The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Tree Density and Experimental Design
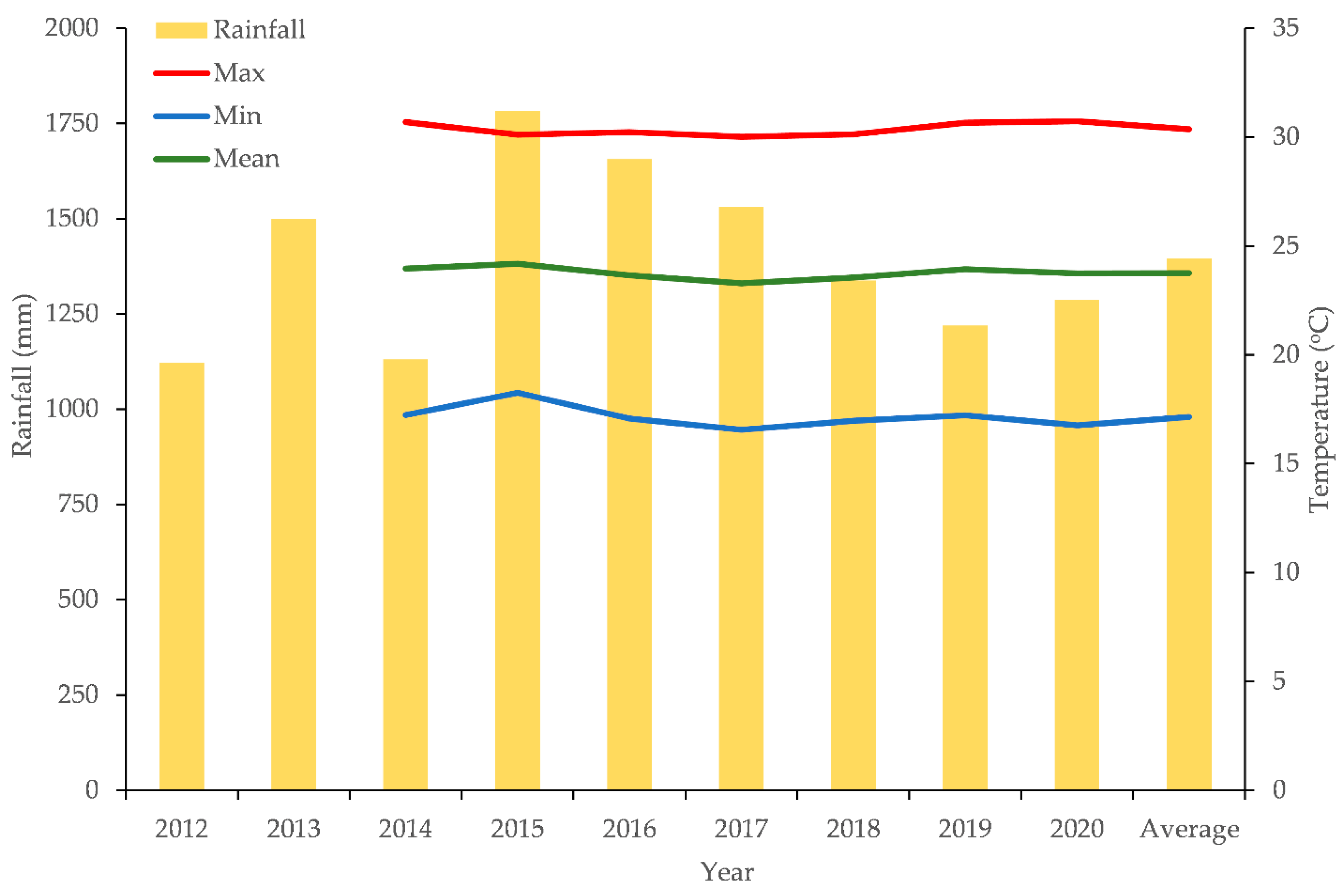
2.3. Experimental Area and Tree Care Conditions
2.4. Tree Size and Row Volume (RV) Estimation
2.5. FY per Tree and Productivity per Hectare
2.6. Production Efficiency and Land Use
2.7. Fruit Quality
2.8. Harvesting Efficiency
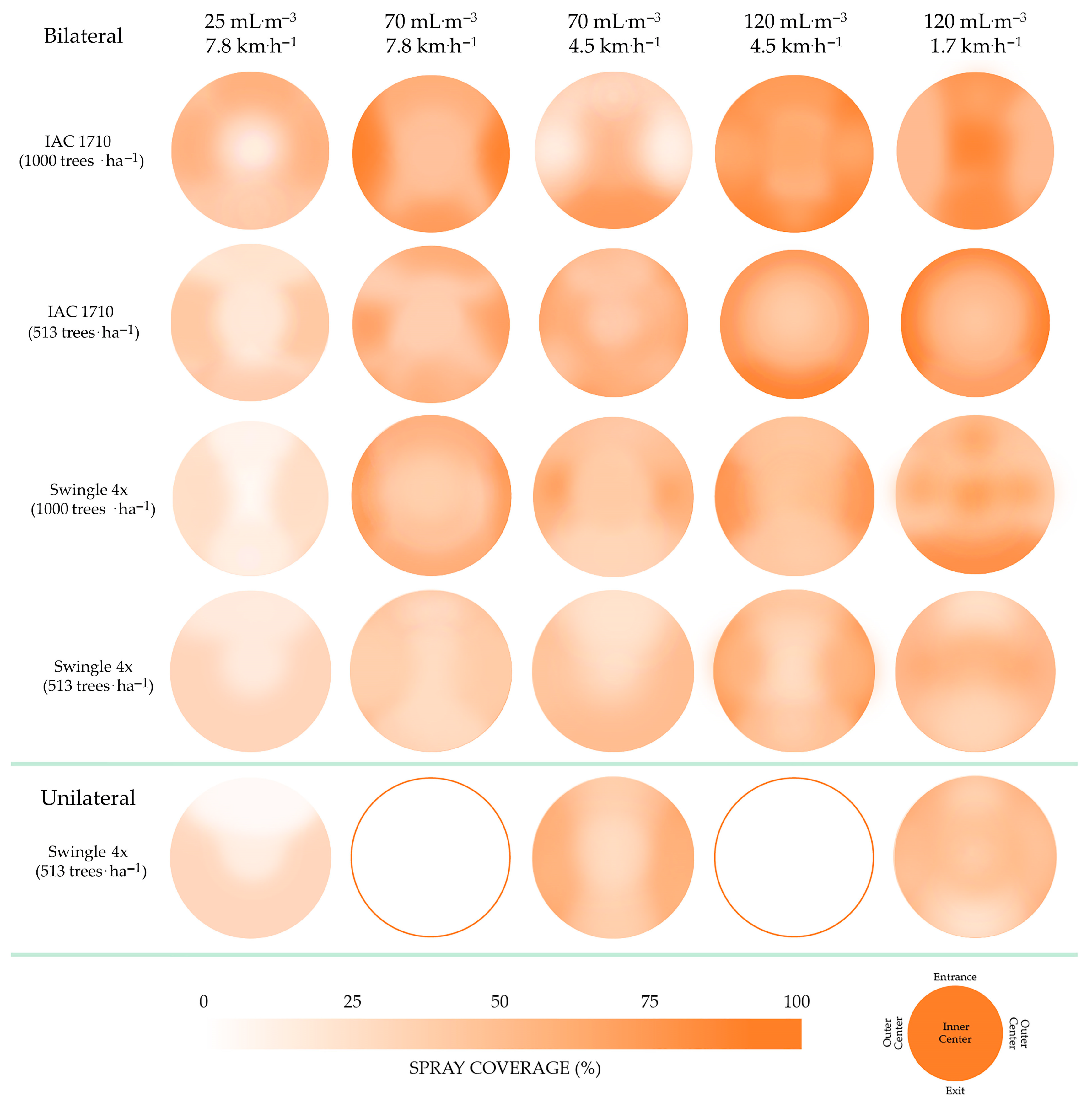
2.9. Spray Coverage
2.10. HLB Incidence
2.11. Statistical Analyses
3. Results
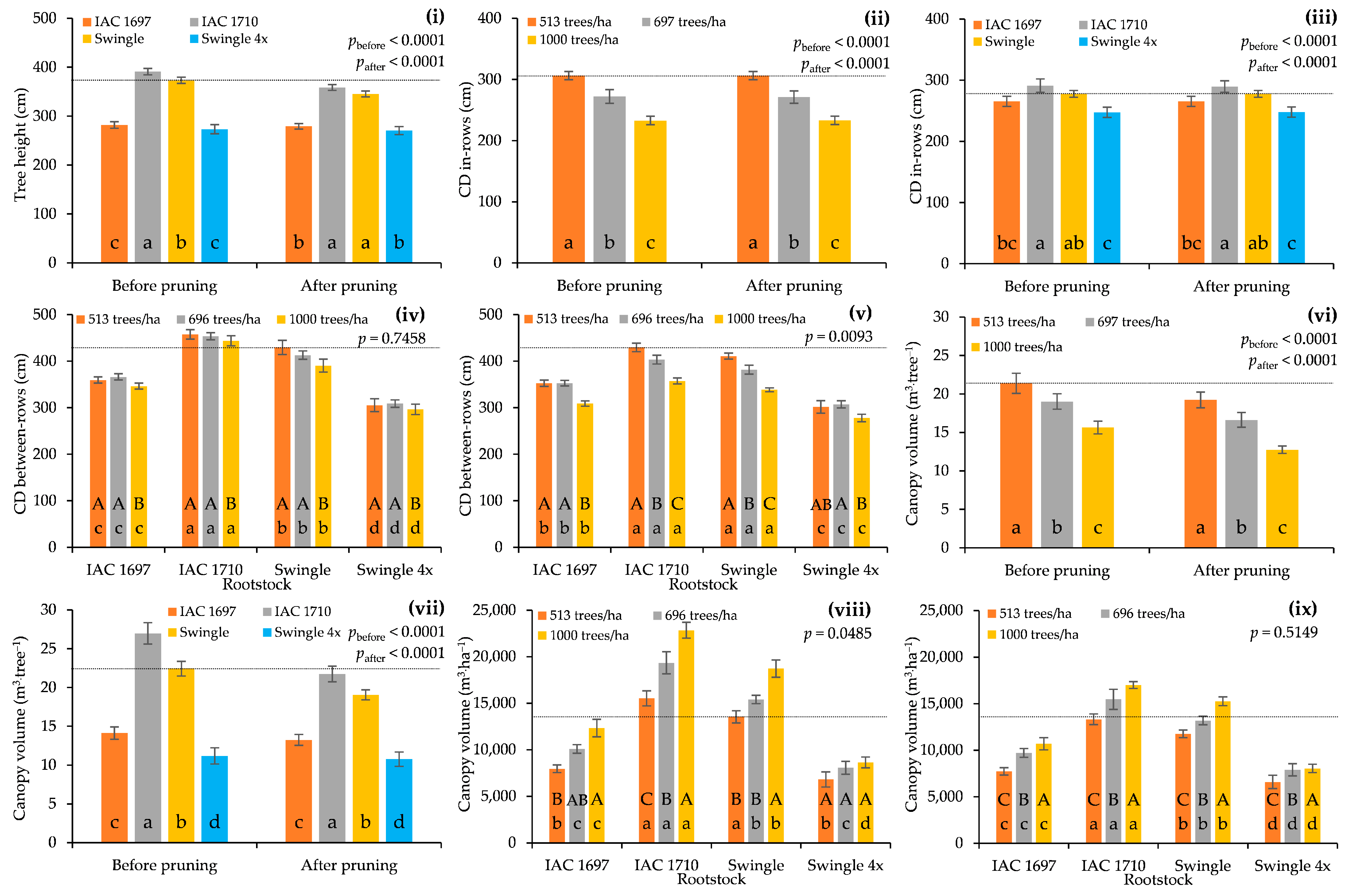
3.1. Tree Size and RV Estimation
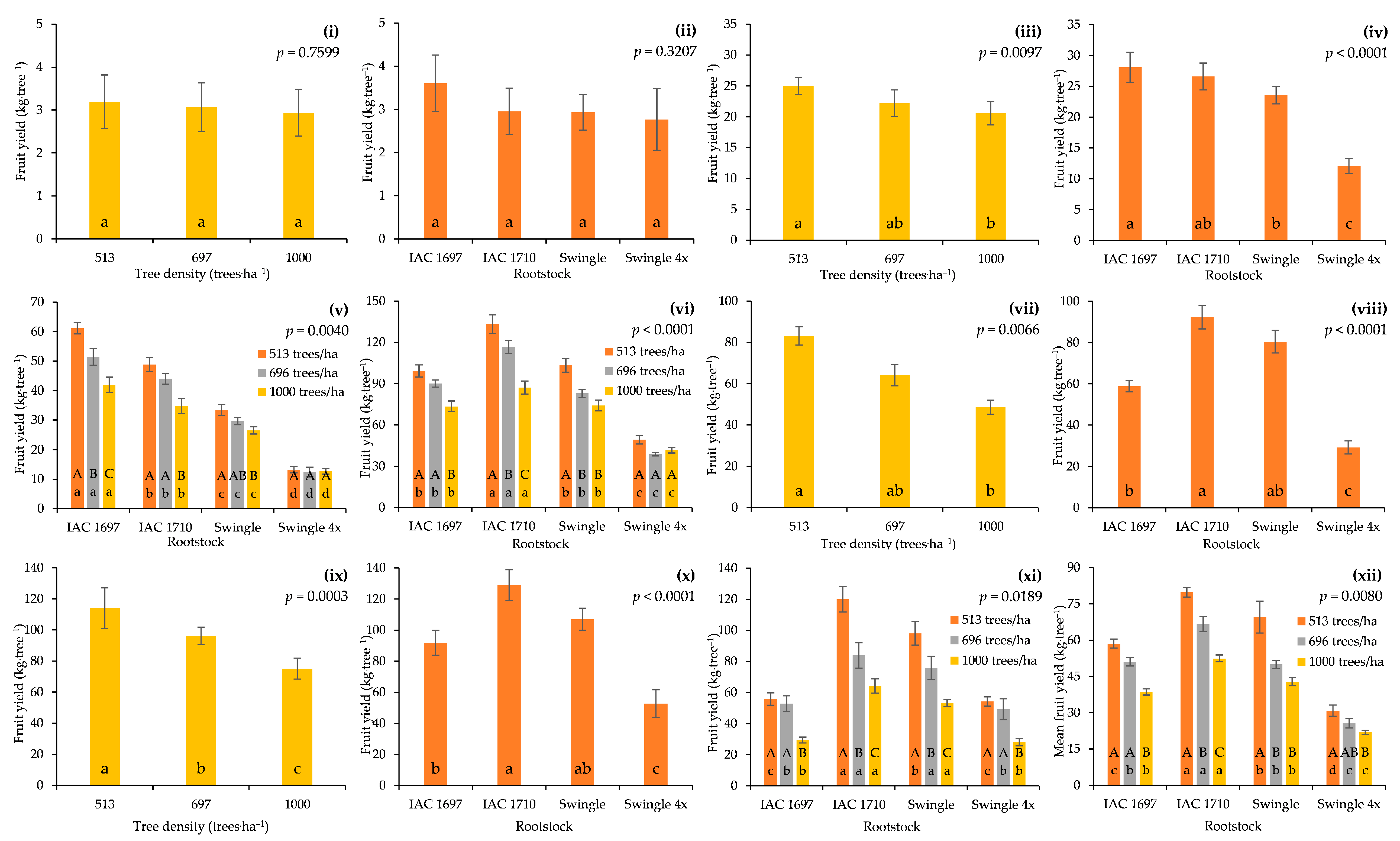
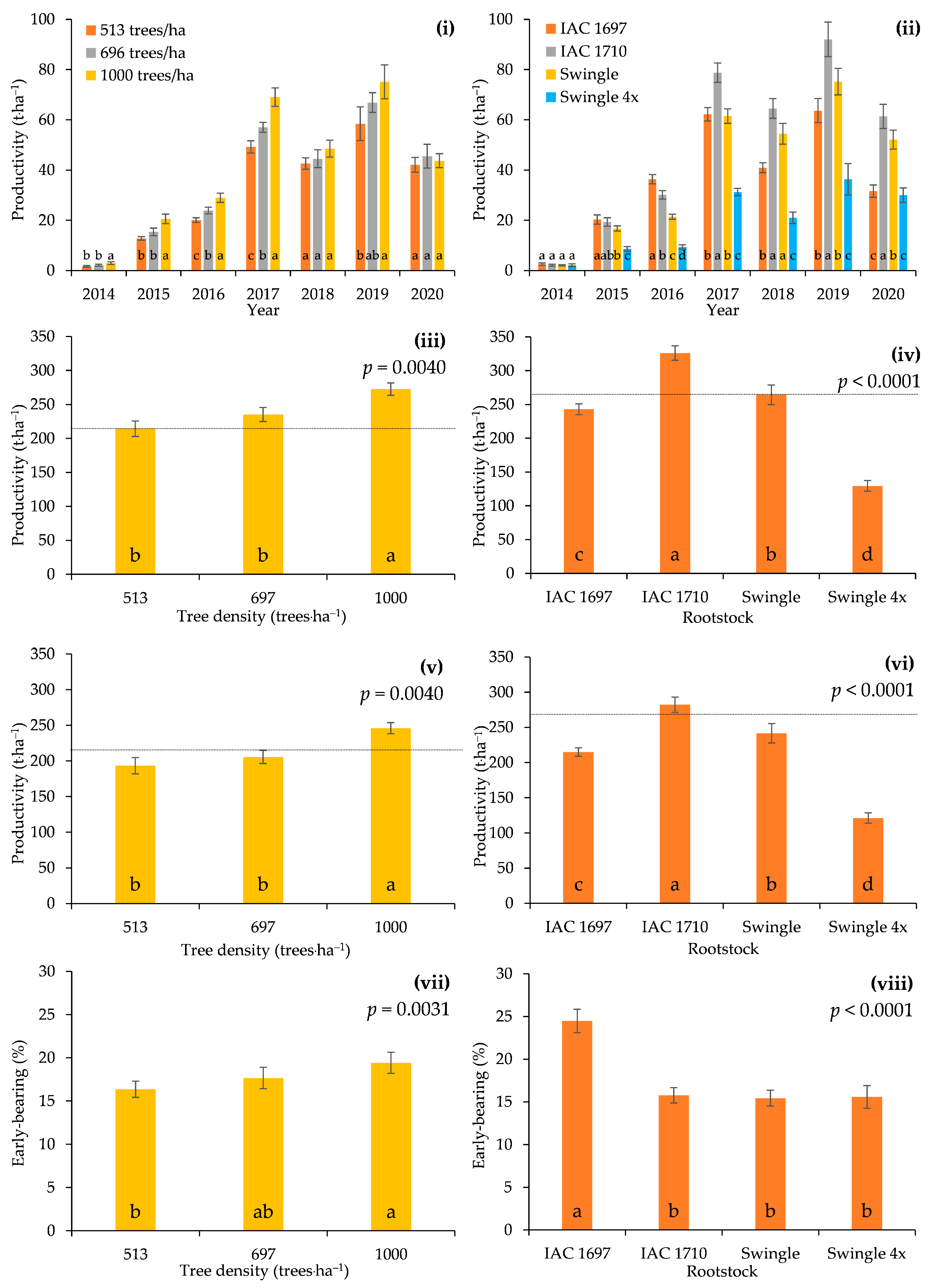
3.2. FY per Tree and FP per Hectare
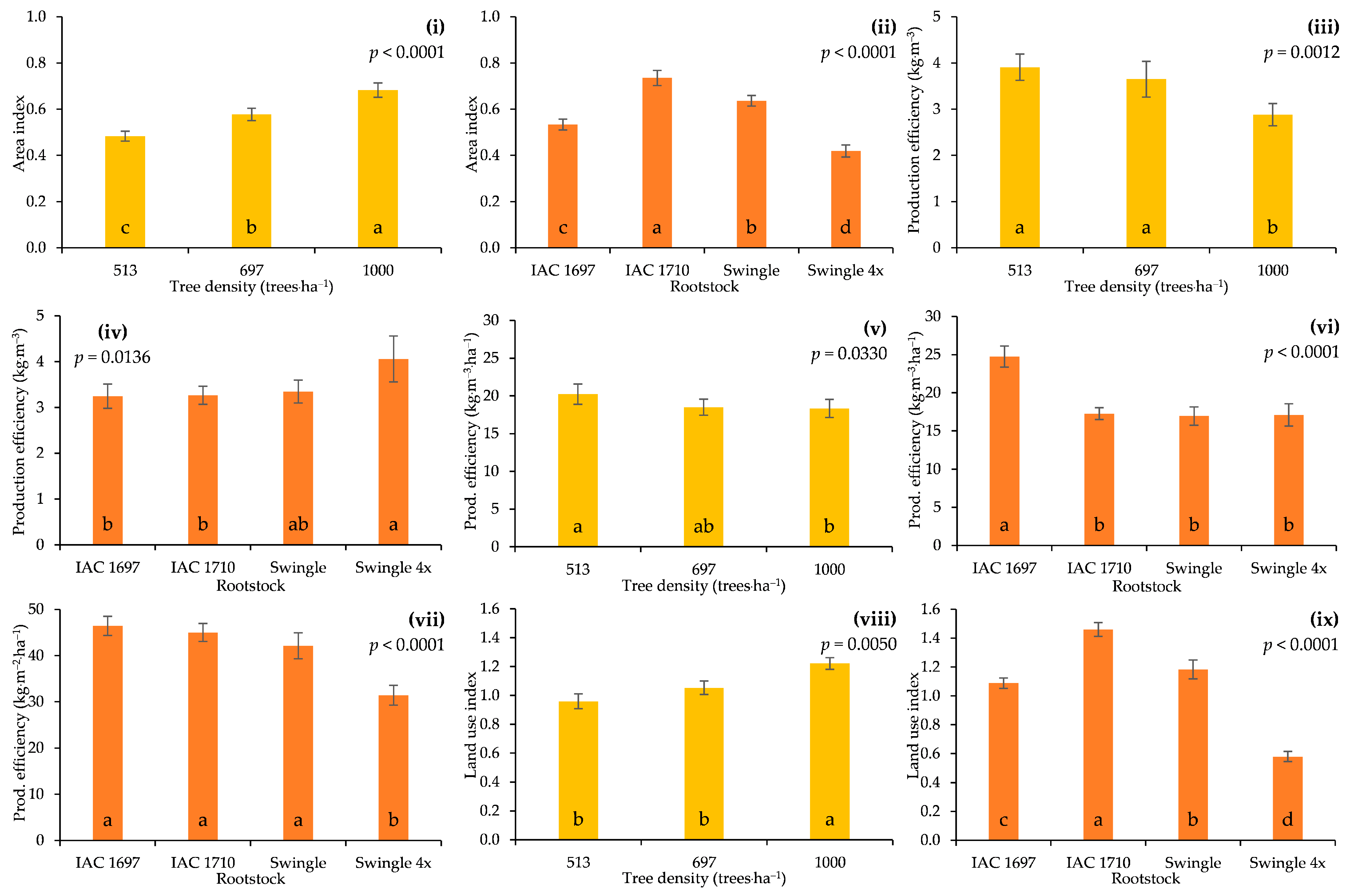
3.3. Production Efficiency and Land Use
3.4. Fruit Quality
3.5. Efficiency of Harvesting
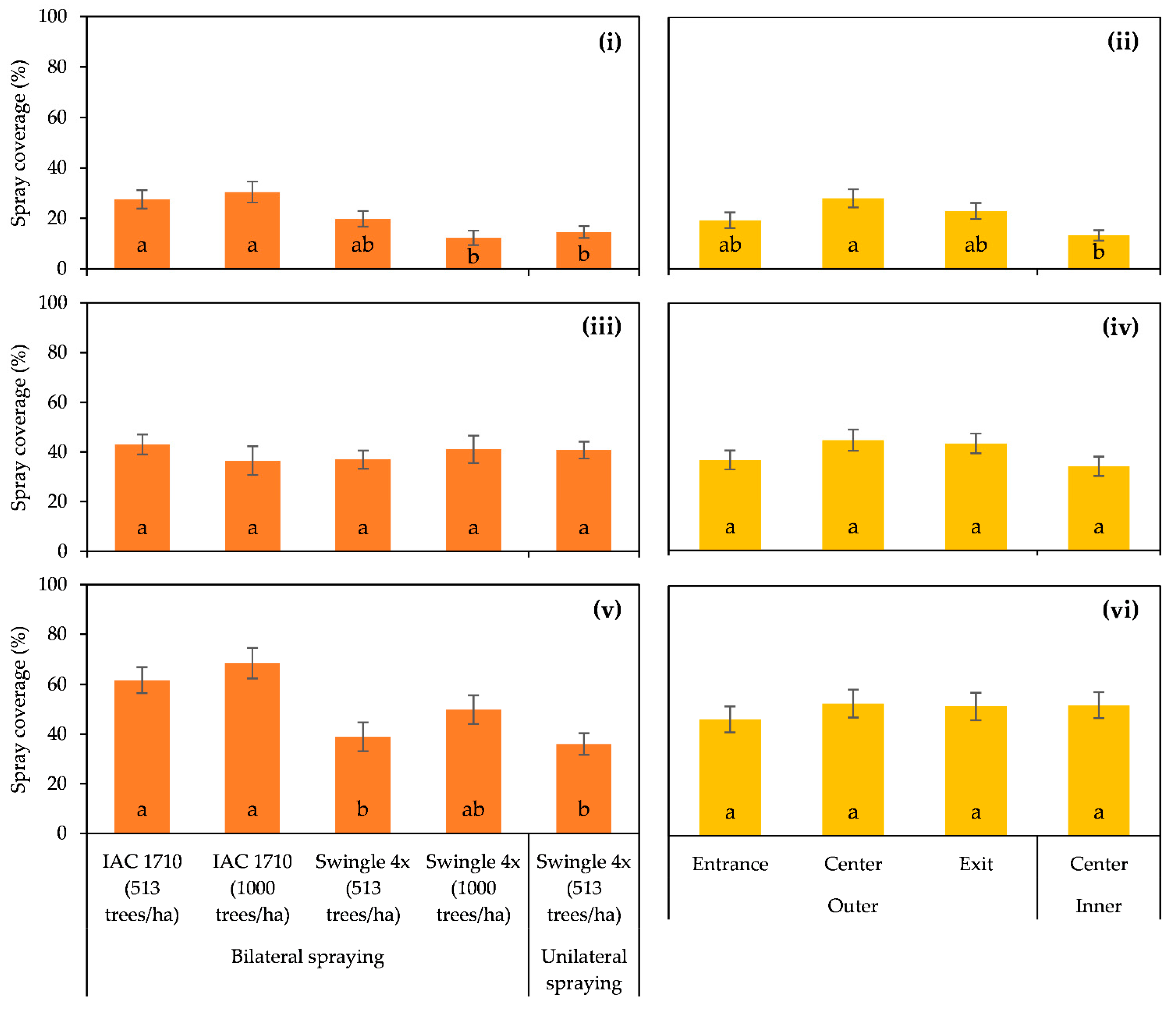
3.6. Spray Coverage
3.7. HLB Incidence
4. Discussion
4.1. Increasing the Tree Density Works Best, as Long as Highly Productive Rootstocks Are Available
4.2. The Rootstock, Rather Than the Tree Density, Is Decisive for Sweet Orange Quality for Processing
4.3. Cropping Practices Can Benefit from Ultra-High Pedestrian Orchards
4.4. Intensively Managed, Improved Rootstocks Increase the Efficiency of Land Use in Citriculture
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Rootstock (Spraying System) | Tree Spacing In-Rows Between-Rows | Tree Row Volume | Spray Volume | Speed | Number of Nozzles | Application Flow | Nozzle Type 1 | Pressure | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (m) | (m3·ha−1) | (mL·m−3) | (L·ha−1) | (km·h−1) | (L·min−1·nozzle−1) | (psi) | ||||
| IAC 1710 (bilateral) | 5.00 | 2.00 | 28,682 | 25 | 717 | 7.8 | 52 | 0.896 | AD2/AC25 | 116 |
| 70 | 2008 | 4.5 | 52 | 1.448 | AD4/AC25 | 101 | ||||
| 70 | 2008 | 7.8 | 52 | 2.510 | AD5/AC25 | 158 | ||||
| 120 | 3442 | 1.7 | 52 | 0.938 | AD2/AC25 | 127 | ||||
| 120 | 3442 | 4.5 | 52 | 2.482 | AD5/AC25 | 154 | ||||
| 6.50 | 3.00 | 21,225 | 25 | 531 | 7.8 | 52 | 0.862 | AD2/AC25 | 108 | |
| 70 | 1486 | 4.5 | 52 | 1.393 | AD3/AC25 | 189 | ||||
| 70 | 1486 | 7.8 | 52 | 2.414 | AD5/AC25 | 146 | ||||
| 120 | 2547 | 1.7 | 52 | 0.902 | AD2/AC25 | 118 | ||||
| 120 | 2547 | 4.5 | 52 | 2.388 | AD5/AC25 | 143 | ||||
| Swingle 4× (bilateral) | 5.00 | 2.00 | 11,048 | 25 | 276 | 7.8 | 30 | 0.598 | AD2/AC23 | 128 |
| 70 | 773 | 4.5 | 30 | 0.967 | AD2/AC25 | 135 | ||||
| 70 | 773 | 7.8 | 30 | 1.676 | AD4/AC25 | 135 | ||||
