Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate
Abstract
:1. Introduction
| Salvia Species | Plant Height | Leaf | Flower | Distribution | Uses/Other Properties |
|---|---|---|---|---|---|
| Salvia fruticosa, Greek sage | up to 120 cm tall and wide | silvery, covered with hairs, often with 1–2 pairs of small lobes below the main one | lilac, pink or sometimes white (1.6–2.5 cm long) in early spring [14] | Endemic to the eastern Mediterranean, including southern Italy, North Africa and the Canary Islands. In Greece, in the Central country, the Peloponnese and the Aegean islands [13] | In Greece, traditionaly used as a medicinal, culinary and melliferous plant since the antiquity [15]. Widely used for the preparation of an herbal tea |
| Salvia officinalis, common sage | up to 60 cm tall and wide | greenish above but white felted beneath, rough, oblong to elliptical, margin finely toothed | violet-blue, pink or white (2.0–3.5 cm long) in May–July [14,17] | Widespread on the Apennines and eastern Adriatic coast [14,17]. In North and Eastern Greece and in the Ionian islands | Medicinal and culinary use, since the antiquity and medieval. Most important Salvia species worldwide, cultivated in many varieties as medicinal and ornamental |
| Salvia pomifera ssp. pomifera | up to 100 cm tall and wide | grayish, hairy, oblong to linear-oblong, rounded or subcordate at base | pink or violet in spring to early summer of intense color on elegantly curving inflorescences [19] | Grows in Crete and in the Peloponnese [13] | The leaves are used in cooking and are rich in essential oil valuable in food industry [20] |
| Salvia ringens | up to 30 cm tall and wide | dark green, pinnatisect or pinnate with 3–6 pairs of small lateral segments, appressed-hairy | tall (60 cm), branching flowering stems with 2–4 large (about 3.8 cm long) violet-blue flowers during late spring through summer [17] | South and Eastern parts of Balkan Peninsula, including North and Central Greece [17] | A hardy herbaceous plant resistant to low temperatures. Not used as a medicinal or culinary herb |
| Salvia tomentosa, Balsamic Sage | up to 80 cm tall and wide | grey-green with a rounded or heart-shaped base | usually violet with reddish-brown calyces in late spring or early summer [14,21] | Southern Europe (mostly Balkan Peninsula and Crimea) and part of Western Asia (Anatolia and Near East) [22]. In Greece: North-Eastern and Eastern Aegean Islands [2] | Drought tolerance. Not used as a medicinal or culinary herb. |
2. Materials and Methods
2.1. Mother Plant Growth and Pollination Conditions
2.2. Hybrids Growth
2.3. Salvia fruticosa Growth
2.4. Statistical Analysis
3. Results
3.1. Interspecific Crosses and Hybrids Production
3.2. Hybrids Growth
3.2.1. Peat-Perlite 2:1 (v/v)
3.2.2. Peat-Perlite 2:1 (v/v) plus 25 g/L Attapulgite
3.3. Salvia fruticosa Growth
3.4. Flowering
4. Discussion
4.1. Interspecific Crosses and Hybrids Production
4.2. Salvia Hybrids and S. fruticosa Growth and Drought Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, S.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanischer Garten und Botanisches Museum: Berlin, Germany, 2013. [Google Scholar]
- Bayramoğlu, E.; Demirel, Ö. Xerophytic landscape. In Environment and Ecology at the Beginning of 21st Century; Efe, R., Bizzarri, C., Cürebal, İ., Nyusupova, N., Eds.; St. Kliment Ohridski University Press: Sofia, Bulgaria, 2015; pp. 180–189. [Google Scholar]
- Kanellou, Ε.; Papafotiou, M.; Economoy, G.; Paraskevopoulou, A.; Kartsonas, E. Response of sowed herbaceous forb mixtures suitable for aesthetic improvement and vegetation management at archaeological sites of the Mediterranean region. Ecol. Eng. 2021, 167, 106256. [Google Scholar] [CrossRef]
- Martinetti, L.; Tosca, A.; Spoleto, P.; Valaguss, M.; Gatt, A. Evaluation of water stress tolerance of some species suitable for extensive green roofs. Acta Hortic. 2018, 1215, 113–116. [Google Scholar] [CrossRef]
- Papafotiou, M.; Pergialioti, N.; Papanastasatos, E.A.; Tassoula, L.; Massas, I.; Kargas, G. Effect of Substrate Type and Depth and the Irrigation Frequency on Growth of Semiwoody Mediterranean species in Green Roofs. Acta Hortic. 2013, 990, 481–486. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Water use efficiency, growth and anatomic-physiological parameters of Mediterranean xerophytes as affected by substrate and irrigation on a green roof. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12283. [Google Scholar] [CrossRef]
- Stathers, R. The Bee and the Stockmarket—An Overview of Pollinator Decline and Its Economic and Corporate Significance. Schroders 2014, 15. Available online: https://www.schroders.com/en/sysglobalassets/staticfiles/schroders/sites/global/pdf/the_bee_and_the_stockmarket.pdf (accessed on 15 April 2021).
