quality relationships. Acta Hort. 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Tian, M.S.; Downs, C.G.; Lill, R.E.; King, G.A. A Role for Ethylene in the Yellowing of Broccoli after Harvest. J. Am. Soc. Hort. Sci. 1994, 119, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.L.; Civello, P.M.; Chaves, A.R.; Martinez, G.A. Effect of hot air treatments on senescence and quality parameters of harvested broccoli (Brassica oleracea L. var Italica) heads. J. Sci. Food Agric. 2005, 85, 1154–1160. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2021. Available online: http://www.fao.org/faostat/en/ (accessed on 11 October 2021).
- Jones, R.; Faragher, J.; Winkler, S. A review of the influence of postharvest treatments on quality and glucosinolate content in broccoli (Brassica oleracea var. italica) heads. Postharvest Biol. Technol. 2006, 41, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Nie, M.; Xiao, Y.; Zhu, L.; Gao, R.; Zhou, C.; Li, D.; Xu, Y.; Dai, Z. Positive effects of ultrasound pretreatment on the bioaccessibility and cellular uptake of bioactive compounds from broccoli: Effect on cell wall, cellular matrix and digesta. LWT 2021, 149. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, D.Y.; Cheng, L.J.; Bayrak, M.; Jegasothy, H.; Sanguansri, L.; Augustin, M.A. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil. LWT 2020, 128. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. Studies on the development of vegetable-based powdered beverages –Effect of the composition and dispersing temperature on potential bioaccessibility of main low-molecular antioxidants and antioxidant properties. LWT 2020, 131. [Google Scholar] [CrossRef]
- Kyere, E.O.; Popovich, D.G.; Palmer, J.; Wargent, J.J.; Fletcher, G.C.; Flint, S. Reduction of the attachment, survival and growth of L. monocytogenes on lettuce leaves by UV-C stress. LWT 2021, 145. [Google Scholar] [CrossRef]
- Feng, X.; Hongfei, W.; Yuechang, T.; Shuanquan, D.; Xing, Q.; Xuehong Ch Yonghua, Z. Effect of 1-methylcyclopropene on senescence and sugar metabolism in harvested broccoli florets. Postharvest Biol. Technol. 2016, 116, 45–49.11. [Google Scholar]
- Costa, L.; Millan Montano, Y.; Carrión, C.; Rolny, N.; Guiamet, J. Application of low intensity light pulses to delay postharvest senescence of Ocimum basilicum leaves. Postharvest Biol. Technol. 2013, 86, 181–191.12. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Chen, Y.; Lin, Y.; Shaw, J. Characterization of senescence-associated proteases in postharvest broccoli florets. Plant Physiol. Biochem. 2004, 42, 663–670. [Google Scholar] [CrossRef]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Ascorbate metabolism in harvested broccoli. J. Exp. Botany 2003, 54, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.; Martinez-Romero, D.; Guillén, F.; Castillo, S.; Valero, D. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
- Costa, M.; Vicente, A.; Civello, P.; Chaves, A.; Martínez, G. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; García-Viguera, C.; Castillo, S.; Serrano, M.; Valero, D. Modified atmosphere packaging for broccoli sprouts affected by film permeability. Acta Hortic. 2015, 1071, 269–274. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Yamauchi, N.; Takino, S.; Shigyo, M. Effect of UV-A and UV-B irradiation on broccoli (Brassica oleracea L. Italica Group) floret yellowing during storage. Postharvest Biol. Technol. 2009, 54, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Lobato, M.; Hasperué, J.; Civello, P.; Chaves, A.; Martínez, G. Effect of 1-MCP on the expression of chlorophyll degrading genes during senescence of broccoli (Brassica Oleracea L.). Sci. Hortic. 2012, 144, 208–211. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P. Yellowing of broccoli in storage is reduced by 1 methylcyclopropene. HortScience 2000, 35, 885–887. [Google Scholar] [CrossRef] [Green Version]
- Vijay, P.; Rakesh, P. Technical information for use of 1-methylcyclopropene (1-MCP) on fruits and vegetables for extending their shelf-life. In Laboratory Manual on “Post-Harvest Physiology of Fruits and Flowers” Division of Plant Physiology; JARJ: New Delhi, India, 2010; pp. 59–64. [Google Scholar]
- Pereira, M.E.C.; Sargent, S.A.; Sims, C.A.; Huber, D.J.; Moretti, C.L.; Crane, J.H. Aqueous 1-methylcyclopropene extends longevity and does not affect sensory acceptability of Guatemalan-West Indian hybrid avocado. HortTechnology 2013, 23, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EECText with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0396 (accessed on 21 October 2021).