| 120 | 1326 | 1.7 | 30 | 0.626 | AD2/AC23 | 140 | ||||
| 120 | 1326 | 4.5 | 30 | 1.657 | AD4/AC25 | 132 | ||||
| 6.50 | 3.00 | 7147 | 25 | 179 | 7.8 | 30 | 0.503 | AD2/AC23 | 90 | |
| 70 | 500 | 4.5 | 30 | 0.813 | AD2/AC25 | 96 | ||||
| 70 | 500 | 7.8 | 30 | 1.409 | AD3/AC25 | 193 | ||||
| 120 | 858 | 1.7 | 30 | 0.526 | AD2/AC23 | 99 | ||||
| 120 | 858 | 4.5 | 30 | 1.394 | AD3/AC25 | 189 | ||||
| Swingle 4× (unilateral) | 25 | 179 | 7.8 | 15 | 0.503 | AD2/AC23 | 90 | |||
| 6.50 | 3.00 | 7147 | 70 | 500 | 4.5 | 15 | 0.813 | AD2/AC25 | 96 | |
| 120 | 858 | 1.7 | 15 | 0.526 | AD2/AC23 | 99 | ||||
| Tree Density | FW | SS | TA | MI 1 | TI 2 | JC | IY 3 | SSY 4 |
|---|---|---|---|---|---|---|---|---|
| (Trees·ha−1) | (g) | (°Brix) | (%) | (kg SS·Box−1) | (%) | (Boxes·t−1) | (kg SS·ha−1) | |
| 2017 | ||||||||
| 513 | 177 a | 12.63 a | 0.77 a | 16.94 a | 2.98 a | 57.94 a | 222 a | 3536 c |
| 697 | 167 a | 12.87 a | 0.81 a | 16.19 a | 2.99 a | 57.02 a | 221 a | 4102 b |
| 1000 | 170 a | 12.89 a | 0.78 a | 16.68 a | 3.07 a | 58.50 a | 216 a | 4891 a |
| Rootstock | ||||||||
| IAC 1697 | 160 b | 13.30 a | 0.81 a | 16.61 a | 3.21 a | 59.10 a | 206 c | 4790 ab |
| IAC 1710 | 187 a | 11.67 c | 0.67 b | 17.45 a | 2.79 c | 58.61 ab | 237 a | 5100 a |
| Swingle | 178 a | 12.60 b | 0.84 a | 15.29 a | 2.95 b | 57.44 bc | 224 b | 4464 b |
| Swingle 4× | 159 b | 13.61 a | 0.82 a | 17.05 a | 3.11 a | 56.13 c | 212 c | 2352 c |
| p-values | ||||||||
| Density (D) | 0.0914 | 0.2097 | 0.4364 | 0.5373 | 0.0851 | 0.0602 | 0.1090 | 0.0002 |
| Rootstock (R) | <0.0001 | <0.0001 | 0.0022 | 0.0760 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D × R | 0.5685 | 0.666 | 0.5361 | 0.6836 | 0.8311 | 0.0597 | 0.8074 | 0.3698 |
| CV% (D) | 6.39 | 3.12 | 10.29 | 11.07 | 3.37 | 2.38 | 3.57 | 9.02 |
| CV% (R) | 7.80 | 3.06 | 13.45 | 12.20 | 3.06 | 2.12 | 3.37 | 10.49 |
| Tree density (trees·ha−1) | 2018 | |||||||
| 513 | 242 a | 10.99 a | 0.82 a | 13.64 a | 2.18 a | 48.48 a | 306 a | 1978 b |
| 697 | 230 a | 11.23 a | 0.87 a | 13.19 a | 2.18 a | 47.55 a | 305 a | 2396 ab |
| 1000 | 234 a | 11.31 a | 0.89 a | 13.02 a | 2.21 a | 47.81 a | 302 a | 2563 a |
| Rootstock | ||||||||
| IAC 1697 | 220 b | 11.82 a | 0.89 a | 13.43 ab | 2.43 a | 50.42 a | 272 b | 2388 b |
| IAC 1710 | 232 ab | 10.75 b | 0.76 b | 14.19 a | 2.16 b | 49.11 ab | 308 a | 3470 a |
| Swingle | 248 a | 10.94 b | 0.91 a | 12.25 b | 2.11 b | 47.12 bc | 316 a | 2389 b |