- Goulson, D.; Nicholls, Ε.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347. [Google Scholar] [CrossRef]
- Salisbury, A.; Armitage, J.; Bostock, H.; Perry, J.; Tatchell, M.; Thompson, K. Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): Should we plant native or exotic species? J. Appl. Ecol. 2015, 52, 1156–1164. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilo, P.; Gullo, M.A.L.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. AoB Plants 2015, 7, plv007. [Google Scholar] [CrossRef] [PubMed]
- Kokkinou, I.; Ntoulas, N.; Nektarios, P.A.; Varela, D. Response of native aromatic and medicinal plant species to water stress on adaptive green roof systems. Hortscience 2016, 51, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of Crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Blamey, M.; Grey-Wilson, C. Mediterranean Wild Flowers; Harper Collins Publishers: London, UK, 1993; pp. 401–402. [Google Scholar]
- Clebsch, B.; Barner, C.D. The New Book of Salvias; Timber Press: Portland, OR, USA, 2003; pp. 125–127. [Google Scholar]
- Hanson, B. Designing an Herb Garden; Brooklyn Botanic Garden: Brooklyn, NY, USA, 2004; p. 58. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 3 Diapenstaceae to Myoporaceae; Cambridge University Press: Great Britain, UK, 1972; pp. 188–190. [Google Scholar]
- Bettaieb, I.; Zakhama, N.; Aidi Wannes, W.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Karousou, R.; Hanlidou, E.; Kokkini, S. II Botany, 2. The sage plants of Greece: Distribution and infraspecific variation. In Sage: The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; p. 39. [Google Scholar]
- Karousou, R.; Vokou, D.; Kokkini, S. Distribution and essential oils of Salvia pomifera subsp. pomifera (Labiatae) on the island of Crete (S Greece). Biochem. Syst. Ecol. 1998, 26, 889–897. [Google Scholar] [CrossRef]
- Hedge, I. Salvia Linnaeus. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 188–192. [Google Scholar]
- Guner, A.; Ozhatay, N.; Ekim, T.; Baser, K.H.C. Supplement II. In Flora of Turkey and the East Aegean Islands; Edinburg University Press: Edinburg, UK, 2000; Volume 11. [Google Scholar]
