1. Introduction
Tomato (
Solanum lycopersicum L.) is widely cultivated and is one of the most important vegetable crops, ranking next to potato in global vegetable cropping area and first amongst processing crops [
1]. Tomato wilt disease, caused by
Fusarium oxysporum f. sp.
lycopersici (FOL), is the most harmful tomato disease leading to severe production losses [
2]. Tomato Fusarium wilt is a soil-borne systemic disease severely affecting all plant growth stages and is the main limiting factor in both greenhouse and field-grown tomato production [
3,
4]. Disease incidence is 100% with complete infection of all plant tissues. The main symptoms observed are yellowing, wilting, stunting of growth, and ultimately death of plants [
5].
F.
oxysporum f. sp.
lycopersici, the causative agent, includes three physiological races, which prove challenging to develop resistance to Fusarium wilt [
6].
Fusarium pathogens have a wide variety of hosts and a large degree of morphological and physiological heterogeneity, enabling them to occupy different ecological niches in diverse geographical regions [
7]. Molecular approaches are now routinely used to identity
Fusarium species, which are often more efficient than those based on morphological characteristics [
8]. The use of internal transcribed spacer (ITS) region DNA sequence analysis has now become a standard routine for the discovery, identification, classification, and phylogenetic analysis of many fungi at the species level [
9]. Molecular methods based on sequence analysis of multiple genes can be useful to classify and identify pathogenic isolate and identify new species [
10].
An objective of modern farming is to produce quality food using sustainable and ecologically friendly approaches. This includes reducing the use of certain chemicals, such as pesticides, to minimize possible environmental harm. Biological agents and natural products, like essential oils, are an attractive choice for growth enhancers or as alternative pesticides for pest management [
11]. The use of biological control agents is considered more sustainable, reduces harmful residues entering the food chain, safer for implementation and economical in cost, depending on the strength of protective antagonistic organisms against plant pathogen [
12].
Bacillus spp. are widespread in soil and have been examined for their capacity as biocontrol agents (BCAs) against plant diseases [
13]. Rhizobacteria play a significant role in plants, suppressing plant diseases and enhancing crop yield [
14]. The identification of effective rhizobacteria isolates can be achieved with the use of 16S rRNA gene-based sequencing methods [
15,
16].
Essential oils produced from various plant species have also been suggested as potential biological control agents and shown to have antimicrobial activity and antioxidant and bio-regulatory properties [
17]. Peppermint (
Mentha × piperita or
Mentha balsamea Wild.) is a medicinal plant with high nutritional value and use in both food and pharmaceutical industries [
18]. The application of essential oils is considered an effective method for preventing Fusarium wilt in banana, muskmelon, and tomato [
19,
20]. Essential oils can be used as a protective biofungicide for
F. oxysporum f. sp.
lycopersici management, with the antifungal properties of the essential oils supporting disease resistance and promoting growth. [
21].
Our study aims to examine the antifungal activity of peppermint oil (PO) and B. amyloliquefaciens (BA) against FOL and analyze the chemical components of PO. We tested the use of PO, BA, and both (BA + PO) in combination to reduce the severity of tomato Fusarium wilt disease caused by FOL under greenhouse and field conditions, as well as to examine their effect on growth and yield parameters.
4. Discussion
In our study, we identified the highly pathogenic isolate FKAU1 previously isolated by [
22] using ITS sequencing, based on the amplification of the rDNA region. FKAU1 isolate showed a 100% sequence similarity with