| Swingle 4× | 241 ab | 11.20 b | 0.87 ab | 13.25 ab | 2.06 b | 45.13 c | 322 a | 1003 c |
| p-values | ||||||||
| Density (D) | 0.3273 | 0.2560 | 0.2696 | 0.6841 | 0.8598 | 0.4797 | 0.8862 | 0.0241 |
| Rootstock (R) | 0.0101 | 0.0003 | 0.0130 | 0.0551 | <0.0001 | <0.0001 | 0.0001 | <0.0001 |
| D × R | 0.5345 | 0.8756 | 0.8059 | 0.9328 | 0.7700 | 0.6584 | 0.8162 | 0.9439 |
| CV% (D) | 8.82 | 4.44 | 12.84 | 15.16 | 7.78 | 4.36 | 8.54 | 19.18 |
| CV% (R) | 8.29 | 4.83 | 12.59 | 12.35 | 7.75 | 4.24 | 7.97 | 24.71 |
| Tree Density | FW | SS | TA | MI 1 | TI 2 | JC | IY 3 | SSY 4 |
|---|---|---|---|---|---|---|---|---|
| (Trees·ha−1) | (g) | (°Brix) | (%) | (kg SS·Box−1) | (%) | (Boxes·t−1) | (kg SS·ha−1) | |
| 2019 | ||||||||
| 513 | 212 a | 11.20 a | 0.59 a | 19.32 a | 2.50 b | 54.70 a | 266 a | 3668 b |
| 697 | 208 a | 11.47 a | 0.58 a | 19.76 a | 2.55 ab | 54.44 a | 261 ab | 4210 ab |
| 1000 | 192 b | 11.67 a | 0.60 a | 19.51 a | 2.64 a | 55.39 a | 252 b | 4877 a |
| Rootstock | ||||||||
| IAC 1697 | 183 c | 12.54 a | 0.62 a | 20.29 ab | 2.95 a | 57.57 a | 224 b | 4583 a |
| IAC 1710 | 199 b | 10.69 c | 0.61 a | 17.45 c | 2.43 b | 55.73 ab | 272 a | 5647 a |
| Swingle | 212 ab | 11.19 b | 0.60 a | 18.70 bc | 2.47 b | 54.20 b | 267 a | 4815 a |
| Swingle 4× | 222 a | 11.37 b | 0.53 b | 21.69 a | 2.41 b | 51.88 c | 275 a | 1962 b |
| p-values | ||||||||
| Density (D) | 0.0151 | 0.1016 | 0.7502 | 0.8529 | 0.0501 | 0.0540 | 0.0488 | 0.0320 |
| Rootstock (R) | <0.0001 | <0.0001 | 0.0013 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D × R | 0.4771 | 0.3575 | 0.0867 | 0.3126 | 0.2693 | 0.5327 | 0.1992 | 0.5011 |
| CV% (D) | 4.64 | 3.44 | 9.64 | 9.71 | 3.53 | 1.21 | 3.67 | 16.29 |
| CV% (R) | 5.75 | 3.15 | 7.13 | 8.56 | 4.72 | 2.93 | 4.81 | 19.11 |
| Tree density (trees·ha−1) | 2020 | |||||||
| 513 | 212 a | 12.44 a | 0.65 a | 19.38 a | 3.15 a | 61.99 a | 211 a | 3162 a |
| 697 | 196 a | 12.72 a | 0.67 a | 19.48 a | 3.19 a | 61.42 a | 208 a | 3652 a |
| 1000 | 193 a | 12.69 a | 0.71 a | 18.07 a | 3.14 a | 60.75 a | 211 a | 3315 a |
| Rootstock | ||||||||
| IAC 1697 | 189 b | 13.18 a | 0.75 a | 17.83 b | 3.28 a | 60.91 a | 202 a | 2563 b |
| IAC 1710 | 191 b | 12.23 b | 0.65 ab | 18.81 ab | 3.06 a | 61.34 a | 217 a | 4377 a |
| Swingle | 203 ab | 12.62 ab | 0.67 ab | 19.14 ab | 3.20 a | 62.12 a | 207 a | 4113 a |
| Swingle 4× | 218 a | 12.44 ab | 0.64 b | 20.13 a | 3.10 a | 61.18 a | 214 a | 2452 b |
| p-values | ||||||||
| Density (D) | 0.1557 | 0.6916 | 0.5500 | 0.3949 | 0.9173 | 0.3950 | 0.9239 | 0.4181 |