- Dudai, N.; Lewinsohn, E.; Larkov, O.; Katzir, I.; Ravid, U.; Chaimovitsh, D.; Diah, S.; Putievsky, E. Dynamics of Yield Components and Essential Oil Production in a Commercial Hybrid Sage (Salvia officinalis × Salvia fruticosa cv. Newe Ya’ar No. 4). J. Agric. Food Chem. 1999, 47, 4341–4345. [Google Scholar] [CrossRef] [PubMed]
- Radosavljević, I.; Bogdanović, S.; Celep, F.; Filipović, M.; Satovic, Z.; Surina, B.; Liber, Z. Morphological, genetic and epigenetic aspects of homoploid hybridization between Salvia officinalis L. and Salvia fruticosa Mill. Sci. Rep. 2019, 9, 3276. [Google Scholar] [CrossRef] [Green Version]
- Putievsky, E.; Ravid, U.; Diwan-Rinzler, N.; Zohary, D. Genetic affinities and essential oil composition of Salvia officinalis L., S. fruticosa Mill., S. tomentosa and their hybrids. Flavour Fragr. J. 1990, 5, 121–123. [Google Scholar] [CrossRef]
- Chen, D.; Du, Y.; Zhu, H.; Deng, Y. Synthesis and characterization of a microfibrous TiO2–CdS/palygorskite nanostructured material with enhanced visible-light photocatalytic activity. Appl. Clay Sci. 2014, 87, 285–291. [Google Scholar] [CrossRef]
- Quan, G.; Zhang, J.; Guo, J.; Lan, Y. Removal of Cr(VI) from aqueous solution bynanoscale zero-valent iron grafted on acid-activated Attapulgite. Water Air Soil Pollut. 2014, 225, 1979. [Google Scholar] [CrossRef]
- Dong, L.; Lin, L.; Li, Q.; Huang, Z.; Tang, X.; Wu, M.; Li, C.; Cao, X.; Scholz, M. Enhanced nitrate-nitrogen removal by modified attapulgite-supported nanoscale zero-valent iron treating simulated groundwater. J. Environ. Manag. 2018, 213, 151–158. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Zhang, H. Removal of rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation. Appl. Catal. 2015, 178, 29–36. [Google Scholar] [CrossRef]
- Liang, X.; Han, J.; Xu, Y.; Sun, Y.; Wang, L.; Tan, X. In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 2014, 235, 9–18. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, D.; Hou, H.; Gu, Q.; Yang, R.; Chen, J.; Yang, J.; Wang, L. Catalyticdegradation of PNP and stabilization/solidification of Cd simultaneously in soil using microwave-assisted Fe-bearing attapulgite. Chem. Eng. J. 2016, 304, 747–756. [Google Scholar] [CrossRef]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef]