- Sisler, E.C.; Blankenship, S.M. Methods of Counteracting an Ethylene Response in Plants. U.S. Patent 5,518,988, 1996. [Google Scholar]
- Sisler, E.C.; Serek, M. Compounds interacting with the ethylene receptor in plants. Plant Biol. 2003, 5, 473–480. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M.; Dupille, E. Comparison of cyclopropene, 1- methylcyclopropene, and 3,3-dimethylcyclopropene as ethylene antagonists in plants. Plant Growth Regul. 1996, 18, 169–174. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Watkins, C.B.; Miller, W.B. A Summary of Physiological Processes or Disorders in Fruits, Vegetables and Ornamental Products that are Delayed or Decreased, Increased, or Unaffected by Application of 1- Methylcyclopropene (1-MCP). 2005. Available online: bhttp://www.hort.cornell.edu/mcp/N) (accessed on 23 October 2021).
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; González-Gómez, D. Different postharvest strategies to preserve broccoli quality during storage and shelf life: Controlled atmosphere and 1-MCP. Food Chem. 2013, 138, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, M. Storage of vegetables. Gard. Rap. 2015, 7, 14–18. (In Polish) [Google Scholar]
- EAPR. Method of Assessment for Potatoes and Potato Products; European Association for Potato Research: Wageningen, The Netherlands, 1974; pp. 68–69. [Google Scholar]
- Talburt, W.F.; Smith, O. Potato Processing; Van Nostrand Reinhold Co.: New York, NY, USA, 1987; pp. 371–474. [Google Scholar]
- Kapur, A.; Haskowić, A.; Čoprajanićijević, A.; Klepo, L.; Topčagić, A.; Tahirović, I.; Sofić, E. Spectrophotometric analysis of total ascorbic acid content in various fruits and vegetables. Bull. Chem. Technol. Bosnia Herzeg. 2012, 38, 39–42. [Google Scholar]
- Sweeney, R.A.; Rexroad, P.R. Comparison of LECO FP-228 “nitrogen determinator” with AOAC copper catalyst Kjeldahl method for crude protein. J. Assoc. Off. Anal Chem. 1987, 70, 1028–1030. [Google Scholar] [CrossRef]
- Baker, W.H.; Thompson, T.L. Determination of nitrate-nitrogen (NO3-N) in plant samples by selective ion electrode. In Plant Analysis Reference Procedures for the Southern Region of the United States- Southern Cooperative Series Bulletin #368; The University of Georgia: Athens, GA, USA, 1992; pp. 23–26. [Google Scholar]
- UNECE STANDARD. FFV-48 Standards Concerning the Marketing and Commercial Quality Control of Broccoli 2017 Edition; United Nations: New York, NY, USA; Geneva, Switzerland, 2017; pp. 1–8. [Google Scholar]
- PN-EN ISO 8586:2014-Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Sensory Evaluation Experts (English version); ISO: Geneva, Switzerland, 2014; pp. 1–42.
- Gębczyński, P. Quantitative changes of selected chemical components in the freezing and freezing process of main and side broccoli florets. Acta Sci. Pol. Technol. Aliment. 2003, 2, 31–39. [Google Scholar]
- Gajewski, M. Storage of Vegetables; SGGW: Warsaw, Poland, 2005; pp. 1–176. [Google Scholar]
- Zsom, P.; Polgari, P.; Nguyen LP, L.; Hitka, G. Zsom-Muha V. Quality maintenance of broccoli by the use of1-MCP treatments. Prog. Agric. Eng. Sci. 2020, 16, 95. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, A.; Kunicki, E.; Libik, A. The effects of different methods of cultivation and plant spacing on the chemical composition of broccoli heads. Folia Hortic. 2009, 21, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Kosterna, E. The yield and quality of broccoli grown under flat covers with soil mulching. Plant Soil Environ. 2014, 60, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.K.Y.; Wearing, A.H.; Rickert, K.G.; Birch, C.J. Broccoli yield and quality can be determined by cultivar and temperature but not photoperiod in south-east Queensland. Aust. J. Exp. Agric. 2000, 39, 901–909. [Google Scholar] [CrossRef]