| Rootstock (R) | <0.0001 | 0.0123 | 0.0345 | 0.0671 | 0.0675 | 0.5341 | 0.0826 | <0.0001 |
| D × R | 0.6294 | 0.5114 | 0.6651 | 0.8442 | 0.9436 | 0.5396 | 0.9425 | 0.0909 |
| CV% (D) | 12.89 | 7.88 | 24.02 | 15.92 | 10.27 | 3.87 | 10.31 | 29.55 |
| CV% (R) | 6.99 | 5.38 | 13.28 | 10.56 | 6.51 | 3.42 | 6.98 | 16.34 |


References
- Food and Agriculture Organization of the United Nations-FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 September 2021).
- Singerman, A.; Rogers, M.E. The economic challenges of dealing with citrus greening: The case of Florida. J. Integr. Pest Manag. 2020, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in Sao Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Girardi, E.A.; Mourão Filho, F.A.A.; Ferrarezi, R.S.; Colleta-Filho, H.D. Advances in citrus propagation in Brazil. Rev. Bras. De Frutic. 2019, 41, e-422. [Google Scholar] [CrossRef] [Green Version]
- Girardi, E.A.; Cerqueira, T.S.; Cantuarias-Avilés, T.E.; Silva, S.R.; Stuchi, E.S. Sunki mandarin and Swingle citrumelo as rootstocks for rain-fed cultivation of late-season sweet orange selections in northern São Paulo state, Brazil. Bragantia 2017, 76, 501–511. [Google Scholar] [CrossRef]
- Girardi, E.A.; Pompeu Junior, J.; Teofilo Sobrinho, J.; Soares Filho, W.S.; Passos, O.S.; Cristofani-Yaly, M.; Sempionato, O.R.; Stuchi, E.S.; Donadio, L.C.; Mattos Junior, D.; et al. Guia de Reconhecimento dos Citros em Campo: Um Guia Prático Para o Reconhecimento em Campo de Variedades de Laranjeira-Doce e Outras Espécies de Citros Cultivadas no Estado de São Paulo e Triângulo Mineiro; Fundecitrus: Araraquara, Brazil, 2021; 158p. [Google Scholar]
- Azevedo, F.A.; Almeida, R.F.; Martinelli, R.; Próspero, A.G.; Licerre, R.; Conceição, P.M.; Arantes, A.C.C.; Dovis, V.L.; Boaretto, R.M.; Mattos Junior, D. No-tillage and high-density planting for Tahiti acid lime grafted onto Flying Dragon trifoliate orange. Front. Sustain. Food Syst. 2020, 4, 108. [Google Scholar] [CrossRef]
- Mattos Junior, D.; Quaggio, J.A.; Cantarella, H.; Boaretto, R.M.; Zambrosi, F.C.B. Nutrient management for high citrus fruit yield in tropical soils. Better Crop. Plant Food 2012, 96, 4–7. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1068.9250&rep=rep1&type=pdf (accessed on 4 March 2021).