- Ren, G.; Wang, F.; Huang, Y.; Fan, R.; Wang, B.; Chen, X. Effects of attapulgite clay on growth and physiological metabolism of sweetpotato. J. Huaiyin Inst. Technol. 2018, 3. Available online: https://oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2018&filename=JSHY201803008&uniplatform=NZKPT&v=gYrP8wQtAurH8H9UmD8uIIx6BUILEgmzgv%25mmd2BU8r8ZLbWcIDGKDhALuUGABsp1GIlg (accessed on 10 April 2021).
- Tychonievich, J.; Warner, R.M. Interspecific crossability of selected Salvia species and potential use for crop improvement. J. Am. Soc. Hortic. Sci. 2011, 136, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Kintzios, S.E. (Ed.) Genetic Improvement of Cultivated Species of the Genus Salvia. In Sage: The Genus Salvia; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; p. 32. ISBN 90-5823-005-8. [Google Scholar]
- Petrova, A.; Vladimirov, V. Chromosome atlas of the Bulgarian vascular plants. Phytol. Balc. 2020, 26, 217–427. [Google Scholar]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Abdalla, M.M.; El-Khoshiban, N.H. The influence of water stress on growth relative water content, photosynthetic pigments, some metabolic and hormonal contents of two Triticum aestivum cultivars. J. Appl. Sci. Res. 2007, 3, 2062–2074. [Google Scholar]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of water stress on lavender and sage biomass production, essential oil composition and biocidal properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Sabry, R.M.; Kandil, M.A.M.; Ahmed, S.S. Growth and quality of sage (Salvia officinalis), parsley (Petroselinum crispum) and nasturtium (Tropaeolum majus) as affected by water deficit. Middle East J. Agric. Res. 2016, 5, 286–294. [Google Scholar]
- Soltanbeigi, A.; Yıldız, M.; Dıraman, H.; Terzi, H.; Sakartepe, E.; Yıldız, E. Growth responses and essential oil profile of Salvia officinalis L. influenced by water deficit and various nutrient sources in the greenhouse. Saudi J. Biol. Sci. 2021, in press. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of Drought Stress on Biomass, Essential Oil Content, Nutritional Parameters, and Costs of Production in Six Lamiaceae Species. Water 2019, 11, 573. [Google Scholar] [CrossRef] [Green Version]