F. oxysporum NIHHS467 and
F. oxysporum strain ATCC 48112.
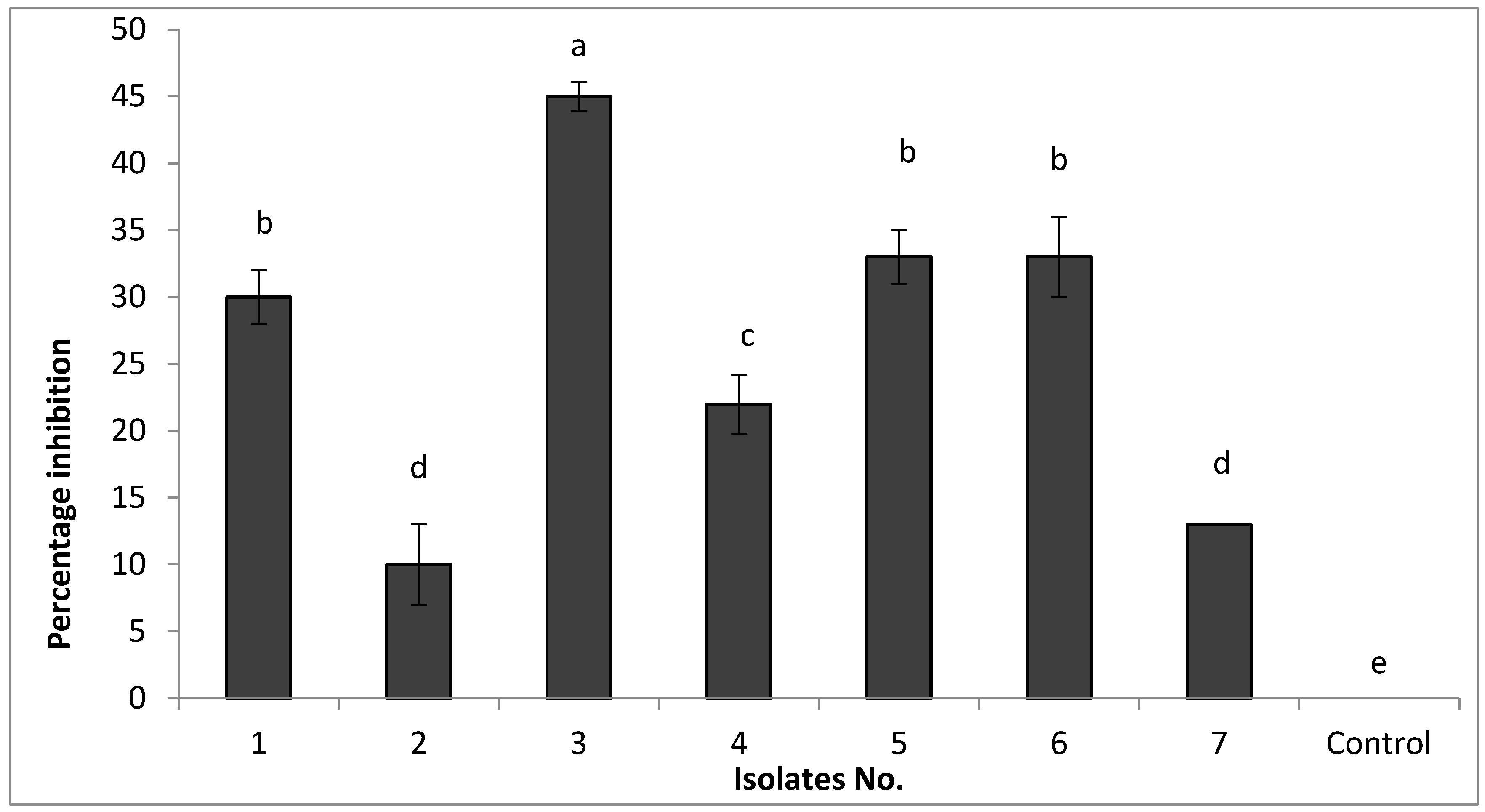
To develop potential bio-control agents against FKAU1, we obtained seven bacterial isolates from the rhizosphere of healthy tomato plants. Rhizosphere microbial populations are known to play essential roles in plant health and disease protection [
30] and are a source of potential bio-control agents. The capability of these seven isolates to inhibit the growth of FKAU1 was tested by the dual culture technique [
31,
32]. Our results agree with those reported by Kang et al. [
33], who mentioned that
Bacillus spp. have been considered potential biocontrol agents for the control of phytopathogens because they have various antifungal activities and are safe to use. Our results indicate that all tested isolates were able to inhibit the growth of FOL to differing degrees. A range of mechanisms are potentially behind this antagonistic activity and the inhibition of FOL, such as a greater potential for nutrient competition or by the release of products in the media, resulting in a reduced growth rate or killing
F. oxysporum [
34], or the production of antibiotics that can suppress the growth of pathogens [
34].
Bacterial isolate No. 3 showed the highest percentage of inhibition of FKAU1 growth in vitro and was selected for further study. To identify the species of this isolate, we sequenced the 16S rRNA region, which suggested close relation to
Bacillus amyloliquefaciens [
16]. Our results agree with those reported previously by [
8,
22].