- Bassanezi, R.B.; Lopes, S.A.; Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Behlau, F.; Belasque, J.; Leite, R.P.; Bergamin Filho, A.; Gottwald, T.R.; Graham, J.H.; Scandelai, L.H.M.; Primiano, I.V.; Bassanezi, R.B.; Ayres, A.J. Relative contribution of windbreak, copper sprays, and leafminer control for citrus canker management and prevention of crop loss in sweet orange trees. Plant Dis. 2021, 105. [Google Scholar] [CrossRef]
- Lanza, F.E.; Metzker, T.G.; Vinhas, T.; Behlau, F.; Silva Junior, G.J. Critical fungicide spray period for citrus black spot control in São Paulo State, Brazil. Plant Dis. 2018, 102, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Perondi, D.; Fraisse, C.W.; Dewdney, M.M.; Cerbaro, V.A.; Andreis, J.H.D.; Gama, A.B.; Silva Junior, G.J.; Amorim, L.; Pavan, W.; Peres, N.A. Citrus advisory system: A web-based postbloom fruit drop disease alert system. Comput. Electron. Agric. 2020, 178, 105781. [Google Scholar] [CrossRef]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P.A.; Lopes, J.R.S. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behaviour of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef]
- Fundo de Defesa da Citricultura. Tree Inventory of the São Paulo and West-Southwest Minas Gerais Citrus Belt: Snapshot of Groves in March 2021; Fundo de Defesa da Citricultura: Araraquara, Brazil, 2021; 143p, Available online: https://www.fundecitrus.com.br/pdf/pes_relatorios/2021_07_30_Tree_Inventory_and_Orange_Crop_Forecast_2021-2022_Plantio_2020_Revisado.pdf (accessed on 4 March 2021).
- Silveira, L.K.; Pavão, G.C.; Dias, C.T.S.; Quaggio, J.A.; Pires, R.C.M. Deficit irrigation effect on fruit yield, quality and water use efficiency: A long-term study on Pêra-IAC sweet orange. Agric. Water Manag. 2020, 231, 106019. [Google Scholar] [CrossRef]
- Fundo de Defesa da Citricultura. Tree Inventory of the São Paulo and West-Southwest Minas Gerais Citrus Belt: Snapshot of Groves in March 2018; Fundo de Defesa da Citricultura: Araraquara, Brazil, 2018; 144p, Available online: https://www.fundecitrus.com.br/pdf/pes_relatorios/Tree_Inventory_and_Orange_Crop_Forecast_2018-2019.pdf (accessed on 4 March 2021).
- Fundo de Defesa da Citricultura. Levantamento da Incidência das Doenças dos Citros: Greening, CVC e Cancro Cítrico no Cinturão Citrícola de São Paulo e Triângulo/Sudoeste Mineiro 2021; Fundo de Defesa da Citricultura: Araraquara, Brazil, 2021; 77p, Available online: https://www.fundecitrus.com.br/pdf/levantamentos/Relatorio_levantamento_de_doencas_2021-greening_CVC_e_cancro_citrico.pdf (accessed on 27 October 2021).