- Idrissi, O.; De Keyser, E.; De Riek, J.; Houasli, C.; Van Damme, P.; Udupa, S.M. Genetic variability for root and shoot traits in a lentil (Lens culinaris Medik.) recombinant inbred line population and their association with drought tolerance. Eucaliptica 2015, 204, 693–709. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mwadzingeni, L.; Zengeni, R.; Mutema, M.; Chaplot, V. Variance components and heritability of traits related to root: Shoot biomass allocation and drought tolerance in wheat. Euphytica 2018, 214, 225. [Google Scholar] [CrossRef]
- Mwenye, O.J.; Van Rensburg, L.; Van Biljon, A.; Van der Merwe, R. Seedling shoot and root growth responses among soybean (Glycine max) genotypes to drought stress. In Soybean—Biomass, Yield and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2018; p. 10. [Google Scholar] [CrossRef]


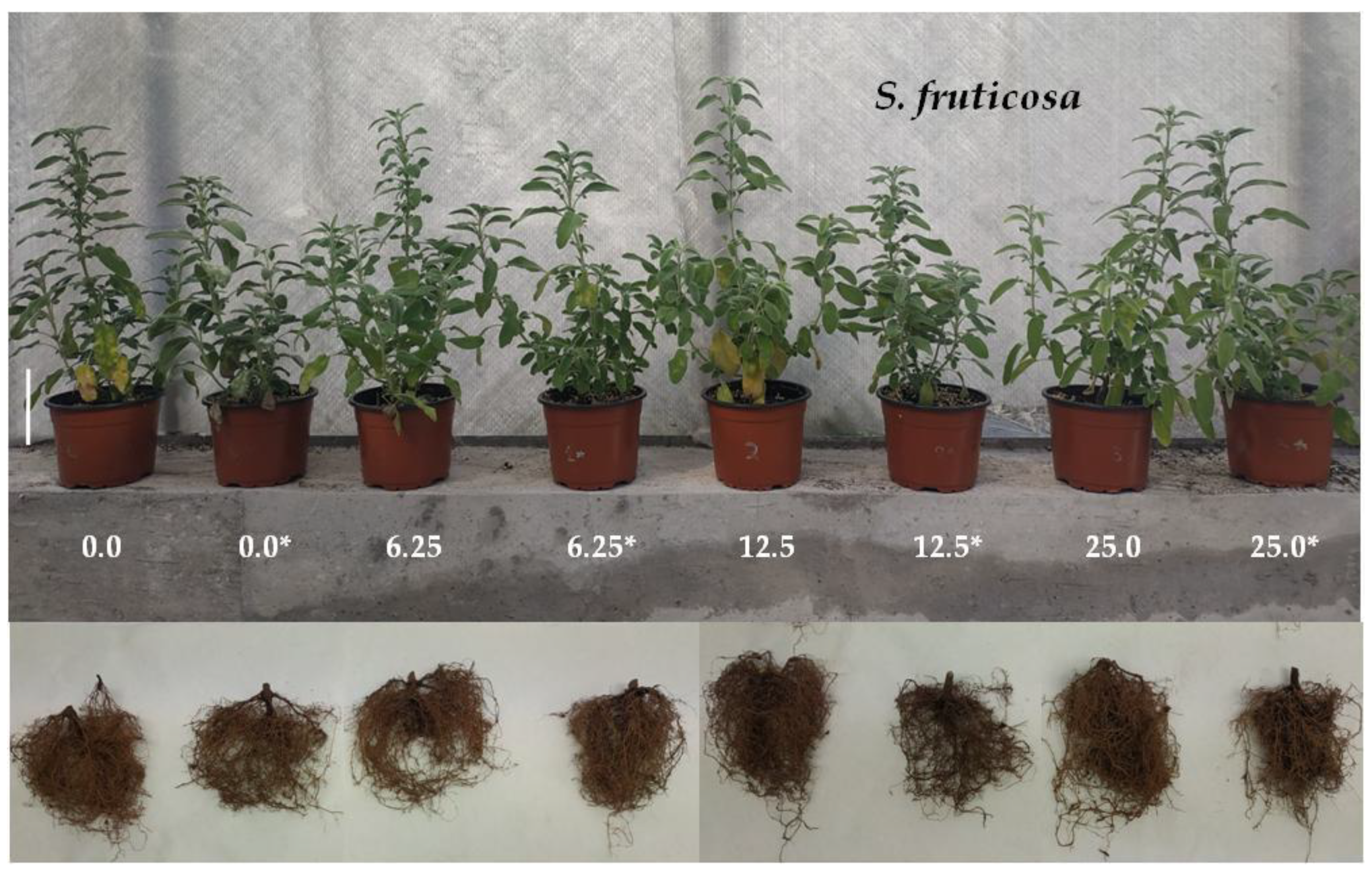
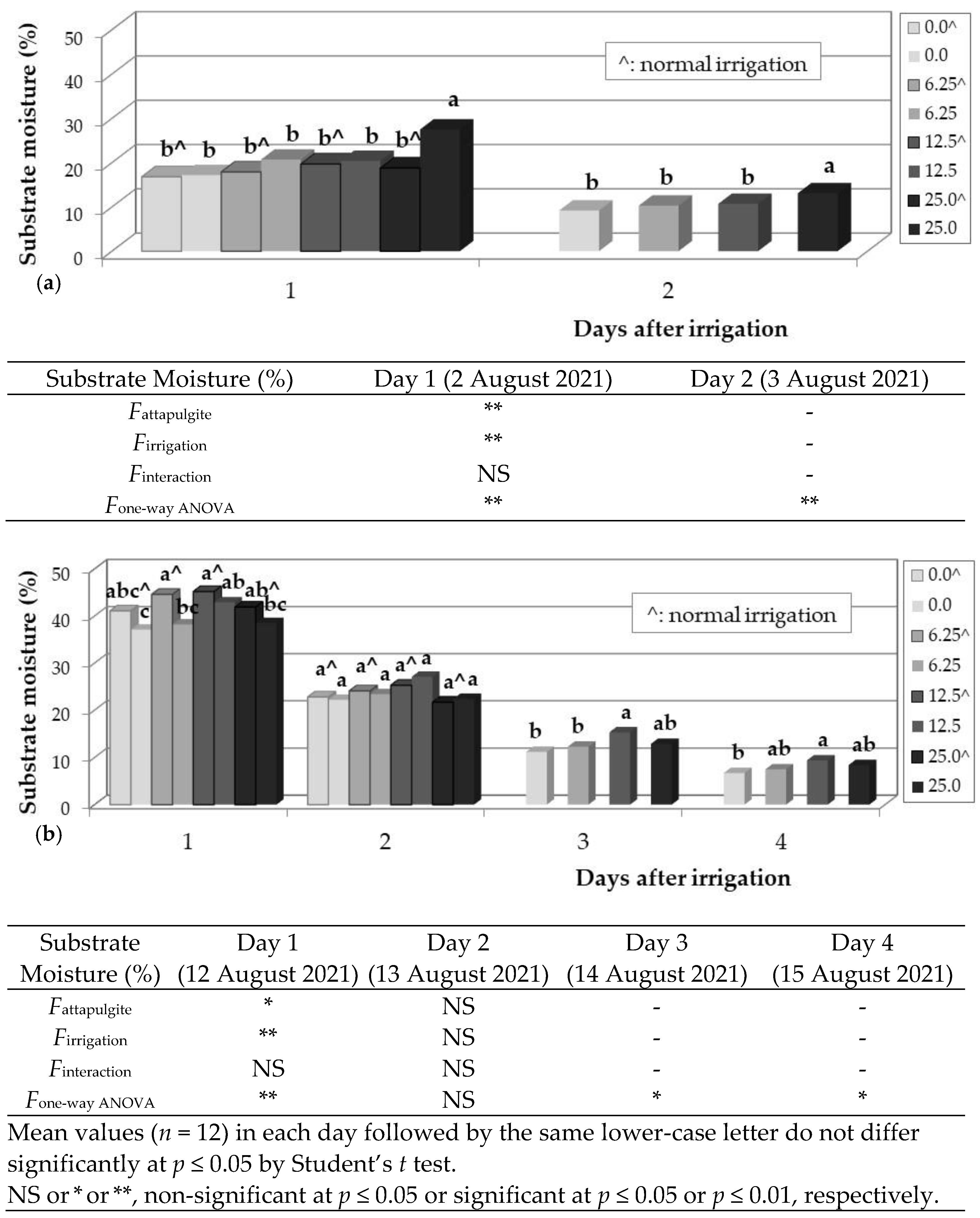
| Cross | Pollinations Number | Successful Crosses (%) | Total Seed Number | Seedling Number * |
|---|---|---|---|---|
| S. officinalis × self | 50 | 80 | 40 | 29 |
| S. officinalis × S. ringens | 502 | 5.7 | 27 | 7 |
| S. officinalis × S. pomifera | 531 | 5.6 | 30 | 6 |
| S. officinalis × S. tomentosa | 389 | 6.4 | 24 | 5 |
| S. ringens × S. officinalis | 200 | 0 | 0 | 0 |
| S. pomifera × S. officinalis | 200 | 0 | 0 | 0 |
| S. tomentosa × S. officinalis | 200 | 0 | 0 | 0 |
| S. fruticosa × self | 50 | 92 | 48 | 39 |
| S. fruticosa × S. ringens | 100 | 19 | 19 | 4 |
| S. fruticosa × S. pomifera | 100 | 8 | 8 | 1 |
| S. fruticosa × S. tomentosa | 100 | 28 | 28 | 0 |
| S. ringens × S. fruticosa | 100 | 0 | 0 | 0 |
| S. pomifera × S. fruticosa | 100 | 0 | 0 | 0 |
| S. tomentosa × S. fruticosa | 100 | 53 | 160 | 11 |
| 3-Way ANOVA | Plant Height (cm) | Lateral Shoot Number | Lateral Shoot Mean Length (cm) | Lateral Shoot Total Length (cm) | Above Ground f.w. (g) | Roots f.w. (g) | Above Ground f.w./Roots f.w. | Above Ground d.w. (g) | Roots d.w. (g) | Above Ground d.w./Roots d.w. |
|---|---|---|---|---|---|---|---|---|---|---|
| S. officinalis × S. pomifera | 32.8 a z | 4.4 b | 11.5 | 49.4 | 22.3 c | 15.4 | 1.5 | 8.4 c | 3.5 | 2.5 |
| S. officinalis × S. ringens | 27.0 b | 7.7 a | 10.1 | 73.2 | 31.4 a | 10.4 | 3.2 | 11.6 a | 3.0 | 4.2 |