- Nishikawa, N.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Botany 2005, 56, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte-Sierra, A.; Forney, C.; Michaud, D.; Angers, P.; Arul, J. Influence of hormetic heat treatment on quality and phytochemical compounds of broccoli florets during storage. Postharvest Biol. Technol. 2017, 128, 44–53. [Google Scholar] [CrossRef]
- Raseetha, S.; Leong, S.; Burritt, D.; Oey, I. Understanding the degradation of ascorbic acid and glutathione in relation to the levels of oxidative stress biomarkers in broccoli (Brassica oleracea L. italica cv. Bellstar) during storage and mechanical processing. Food Chem. 2013, 138, 1360–1369. [Google Scholar] [CrossRef]
- Kwasniewska-Karolak, I.; Mostowski, R. Influence of cultivation method and refrigerated storage on changes in vitamin C and chlorophyll content in sweet pepper and broccoli. Ferment. Fruit Veg. Ind. 2017, 61, 10. (In Polish) [Google Scholar]
- Ma, G.; Zhang, L.C.; Kato, M.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Ikoma, Y.; Matsumoto, H. Effect of 1-methylcyclopropene on the expression of genes for ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2010, 58, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Kamisako, T. Effect of electrostatic atomization on ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2012, 74, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, B. Role of ascorbic acid in photosynthesis. Biochemistry 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Elshima, N.M.; Shekib, L.A.; Sharara, M.S. Effect of Domestic Processing Methods on the Chemical Composition and Organoleptic Properties of Broccoli and Cauliflower. Am. J. Food Nutr. 2015, 3, 125–130. [Google Scholar]
- Raubenheimer, K.; Bondonno, C.; Blekkenhorst, L.; Wagner, K.H.; Peake, J.M.; Neubauer, O. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr. ReviewsVR 2019, 77, 584–599. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Sinskey, A.J.; Weisman, M.; Bishop, W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J. Natl. Cancer Inst. 1974, 53, 79–84. [Google Scholar]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Wichrowska, D. The content of nitrate (V) in potato tubers storage in temperature 4 °C. Polish J. Nat. Sc. 2007, 4, 121–127. [Google Scholar]
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 2010, 118, 774–781. [Google Scholar] [CrossRef]
- Cefola, M.; Amodio, M.L.; Rinaldi, R.; Vanadia, S.; Colelli, G. Exposure to 1-methylcyclopropen (1-MCP) delays the effects of ethylene on fresh-cut broccoli raab (Brassica rapa L.). Postharvest Biol. Technol. 2010, 58, 29–35. [Google Scholar] [CrossRef]

| Broccoli in Class I | Broccoli in Class II |
|---|---|
The following slight defects, however, may be allowed, provided these do not affect the general appearance of the produce, quality, maintaining the quality and presentation in the package:
| class II includes broccoli that does not qualify for inclusion in class I but satisfy the minimum requirements specified above; broccoli may be: slightly loose and less compact, less tightly-grained; buds must be practically closed; the floral stem must be reasonably tender, and may have a trace of woodiness; The following defects may be allowed, provided broccoli retains its essential characteristics as regards the quality, maintaining the quality and presentation in the package: defects in shape defects in coloring slight bruising and injury. |
| Factors | After 10 Days | After 20 Days | After 30 Days | |
|---|---|---|---|---|
| Cultivars | Naxos | 1.86 ± 0.001 a | 3.22 ± 0.003 b | 3.70 ± 0.001 c |
| Monaco | 0.71 ± 0.002 d | 2.56 ± 0.001 c | 3.12 ± 0.002 d | |
| Vicario | 1.46 ± 0.001 c | 3.41 ± 0.004 a | 4.02 ± 0.002 a | |
| Bay Meadows | 1.61 ± 0.001 b | 3.40 ± 0.002 a | 3.88 ± 0.001 b | |
| Storage technology | NSF | 1.80 ± 0.002 A | 3.77 ± 0.001 A | 4.36 ± 0.003 A |