- Wheaton, T.A.; Whitney, J.D.; Castle, W.S.; Muraro, R.P.; Browning, H.W.; Tucker, D.P.H. Citrus scion and rootstock, topping height, and tree spacing affect tree size, yield, fruit quality, and economic return. J. Am. Soc. Hortic. Sci. 1995, 120, 861–870. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Jani, A.D.; James III, H.D.; Gil, C.; Ritenour, M.A.; Wright, A.L. Sweet orange orchard architecture design, fertilizer, and irrigation management strategies under huanglongbing-endemic conditions in the Indian River citrus district. HortScience 2020, 55, 2028–2036. [Google Scholar] [CrossRef]
- Singerman, A.; Burani-Arouca, M.; Futch, S.H. The profitability of new citrus plantings in Florida in the era of huanglongbing. HortScience 2018, 53, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.S.; Stuchi, E.S.; Silva, P.R.B.; Bassanezi, R.B.; Girardi, E.A.; Laranjeira, F.F. Could tree density play a role in managing Citrus Huanglongbing epidemics? Trop. Plant Pathol. 2019, 44, 268–274. [Google Scholar] [CrossRef]
- Ladaniya, M.S.; Marathe, R.A.; Murkute, A.A.; Huchche, A.D.; Das, A.K.; George, A.; Kolwadkar, J. Response of Nagpur mandarin (Citrus reticulata Blanco) to high density planting systems. Sci. Rep. 2021, 11, 10845. [Google Scholar] [CrossRef]
- Cronje, R.; Human, C.; Ratlapane, I. Pruning strategies for young ‘Nadorcott’ mandarin trees planted in high density orchards in South Africa. Int. J. Fruit Sci. 2021, 21, 921–931. [Google Scholar] [CrossRef]
- Joubert, F.J.; du Plessis, M.H.; Steenkamp, E.D.; Stassen, P.J.C. Manipulation of citrus trees for new higher density orchards. J. Appl. Hortic. 2002, 4, 17–20. Available online: http://www.horticultureresearch.net/jah/2002_4_1_17_20.PDF (accessed on 4 March 2021). [CrossRef]
- Hamido, S.A.; Morgan, K.T. Effect of various irrigation rates on growth and root development of young citrus trees in high-density planting. Plants 2020, 9, 1462. [Google Scholar] [CrossRef]
- Mademba-Sy, F.; Lemerre-Desprez, Z.; Lebegin, S. Use of ‘Flying Dragon’ trifoliate orange as dwarfing rootstock for citrus under tropical climatic conditions. Hortscience 2012, 47, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Arenas, F.J.; González-Chimeno, A.B.; Romero-Rodríguez, E.; Casado, G.; Bordas, M.; Torrents, J.; Hervalejo, A. Adaptation of two citrus cultivars grafted on Forner-Alcaide Nº 517 to super high-density system and evaluation of mechanized harvesting. Citrus Res. Technol. 2016, 37, 122–131. [Google Scholar] [CrossRef]
- Díez, C.M.; Moral, J.; Cabello, D.; Morello, P.; Rallo, L.; Barranco, D. Cultivar and tree density as key factors in the long-term performance of super high-density olive orchards. Front. Plant Sci. 2016, 7, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig, G.; Lordan, J.; Sazo, M.M.; Hoying, S.; Fargione, M.; Reginato, G.; Donahue, D.J.; Francescatto, P.; Fazio, G.; Robinson, T. Long-term performance of ‘Gala’, Fuji’ and ‘Honeycrisp’ apple trees grafted on Geneva® rootstocks and trained to four production systems under New York State climatic conditions. Sci. Hortic. 2019, 244, 277–293. [Google Scholar] [CrossRef]
- Adiga, D.J.; Veena, G.L.; Thondaiman, V.; Babli, M. An overview of canopy management in cashew (Anacardium occidentale L.). J. Hortic. Sci. 2020, 15, 127–135. Available online: https://jhs.iihr.res.in/index.php/jhs/article/view/905/556 (accessed on 4 August 2021). [CrossRef]
- Stuchi, E.S.; Donadio, L.C.; Sempionato, O.R. Performance of Tahiti lime on Poncirus trifoliata var. monstrosa Flying Dragon in four densities. Fruits 2003, 58, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Hervalejo, A.; Suárez, M.P.; Arenas-Arenas, F.J. Substandard and semi-dwarfing citrus rootstocks for more intensive, higher-density, and sustainable plantation systems. Agronomy 2021, 11, 660. [Google Scholar] [CrossRef]
- Costa, D.P.; Stuchi, E.S.; Girardi, E.A.; Moreira, A.S.; Gesteira, A.S.; Coelho Filho, M.A.; Ledo, C.A.S.; Silva, A.L.V.; Leão, H.C.; Passos, O.S.; et al. Less is more: A hard way to get potential dwarfing hybrid rootstocks for Valencia sweet orange. Agriculture 2021, 11, 354. [Google Scholar] [CrossRef]
- Schinor, E.H.; Cristofani-Yaly, M.; Bastianel, M.; Machado, M.A. Sunki mandarin vs. Poncirus trifoliata hybrids as rootstocks for Pera sweet orange. J. Agric. Sci. 2013, 5, 190–200. [Google Scholar] [CrossRef]
- Grosser, J.W.; Gmitter, F.G., Jr. Protoplast fusion for production of tetraploids and triploids: Applications for scion and rootstock breeding in citrus. Plant Cell Tissue Organ Cult. 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Kunwar, S.; Grosser, J.; Gmitter, F.G., Jr.; Castle, W.S.; Albrecht, U. Field performance of ‘Hamlin’ orange trees grown on various rootstocks in Huanglongbing-endemic conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Fagoaga, C.; Tadeo, F.R.; Iglesias, D.J.; Huerta, L.; Lliso, I.; Vidal, A.M.; Talon, M.; Navarro, L.; Martínez, J.L.G.; Pena, L. Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA 20-oxidase gene modifies plant architecture. J. Exp. Bot. 2007, 58, 1407–1420. [Google Scholar] [CrossRef] [Green Version]
- Aznar-Sánchez, J.A.; Piquer-Rodríguez, M.; Velasco-Muñoz, J.F.; Manzano-Agugliaro, F. Worldwide research trends on sustainable land use in agriculture. Land Use Policy 2019, 9. [Google Scholar] [CrossRef]
- Dias, L.C.P.; Pimenta, F.M.; Santos, A.B.; Costa, M.H.; Ladle, R.J. Patterns of land use, extensification, and intensification of Brazilian agriculture. Glob. Chang. Biol. 2016, 22, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.P.; Schmitz, C.; Lotze-Campen, H.; Popp, A.; Müller, C. Forecasting technological change in agriculture—An endogenous implementation in a global land use model. Technol. Forecast. Soc. 2014, 81, 236–249. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, P.; Long, S.P.; Smith, P.; Banwart, S.A.; Beerling, D.J. Technologies to deliver food and climate security through agriculture. Nat. Plants 2021, 7, 250–255. [Google Scholar] [CrossRef]
- Balmford, A.; Amano, T.; Bartlett, H.; Chadwick, D.; Collins, A.; Edwards, D.; Field, R.; Garnsworthy, P.; Green, R.; Smith, P.; et al. The environmental costs and benefits of high-yield farming. Nat. Sustain. 2018, 1, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fundo de Defesa da Citricultura. Tree Inventory of the São Paulo and West-Southwest Minas Gerais Citrus Belt: Snapshot of Groves in March 2020; Fundo de Defesa da Citricultura: Araraquara, Brazil, 2021; 141p, Available online: https://www.fundecitrus.com.br/pdf/pes_relatorios/2020_06_25_Tree_Inventory_and_Orange_Crop_Forecast_2020-2021.pdf (accessed on 4 March 2021).
- Pompeu Junior, J.; Laranjeira, F.F.; Blumer, S. Laranjeiras Valência enxertadas em híbridos de trifoliata. Sci. Agric. 2002, 59, 93–97. [Google Scholar] [CrossRef]
- Pompeu Junior, J.; Blumer, S. Híbridos de trifoliata como porta-enxertos para laranjeira Pêra. Pesqui. Agropecuária Trop. 2014, 44, 9–14. [Google Scholar] [CrossRef]
- Blumer, S.; Pompeu Junior, J. Avaliação de citrandarins e outros híbridos de trifoliata como porta-enxertos para citros em São Paulo. Rev. Bras. Frutic. 2005, 27, 264–267. [Google Scholar] [CrossRef]
- Calvez, L.; Dereeper, A.; Mournet, P.; Froelicher, Y.; Bruyère, S.; Morillon, R.; Ollitrault, P. Intermediate inheritance with disomic tendency in tetraploid intergeneric Citrus × Poncirus hybrids enhances the efficiency of citrus rootstock breeding. Agronomy 2020, 10, 1961. [Google Scholar] [CrossRef]
- Ruiz, M.; Oustric, J.; Santini, J.; Morillon, R. Synthetic polyploidy in grafted crops. Front. Plant Sci. 2020, 11, 540894. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.; Wittmann, M.T.S.; Schwarz, S.F.; De Souza, P.V.D.; Gonzatto, M.P.; Weiler, R.L. Comparison between diploid and tetraploid citrus rootstocks: Morphological characterization and growth evaluation. Bragantia 2014, 73, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Peña, L.; Pérez, R.M.; Cervera, M.; Juárez, J.A.; Navarro, L. Early Events in Agrobacterium-mediated genetic transformation of citrus explants. Ann. Bot. 2004, 94, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Rolim, G.S.; Camargo, M.B.P.; Lania, D.G.; Moraes, J.F.L. Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas agroclimáticas para o estado de São Paulo. Bragantia 2007, 66, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M. Mapa pedológico do Estado de São Paulo: Revisado e ampliado; Instituto Florestal: São Paulo, Brazil, 2017; 118p, Available online: https://smastr16.blob.core.windows.net/iflorestal/2017/11/Livro_Solos1.pdf (accessed on 4 March 2021).