| S. officinalis × S. tomentosa | 26.9 b | 8.4 a | 9.6 | 81.6 | 27.8 b | 10.0 | 2.9 | 9.3 b | 2.6 | 3.6 |
| S. fruticosa × S. ringens | 32.4 a | 8.5 a | 8.9 | 76.6 | 27.5 b | 15.5 | 1.9 | 9.6 b | 3.7 | 2.7 |
| without attapulgite | 30.0 a | 7.7 a | 10.2 | 75.9 | 28.1 a | 13.2 a | 2.5 a | 10.1 a | 3.3 a | 3.5 |
| with 25 g/L attapulgite | 29.5 a | 6.8 b | 9.9 | 64.5 | 26.4 b | 12.4 b | 2.3 b | 9.4 b | 3.1 a | 3.0 |
| normal irrigation | 31.9 a | 7.6 a | 10.4 | 75.7 | 28.5 a | 13.5 | 2.4 | 10.3 a | 3.4 | 3.3 |
| sparse irrigation | 27.6 b | 6.9 a | 9.6 | 64.6 | 25.9 b | 12.1 | 2.4 | 9.2 b | 3.0 | 3.2 |
| Significance § | ||||||||||
| Fhybrid | ** | ** | - | - | ** | - | - | ** | - | - |
| Fattapulgite | NS | * | - | - | * | * | * | ** | NS | - |
| Firrigation | ** | NS | - | - | ** | - | - | ** | - | - |
| Fhybrid x attapulgite | NS | NS | - | - | NS | NS | NS | NS | NS | NS |
| Fhybrid x irrigation | NS | NS | - | - | NS | ** | ** | NS | ** | ** |
| Fattapulgite x irrigation | NS | NS | - | - | NS | NS | NS | NS | NS | ** |
| Fhybrid x attapulgite x irrigation | NS | NS | * | * | NS | NS | NS | NS | NS | NS |
| Hybrid Type | Irrigation Frequency | Plant Height (cm) | Lateral Shoot Number | Lateral Shoot Mean Length (cm) | Lateral Shoot Total Length (cm) | Above Ground f.w. (g) | Root f.w. (g) | Above Ground f.w./Roots f.w. | Above Ground d.w. (g) | Root d.w. (g) | Above Ground d.w./Root d.w. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. officinalis × S. pomifera | normal | 36.9 a † | 4.6 c | 12.8 a | 53.8 cd | 24.7 cd | 14.2 bc | 1.8 de | 9.4 bc | 3.5 b | 2.7 cde |
| sparse | 30.2 bc | 4.3 c | 10.2 b | 44.4 d | 20.7 d | 15.0 b | 1.4 e | 8.1 c | 3.2 b | 2.5 e | |
| S. officinalis × S. ringens | normal | 27.5 cd | 9.0 ab | 10.1 b | 87.4 ab | 34.6 a | 12.9 cd | 2.7 c | 12.9 a | 3.4 b | 3.8 b |
| sparse | 27.0 cd | 7.6 b | 10.6 b | 75.0 bc | 33.0 ab | 8.3 f | 4.1 a | 12.1 a | 2.4 c | 5.8 a | |
| S. officinalis × S. tomentosa | normal | 30.1 bc | 10.3 a | 10.2 b | 105.1 a | 29.0 bc | 8.5 f | 3.5 b | 9.4 bc | 2.6 c | 3.7 b |
| sparse | 24.6 d | 8.1 b | 9.1 b | 73.5 bc | 26.2 c | 10.9 e | 2.5 c | 9.0 bc | 2.6 c | 3.6 bcd | |
| S. fruticosa × S. ringens | normal | 32.8 ab | 8.7 ab | 8.7 b | 78.4 b | 28.5 c | 17.3 a | 1.7 e | 10.4 b | 4.1 a | 2.6 de |