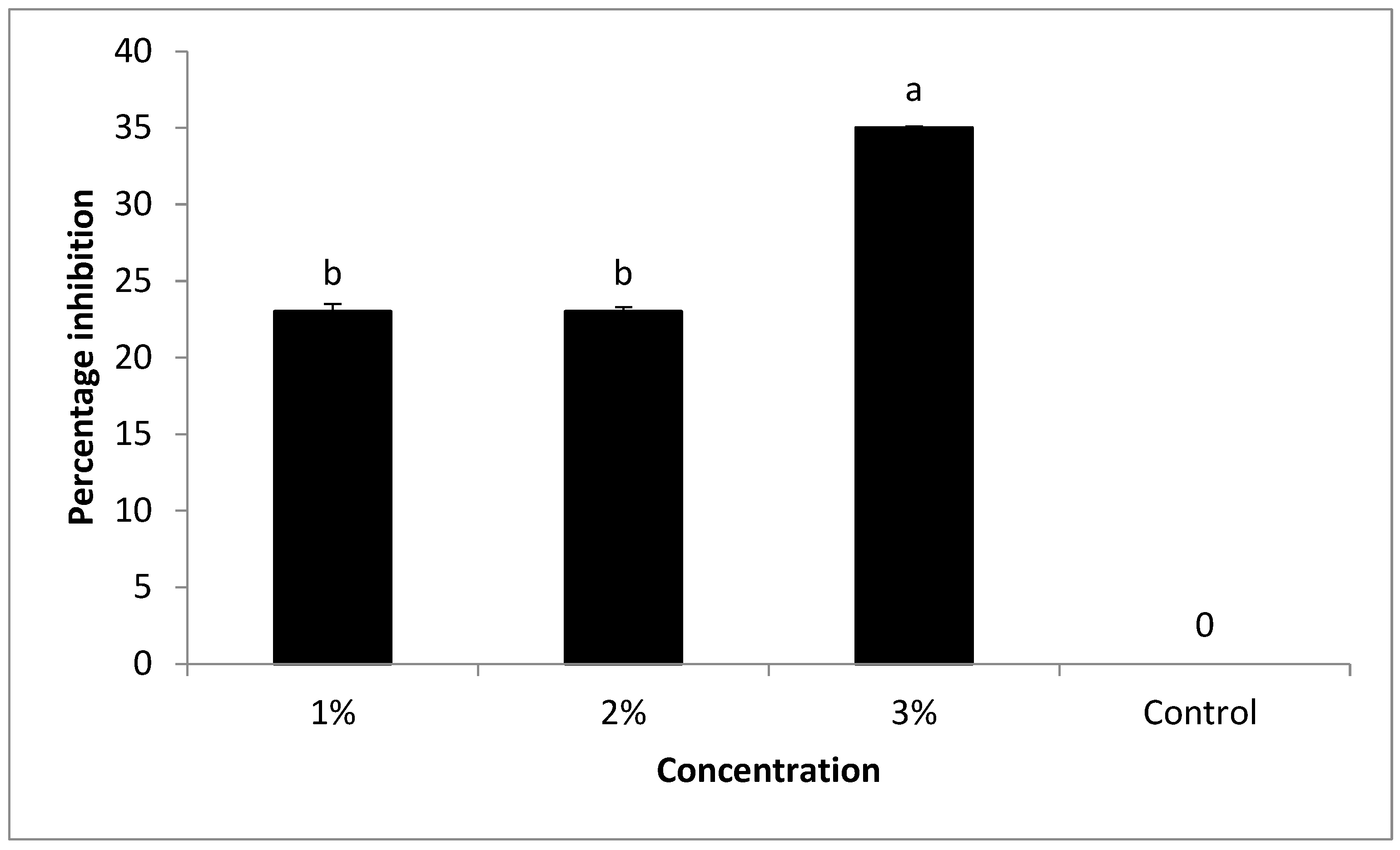
Different concentrations (1, 2, and 3%) of peppermint oil were tested in vitro against the growth of FKAU1. The findings in the current study are based on that by increasing oil concentration, the antifungal activity of the oil increased. Under laboratory screening, the oil has previously been shown to be effective against
F. oxysporum [
35]. These results are agree with [
36], who reported that peppermint oil has shown potent inhibitory activity against several microorganisms, such as
Penicillium digitatum, Saccharomyces cerevisiae,
Aspergillus niger,
A. flavus,
Mucor spp.,
Candida albicans, and
Fusarium oxysporum. The chemical analysis of peppermint oil was conducted using GC-MS. Antimicrobial activity is attributed to some of the main components and the resulting synergistic or antagonistic action. However, minor components may also contribute to the biological activity. The antimicrobial activity of essential oil is perhaps attributable to α-Pinene, γ-Terpinene, Estragole, Methsuximide, Undecane, (3, 4-Dimethoxyphenyl) (3H-imidazol-4-yl) Methanone, Caryophyllene, Eugenol, Phenol, Azulene, 4, 8-dimethyl-6-phenyl-, 1-eicosene, D-limonene, Aceptophenone, 1H-Indole, 5-methyl-2-phenyl-, acetic acid, [4-(1, 1-dimethyl) phenoxy], methyl ester, and Silane, triethyl (2-phenylethoxy). These compounds have known antimicrobial properties [
37]. Some of the other detected components of investigated essential oils are known bactericides [
38] and may contribute to antimicrobial activity.