- Mattos Júnior, D.; De Negri, J.D.; Pompeu Júnior, J.; Ghilardi, A.A.; Azevedo, F.A.; Bastianel, M. Citros. Instruções Agrícolas Para as Principais Culturas Econômicas, 7th ed.; Aguiar, A.T.E., Gonçalves, C., Paterniani, M.E.A.G.Z., Tucci, M.L.S.A., Castro, C.E.F., Eds.; Instituto Agronômico: Campinas, Brazil, 2014; pp. 138–149. Available online: https://www.iac.sp.gov.br/publicacoes/arquivos/iacboletim200.pdf (accessed on 14 April 2020).
- Turrell, F.M. Tables of Surfaces and Volumes of Spheres and of Prolate and Oblate Spheroids and Spheroidal Coefficients; University of California: Berkeley, CA, USA, 1946; 153p. [Google Scholar]
- Pearce, S.C.; Dobersek-Urbanc, S. The measurements of irregularity in growth and cropping. J. Hortic. Sci. 1967, 42, 295–305. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Sistema IBGE de Recuperação Automática–SIDRA: Levantamento Sistemático da Produção Agrícola 2021. Available online: https://sidra.ibge.gov.br/tabela/6588 (accessed on 27 October 2021).
- Dietrich, J.P.; Schmitz, C.; Müller, C.; Fader, M.; Lotze-Campena, H.; Popp, A. Measuring agricultural land-use intensity–A global analysis using a model-assisted approach. Ecol. Model. 2012, 232, 109–118. [Google Scholar] [CrossRef]
- Scapin, M.; Ramos, H. Manual de Tecnologia de Aplicação em Citros; Fundo de Defesa da Citricultura: Araraquara, Brazil, 2018; 28p, Available online: https://www.fundecitrus.com.br/comunicacao/manual_detalhes/manual-de-tecnologia-de-aplicacao-em-citros/63 (accessed on 4 March 2021).
- Scapin, M.S. Adequação de Volume de Calda e Dose de Bactericida Cúprico Para o Controle de Cancro Cítrico. Master’s Thesis, Fundo de Defesa da Citricultura, Araraquara, Brazil, 2014. Available online: https://www.fundecitrus.com.br/pdf/projetos/MarceloZdaZSilvaZScapin.pdf (accessed on 2 February 2019).
- Bassanezi, R.B.; Primiano, I.V. Huanglongbing and citrus variegated chlorosis integrated management based on favorable periods for vector population increase and symptom expression. Plant Dis. 2021, PDIS-06. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. Available online: https://www.jstor.org/stable/41998278 (accessed on 30 October 2021).
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Meth. 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Maldonado Junior, W. Experimentação Agronômica & AgroEstat: Sistema Para Análises Estatísticas de Ensaios Agronômicos; Gráfica Multipress Ltd.a.: Jaboticabal, Brazil, 2015; 396p. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley: New York, NY, USA, 1990. [Google Scholar]
- The Jamovi Project–Jamovi 2020. (Version 1.2). Available online: https://www.jamovi.org (accessed on 4 September 2021).
- Castle, W.S. A career perspective on citrus rootstocks, their development, and commercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Citrus, B.R. Rendimento Industrial. Available online: https://citrusbr.com/estatisticas/rendimento-industrial/ (accessed on 4 September 2021).
- Tucker, D.P.H.; Wheaton, T.A.; Muraro, R.P. Citrus Tree Pruning Principles and Practices; University of Florida, Florida Cooperative Extension Service: Gainesville, FL, USA, 1994; 9p, Available online: https://ufdcimages.uflib.ufl.edu/IR/00/00/46/22/00001/CH02700.PDF (accessed on 4 March 2021).
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef] [Green Version]
- García-Tejero, I.; Jiménez-Bocanegra, J.A.; Martínez, G.; Romero, R.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L. Positive impact of regulated deficit irrigation on yield and fruit quality in a commercial citrus orchard [Citrus sinensis (L.) Osbeck, cv. salustiano]. Agric. Water Manag. 2010, 97, 614–622. [Google Scholar] [CrossRef]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality, a Review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef] [Green Version]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Zeal. J. Crop. Hortic. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Castle, W.S.; Bowman, K.D.; Grosser, J.W.; Ferrarezi, R.S.; Futch, S.H.; Rogers, S. Florida Citrus Rootstock Selection Guide, 4th ed.; Universiy of Florida: Gainesville, FL, USA, 2016; 4p, Available online: https://edis.ifas.ufl.edu/pdf/HS/HS126000.pdf (accessed on 4 March 2021).
- Costa, D.P.; Stuchi, E.S.; Girardi, E.A.; Gesteira, A.S.; Coelho Filho, M.A.; Ledo, C.A.S.; Fadel, A.L.; Silva, A.L.V.; Leão, H.C.; Ramos, Y.C.; et al. Hybrid Rootstocks for Valencia Sweet Orange in Rainfed Cultivation Under Tropical Savannah Climate. J. Agric. Sci. 2020, 12, 40–55. [Google Scholar] [CrossRef]
- Domingues, A.R.; Marcolini, C.D.M.; Gonçalves, C.H.d.S.; Resende, J.T.V.d.; Roberto, S.R.; Carlos, E.F. Rootstocks genotypes impact on tree development and industrial properties of ‘Valencia’ sweet orange juice. Horticulturae 2021, 7, 141. [Google Scholar] [CrossRef]
- Strik, B.; Buller, G.; Hellman, E. Pruning severity affects yield, berry weight, and hand harvest efficiency of highbush blueberry. Hortscience 2003, 38, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Whitney, J.D.; Wheaton, T.A.; Castle, W.S.; Tucker, D.P.H. Citrus tree size management affects fruit yields and mechanical harvesting efficiency. Trans. ASAE 1983, 26, 704–709. [Google Scholar] [CrossRef]
- Zhou, J.; He, L.; Whiting, M.; Amatya, S.; Larbi, P.A.; Karkee, M.; Zhang, Q. Field evaluation of a mechanical-assist cherry harvesting system. Eng. Agric. Environ. Food 2016, 9, 324–331. [Google Scholar] [CrossRef]
- Bordas, M.; Torrents, J.; Arenas, F.J.; Hervalejo, A. High density plantation system of the Spanish citrus industry. Acta Hortic. 2012, 965, 123–130. [Google Scholar] [CrossRef]
- Brown, G.K. Overview of mechanical harvesting in Florida citrus. In Proceedings of the ASME 2001 Citrus Engineering Conference, Lake Alfred, FL, USA, 22 March 2001; pp. 73–84. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.P.; Scapin, M.S.; Vizoni, M.C.; Zanardi, O.Z.; Eduardo, W.I.; Volpe, H.X.L. Spray volumes and frequencies of insecticide applications for suppressing Diaphorina citri populations in orchards. Crop Prot. 2021, 140, 105406. [Google Scholar] [CrossRef]
- Silva Junior, G.J.; Scapin, M.S.; Silva, F.P.; Silva, A.R.P.; Behlau, F.; Ramos, H.H. Spray volume and fungicide rates for citrus black spot control based on tree canopy volume. Crop Prot. 2016, 85, 38–45. [Google Scholar] [CrossRef]
- Behlau, F.; Lanza, F.E.; Scapin, M.S.; Scandelai, L.H.M.; Silva Junior, G.J. Spray volume and rate based on the tree row volume for a sustainable use of copper in the control of citrus canker. Plant Dis. 2021, 105, 183–192. [Google Scholar] [CrossRef]
- Scapin, M.; Behlau, F.; Scandelai, L.H.M.; Fernando, R.S.; Silva Junior, G.J.; Ramos, H. Tree-row-volume-based sprays of copper bactericide of control of citrus canker. Crop Prot. 2015, 77, 119–126. [Google Scholar] [CrossRef]
- Ramos, H.H.; Yanai, K.; Corrêa, I.M.; Bassanezi, R.B.; Garcia, L.C. Características da pulverização em citro em função do volume de calda aplicado com turbopulverizador. Eng. Agrícola 2007, 27, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Araújo, D.; Raetano, C.G.; Ramos, H.H.; Rocha, D.R., Sr.; Prado, E.P.; Aguiar, V.C. Interference of spray volume, fruit growth and rainfall on spray deposits in citrus black spot control periods. Cienc. Rural 2016, 46, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.D.B.; Moreira, A.S.; Stuchi, E.S.; Bassenezi, R.B.; Laranjeira, F.F.; Girardi, E.A. Huanglongbing incidence, canopy volume, and sprouting dynamics of ‘Valencia’ sweet orange grafted onto 16 rootstocks. Trop. Plant. Pathol. 2020, 45, 611–619. [Google Scholar] [CrossRef]
- Carvalho, E.V.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L.; Stuchi, E.S.; Girardi, E.A.; Lopes, S.A. Modeling seasonal flushing and shoot growth on different citrus scion-rootstock combinations. Sci. Hortic. 2021, 288, 110358. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G.; Albrecht, U. Performance of ‘Valencia orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by hunglongbing. Sci. Hortic. 2016, 201, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Curk, F.; Dhuique-Mayer, C.; Urban, L.; Ollitrault, P.; Luro, F.; Morillon, R. Autotetraploid trifoliate orange (Poncirus trifoliata) rootstocks do not impact clementine quality but reduce fruit yields and highly modify rootstock/scion physiology. Sci. Hortic. 2012, 134, 100–107. [Google Scholar] [CrossRef]
- Bruyère, S.; Luro, F.; Froelicher, Y.; Morillon, R.; Ollitrault, P. Poncirus phylogenetic diagnostic SNPs markers are useful to analyse zygotic rates in diploid and tetraploid Citrus × Poncirus rootstock seedlings. In Proceedings of the International Citrus Congress, Foz do Iguaçu, Brazil, 18–23 September 2016; Mattos, D., Jr., Carlos, E.F., Novelli, V.M., Azevedo, F.A., Della-Coletta Filho, H., Vicente, P.Z.C., Eds.; IAC: New York, NY, USA; IAPAR: Gokhale Nagar, India, 2016; p. 125. Available online: https://agritrop.cirad.fr/583551/ (accessed on 30 October 2021).