| sparse | 30.7 bc | 9.2 ab | 9.8 b | 89.4 ab | 27.7 c | 12.3 de | 2.3 cd | 9.8 b | 3.2 b | 3.1 bcde | |
| Significance § | |||||||||||
| Fhybrid | ** | ** | * | ** | ** | - | ** | ** | - | - | |
| Firrigation | ** | NS | NS | NS | * | - | NS | ** | - | - | |
| Finteraction | NS | NS | NS | NS | NS | ** | NS | NS | * | * | |
| Fone-way ANOVA | ** | ** | * | ** | ** | ** | ** | ** | ** | ** | |
| Hybrid Type | Irrigation Frequency | Plant Height (cm) | Lateral Shoot Number | Lateral Shoot Mean Length (cm) | Lateral Shoot Total Length (cm) | Above Ground f.w. (g) | Roots f.w. (g) | Above Ground f.w./Roots f.w. | Above Ground d.w. (g) | Roots d.w. (g) | Above Ground d.w./Roots d.w. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. officinalis × S. pomifera | normal | 36.0 a † | 4.2 c | 11.6 a | 48.3 c | 22.3 de | 15.5 bc | 1.5 de | 8.4 cd | 3.5 b | 2.4 d |
| sparse | 28.0 cd | 4.5 c | 11.6 a | 50.9 c | 21.3 e | 16.7 ab | 1.3 e | 7.8 d | 3.5 b | 2.2 d | |
| S. officinalis × S. ringens | normal | 29.1 bcd | 8.0 ab | 10.8 ab | 78.2 a | 33.0 a | 11.8 de | 2.9 ab | 12.1 a | 3.4 b | 3.8 ab |
| sparse | 24.3 d | 6.2 bc | 8.9 bc | 52.0 c | 25.1 cd | 8.4 g | 3.2 a | 9.3 bc | 2.7 c | 3.6 ab | |
| S. officinalis × S. tomentosa | normal | 28.3 cd | 7.4 ab | 9.9 ab | 72.4 ab | 29.1 b | 9.4 fg | 3.4 a | 10.0 b | 2.5 c | 4.1 a |
| sparse | 24.7 d | 7.9 ab | 9.3 bc | 75.4 ab | 26.7 bc | 11.2 ef | 2.4 bc | 9.0 bcd | 2.9 c | 3.2 bc | |
| S. fruticosa × S. ringens | normal | 34.6 ab | 8.8 a | 9.3 bc | 81.9 a | 26.9 bc | 18.6 a | 1.5 de | 9.5 bc | 4.2 a | 2.3 d |
| sparse | 31.3 abc | 7.4 ab | 7.6 c | 56.6 bc | 26.7 bc | 13.8 cd | 2.0 cd | 8.7 bcd | 3.4 b | 2.6 cd | |
| Significance § | |||||||||||
| Fhybrid | ** | ** | ** | - | - | - | - | ** | - | - | |
| Firrigation | ** | NS | * | - | - | - | - | ** | - | - | |
| Finteraction | NS | NS | NS | * | ** | ** | ** | NS | ** | * | |
| Fone-way ANOVA | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Attapulgite Concentration (g/L) | Irrigation Frequency | Plant Height (cm) | Lateral Shoot Number | Lateral Shoot Mean Length (cm) | Lateral Shoot Total Length (cm) | Above Ground f.w. (g) | Roots f.w. (g) | Above Ground f.w./Roots f.w. | Above Ground d.w. (g) | Roots d.w. (g) | Above Ground d.w./Roots d.w. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | normal | 36.1 ab † | 7.0 a | 15.8 abc | 108.4 a | 36.4 ab | 9.2 d | 4.1 ab | 10.5 a | 2.6 bc | 4.2 ab |