Volatile oil compounds have antimicrobial effects by either directly harming pathogen cells or by disrupting cellular metabolism [
39]. It was reported that by responding to the active sites of cellular enzymes, volatile compounds may act as H+-carriers, leading to adenosine triphosphate depletion and disturbing cellular membranes [
40,
41]. Due to these antimicrobial activities, volatile oil compounds may also hurt beneficial bacteria.
The antifungal activity of BA, PO, or both in combination were tested on tomato seed germination and seedling vigor in vitro. Pre-treatment of seeds before infection with FKAU1 displayed increased seed germination, MSL, and MRL when compared to the mock treatment. BA gave the highest seed germination and seedling vigor followed by PO. Treatment of seeds with
Bacillus spp cultures has previously been shown to lead to increased shoot and root dry weight of tomato plants compared to untreated and infected seeds with
Fusarium oxysporum [
42]. It has been proposed that the production of antibiotics by
Bacillus spp. plays an essential part in the suppression of plant diseases since these strains can synthesize a wide range of antifungal metabolites. Among them are cyclic lipopeptides (CLPs) that include members of the fengycin surfactin, and iturin families [
34]. The results herein agree with previous research [
12,
30]
In our greenhouse experiment, we determined that BA, PO, and BA + PO seedling treatments were all effective in reducing the severity of Fusarium wilt compared with untreated plants (
Table 5). Treating seedlings with BA or PO separately resulted in a greater reduction in disease severity than in plants treated with BA and PO combined. The most effective treatment showing the lowest Fusarium wilt disease severity was BA. The results of Ajilogba et al. [
42] showed that tomato plant treated with
B. amyloliquefaciens reduced tomato Fusarium wilt by 75% compared to the infected control. Previously,
Bacillus spp. has shown significant reductions in the incidence or severity of various diseases on a diversity of hosts via some mechanisms [
43]. Roots treated with
Bacillus isolates had more root hair compared to those untreated [
42]. Suppression of disease by
Bacillus spp. may be based on the production of a wide array of secondary metabolites (antibiotics, nonvolatile and volatile compounds) and show great potential by colonizing the root surface, causing fungal mycelia lysis, and promoting plant growth [
44]. Major components of essential oils, such as menthone and menthol, may also be responsible for inhibiting the growth of the fungal pathogen. In addition, as has already been proposed, other minor components may work together synergistically in oil [
36,
45].
The treatments trialed in the greenhouse experiment were further tested under field conditions. Similar to previous experiments, all treatments showed BA seedling pre-treatment was best at reducing disease severity, followed by PO, and again the combination of BA + PO ranking third.
Our results are similar to [
46], who reported that
B. amyloliquefaciens SN16-1, isolated from soil, can prevent tomato wilt disease. This isolate is an important biocontrol agent as it can reduce the Fusarium wilt disease rate by 44.44% [
47]. Rhizobacteria biocontrol agents are capable of solubilizing phosphate, aiding with plant nutrition, and generating phytohormone IAA, inducing systemic acquired resistance in plants and as such show promise as biocontrol agents [
47,
48].
Bacillus isolates that control plant disease and increase crop yield are highly desirable to improve the sustainability of agro-ecosystems [
40].