- Pacheco, C.A.; Moreira, A.S.; Girardi, E.A.; Bassanezi, R.B.; Stuchi, E.S. Tree growth, production and huanglongbing incidence of sweet orange varieties using different nursery tree standards. Sci. Hortic. 2021, 284, 110023. [Google Scholar] [CrossRef]
| Tree Density | FW | SS | TA | MI 1 | TI 2 | JC | IY 3 | SSY 4 |
|---|---|---|---|---|---|---|---|---|
| (Trees·ha−1) | (g) | (°Brix) | (%) | (kg SS·Box−1) | (%) | (Boxes·t−1) | (kg SS·ha−1) | |
| 513 | 211 a | 11.86 b | 0.72 a | 17.13 a | 2.72 a | 55.83 a | 250 a | 12,171 c |
| 697 | 200 b | 12.13 ab | 0.74 a | 17.02 a | 2.75 a | 55.21 a | 248 a | 14,136 b |
| 1000 | 198 b | 12.18 a | 0.76 a | 16.57 a | 2.78 a | 55.65 a | 244 a | 15,392 a |
| Rootstock | ||||||||
| IAC 1697 | 189 b | 12.71 a | 0.78 a | 16.78 b | 2.96 a | 56.94 a | 226 b | 13,974 c |
| IAC 1710 | 203 a | 11.38 c | 0.68 c | 16.90 b | 2.62 b | 56.22 ab | 257 a | 18,295 a |
| Swingle | 210 a | 11.90 b | 0.77 ab | 16.14 b | 2.70 b | 55.33 b | 252 a | 15,590 b |
| Swingle 4× | 210 a | 12.22 b | 0.73 b | 17.79 a | 2.70 b | 53.77 c | 254 a | 7739 d |
| p-values | ||||||||
| Density (D) | 0.0071 | 0.0303 | 0.2749 | 0.5988 | 0.1983 | 0.1969 | 0.1839 | 0.0003 |
| Rootstock (R) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D × R | 0.9796 | 0.6517 | 0.2961 | 0.1998 | 0.9213 | 0.3285 | 0.9001 | 0.0963 |
| CV% (D) | 4.02 | 2.21 | 9.35 | 9.36 | 3.07 | 1.57 | 3.31 | 7.05 |
| CV% (R) | 3.62 | 2.68 | 5.12 | 4.37 | 3.61 | 1.64 | 3.79 | 8.27 |
| Tree Density | HET | HEW | HEF |
|---|---|---|---|
| (Trees·ha−1) | (min·Tree−1) | (kg·min−1) | [min·(1000 Fruits−1)] |
| 513 | 21.78 a | 4.72 a | 38.54 a |
| 697 | 17.98 b | 4.56 a | 37.31 a |
| 1000 | 16.53 b | 4.40 a | 40.54 a |
| Rootstock | |||
| IAC 1697 | 19.2 b | 4.61 b | 35.38 b |
| IAC 1710 | 26.2 a | 4.34 b | 44.33 a |
| Swingle | 20.9 b | 4.19 b | 43.54 a |
| Swingle 4× | 8.6 c | 5.11 a | 31.95 b |
| p-values | |||
| Density (D) | 0.0005 | 0.1503 | 0.1313 |
| Rootstock (R) | <0.0001 | <0.0001 | <0.0001 |
| D × R | 0.1846 | 0.0649 | 0.5100 |
| CV% | 15.98 | 12.68 | 14.15 |
| Tree Density | Cumulative HLB Incidence 1 | Mean AUDPC 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| (Trees·ha−1) | 2014 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
| 513 | 0.03 a | 0.08 a | 0.08 a | 0.10 a | 0.10 a | 0.11 a | 0.16 a | 0.62 a |
| 697 | 0.04 a | 0.09 a | 0.09 a | 0.13 a | 0.14 a | 0.15 a | 0.21 a | 0.78 a |
| 1000 | 0.02 a | 0.06 a | 0.07 a | 0.11 a | 0.13 a | 0.14 a | 0.21 a | 0.66 a |
| Rootstock | ||||||||
| IAC 1697 | 0.04 a | 0.08 a | 0.10 a | 0.14 a | 0.14 a | 0.15 a | 0.18 ab | 0.78 a |
| IAC 1710 | 0.04 a | 0.11 a | 0.11 a | 0.14 a | 0.15 a | 0.17 a | 0.27 a | 0.92 a |
| Swingle | 0.04 a | 0.07 ab | 0.07 ab | 0.09 ab | 0.10 a | 0.11 a | 0.18 ab | 0.60 ab |
| Swingle 4× | 0.01 b | 0.04 b | 0.05 b | 0.07 b | 0.09 a | 0.10 a | 0.14 b | 0.45 b |
| p-values | ||||||||
| Density (D) | 0.4340 | 0.1920 | 0.3100 | 0.3100 | 0.1030 | 0.1970 | 0.1770 | 0.433 |
| Rootstock (R) | 0.0440 | 0.0080 | 0.0060 | 0.0020 | 0.0550 | 0.1120 | 0.0020 | <0.0001 |
| D × R | 0.6220 | 0.1730 | 0.0910 | 0.1650 | 0.4000 | 0.1340 | 0.6790 | 0.0800 |
| CV% | 151.46 | 91.93 | 84.48 | 69.93 | 61.99 | 61.65 | 56.76 | 44.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girardi, E.A.; Sola, J.G.P.; Scapin, M.d.S.; Moreira, A.S.; Bassanezi, R.B.; Ayres, A.J.; Peña, L. The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange. Agronomy 2021, 11, 2569. https://doi.org/10.3390/agronomy11122569
Girardi EA, Sola JGP, Scapin MdS, Moreira AS, Bassanezi RB, Ayres AJ, Peña L. The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange. Agronomy. 2021; 11(12):2569. https://doi.org/10.3390/agronomy11122569
Chicago/Turabian StyleGirardi, Eduardo Augusto, João Gabriel Panegossi Sola, Marcelo da Silva Scapin, Alécio Souza Moreira, Renato Beozzo Bassanezi, Antonio Juliano Ayres, and Leandro Peña. 2021. "The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange" Agronomy 11, no. 12: 2569. https://doi.org/10.3390/agronomy11122569
APA StyleGirardi, E. A., Sola, J. G. P., Scapin, M. d. S., Moreira, A. S., Bassanezi, R. B., Ayres, A. J., & Peña, L. (2021). The Perfect Match: Adjusting High Tree Density to Rootstock Vigor for Improving Cropping and Land Use Efficiency of Sweet Orange. Agronomy, 11(12), 2569. https://doi.org/10.3390/agronomy11122569