| sparse | 33.8 abc | 6.1 a | 14.3 c | 85.9 b | 28.9 d | 9.2 d | 3.2 cd | 6.7 c | 2.1 e | 3.4 cd | |
| 6.25 | normal | 36.8 a | 7.2 a | 16.5 abc | 112.8 a | 39.7 a | 9.5 cd | 4.4 a | 11.4 a | 2.4 cde | 4.8 a |
| sparse | 32.6 bc | 6.6 a | 13.9 c | 89.7 b | 33.3 bc | 9.5 cd | 3.7 bc | 8.4 b | 2.1 de | 4.2 ab | |
| 12.5 | normal | 37.0 a | 6.5 a | 18.4 a | 112.1 a | 36.8 ab | 10.4 bcd | 3.6 bc | 10.8 a | 2.6 abc | 4.2 ab |
| sparse | 33.3 abc | 5.8 a | 15.3 bc | 87.9 b | 30.5 cd | 11.5 abc | 2.8 de | 7.8 b | 2.5 bcd | 3.2 cd | |
| 25.0 | normal | 37.3 a | 6.6 a | 17.4 ab | 114.5 a | 37.7 a | 12.1 ab | 3.3 cd | 10.8 a | 3.0 a | 3.7 bc |
| sparse | 31.6 c | 5.3 a | 14.2 c | 74.8 b | 28.0 d | 12.8 a | 2.3 e | 7.5 bc | 2.9 ab | 2.7 d | |
| Significance § | |||||||||||
| Fattapulgite | NS | NS | NS | NS | * | ** | ** | * | ** | ** | |
| Firrigation | ** | * | ** | ** | ** | NS | ** | ** | * | ** | |
| Finteraction | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Fone-way ANOVA | * | NS | * | ** | ** | ** | ** | ** | ** | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papafotiou, M.; Martini, A.N.; Papanikolaou, E.; Stylias, E.G.; Kalantzis, A. Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate. Agronomy 2021, 11, 2401. https://doi.org/10.3390/agronomy11122401
Papafotiou M, Martini AN, Papanikolaou E, Stylias EG, Kalantzis A. Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate. Agronomy. 2021; 11(12):2401. https://doi.org/10.3390/agronomy11122401
Chicago/Turabian StylePapafotiou, Maria, Aikaterini N. Martini, Eleonora Papanikolaou, Eleftherios G. Stylias, and Anastasios Kalantzis. 2021. "Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate" Agronomy 11, no. 12: 2401. https://doi.org/10.3390/agronomy11122401
APA StylePapafotiou, M., Martini, A. N., Papanikolaou, E., Stylias, E. G., & Kalantzis, A. (2021). Hybrids Development between Greek Salvia Species and Their Drought Resistance Evaluation along with Salvia fruticosa, under Attapulgite-Amended Substrate. Agronomy, 11(12), 2401. https://doi.org/10.3390/agronomy11122401