| SF | 1.02 ± 0.001 B | 2.53 ± 0.001 B | 3.01 ± 0.001 B | |
| Mean | 1.41 | 3.15 | 3.68 | |
| LSD p ≤ 0.05 Interaction Cultivar/storage technology | 0.007 | 0.008 | 0.103 | |
| Cultivar | Difference (Score %) ** | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | −1.9 | −0.4 | −1.5 | −1.6 | −7.6 | −2.3 |
| Monaco | −5.6 | −1.7 | −11.3 | −7.6 | −13.9 | −9.0 |
| Vicario | −5.9 | −3.2 | −12.4 | −6.8 | −19.1 | −11.5 |
| Bay Meadows | −7.1 | −1.2 | −9.1 | −0.3 | −14.3 | −3.4 |
| Means | −5.1 | −1.6 | −8.6 | −4.1 | −13.7 | −6.5 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | −9.5 | −12.6 | −25.5 | −12.0 | −30.8 | −21.8 |
| Monaco | −11.2 | +8.5 | −18.1 | −15.2 | −22.1 | −32.0 |
| Vicario | −22.8 | −24.1 | −24.8 | −18.8 | −29.0 | −22.3 |
| Bay Meadows | −14.4 | −7.6 | −21.7 | −20.5 | −30.8 | −28.7 |
| Means | −14.5 | −9.0 | −22.5 | −21.6 | −28.2 | −21.8 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | +9.3 | +4.1 | +10.4 | +12.4 | +21.0 | +16.5 |
| Monaco | +9.3 | +3.9 | +14.8 | +6.7 | +19.1 | +12.2 |
| Vicario | +12.6 | +2.8 | +16.8 | +9.2 | +21.9 | +15.2 |
| Bay Meadows | +5.2 | +2.1 | +14.8 | +8.9 | +24.0 | +13.6 |
| Means | +9.1 | +3.2 | +14.2 | +9.3 | +21.5 | +14.4 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | −30.4 | −19.2 | −36.9 | −21.9 | −41.9 | −24.2 |
| Monaco | −17.3 | −12.8 | −30.2 | −19.9 | −41.1 | −22.7 |
| Vicario | −30.8 | −8.3 | −39.2 | −10.5 | −48.2 | −25.4 |
| Bay Meadows | −23.0 | −12.7 | −24.7 | −16.0 | −42.5 | −21.3 |
| Means | −25.4 | −13.3 | −32.8 | −17.1 | −43.4 | −21.2 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | −6.5 | −0.8 | −8.5 | −1.2 | −10.1 | −2.9 |
| Monaco | −1.1 | −1.0 | −4.9 | −3.5 | −10.4 | −4.8 |
| Vicario | −6.8 | −1.1 | −12.6 | −2.9 | −14.7 | −4.1 |
| Bay Meadows | −4.5 | −1.8 | −5.4 | −2.2 | −7.0 | −2.8 |
| Means | −4.7 | −1.2 | −7.9 | −2.5 | −10.6 | −3.7 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | −2.1 | −3.7 | −4.1 | −5.6 | −15.9 | −16.2 |
| Monaco | −4.7 | −6.1 | −6.8 | −7.4 | −11.6 | −13.4 |
| Vicario | −6.9 | −9.2 | −7.8 | −11.0 | −11.7 | −12.6 |
| Bay Meadows | −2.2 | −1.5 | 3.0 | −3.6 | −5.9 | −6.1 |
| Means | −4.0 | −5.1 | −5.4 | −6.9 | −11.3 | −12.1 |
| Cultivar | Difference (Score %) | |||||
|---|---|---|---|---|---|---|
| After 10 Days of Storage | After 20 Days of Storage | After 30 Days of Storage | ||||
| NSF | SF | NSF | SF | NSF | SF | |
| Naxos | +14.4 | +14.2 | +16.7 | +14.9 | +36.8 | +19.4 |
| Monaco | +5.5 | +4.9 | +7.8 | +6.4 | +17.6 | +13.3 |
| Vicario | +4.5 | +0.7 | +5.5 | +2.7 | +17.6 | +13.1 |
| Bay Meadows | +3.1 | +2.4 | +4.0 | +4.7 | +13.0 | +6.2 |
| Means | +6.9 | +5.6 | +8.5 | +7.2 | +21.3 | +13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichrowska, D.; Kozera, W.; Knapowski, T.; Prus, P.; Ligocka, A. Assessment of the Interactive Effect of the Use of 1-Methylcyclopropene and Cultivars on the Nutritional Value of Broccoli during Storage. Agronomy 2021, 11, 2575. https://doi.org/10.3390/agronomy11122575
Wichrowska D, Kozera W, Knapowski T, Prus P, Ligocka A. Assessment of the Interactive Effect of the Use of 1-Methylcyclopropene and Cultivars on the Nutritional Value of Broccoli during Storage. Agronomy. 2021; 11(12):2575. https://doi.org/10.3390/agronomy11122575
Chicago/Turabian StyleWichrowska, Dorota, Wojciech Kozera, Tomasz Knapowski, Piotr Prus, and Anna Ligocka. 2021. "Assessment of the Interactive Effect of the Use of 1-Methylcyclopropene and Cultivars on the Nutritional Value of Broccoli during Storage" Agronomy 11, no. 12: 2575. https://doi.org/10.3390/agronomy11122575
APA StyleWichrowska, D., Kozera, W., Knapowski, T., Prus, P., & Ligocka, A. (2021). Assessment of the Interactive Effect of the Use of 1-Methylcyclopropene and Cultivars on the Nutritional Value of Broccoli during Storage. Agronomy, 11(12), 2575. https://doi.org/10.3390/agronomy11122575









