Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Fresh Fruit Weight, Length, Diameter, and Fruit Shape Index
2.3. Fruit Peel Thickness, Number of Segments, Pulp Weight, Pulp Color, Soluble Solids, and Vitamin C
2.4. Soluble Sugars Extraction through HPLC
2.5. Organic Acids Determination through UPLC
2.6. Statistical Analysis
3. Results
3.1. Fresh Fruit Weight, Length, Diameter, and Fruit Shape Index
3.2. Fruit Peel Thickness, Number of Segments, Pulp Weight, Pulp Color, Soluble Solids, and Vitamin C
3.3. Soluble Sugars
3.4. Organic Acids
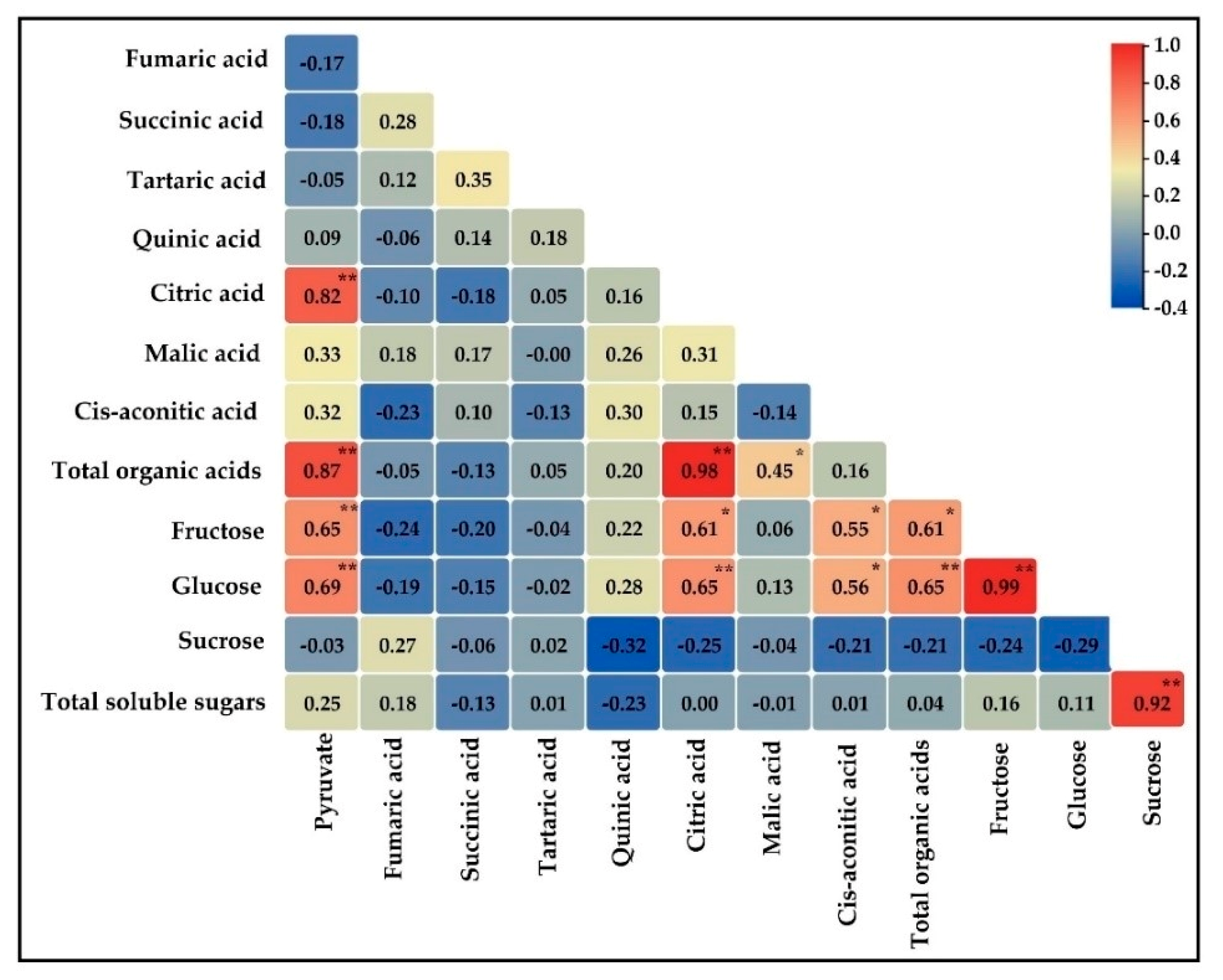
3.5. Correlation among Organic Acid and Soluble Sugar Contents
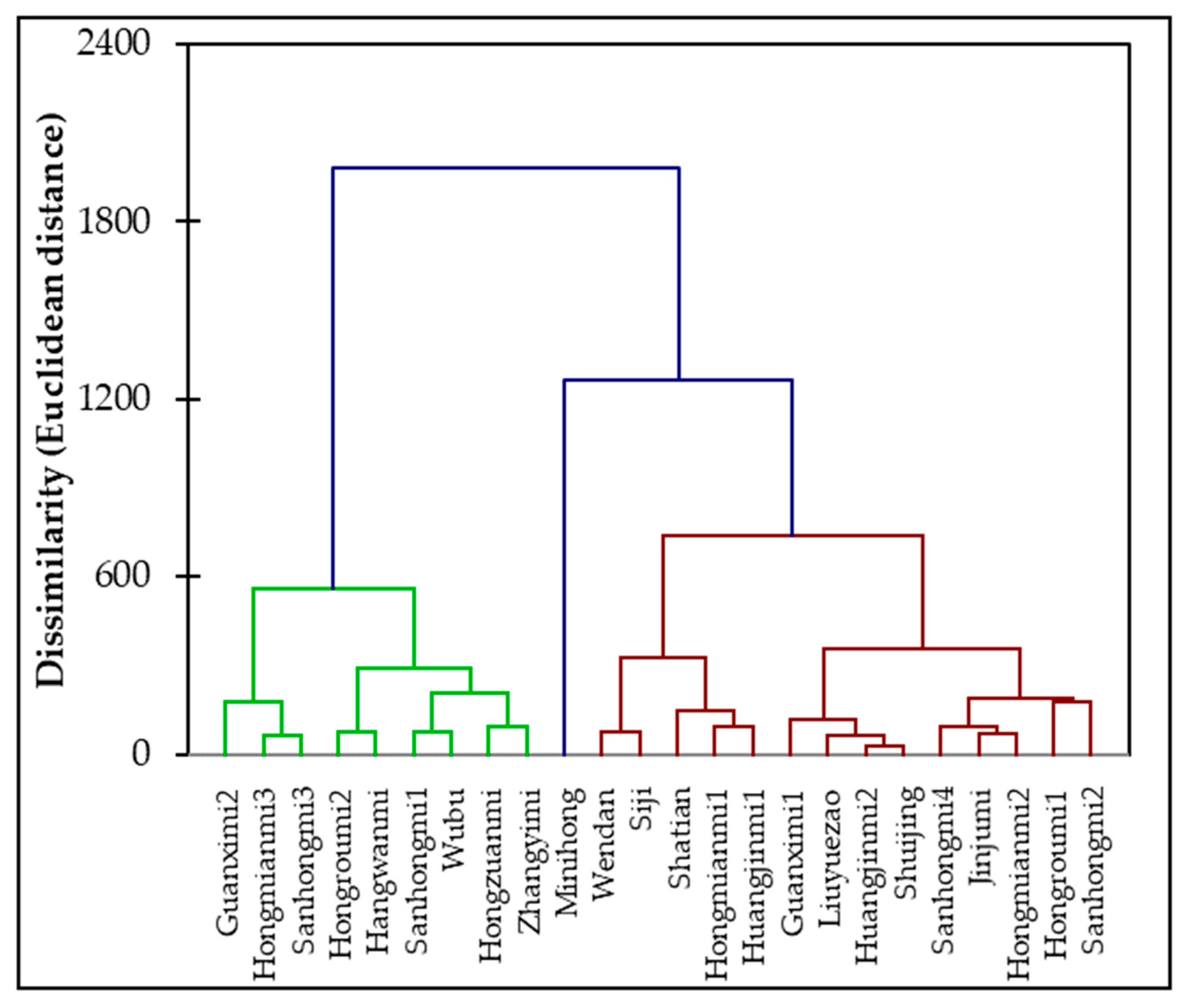
3.6. AHC Analysis of 24 Pomelo Cultivars Based on their Physiology and Sugar-Acid Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barboni, T.; Luro, F.; Chiaramonti, N.; Desjobert, J.-M.; Muselli, A.; Costa, J. Volatile composition of hybrids Citrus juices by headspace solid-phase micro extraction/gas chromatography/mass spectrometry. Food Chem. 2009, 116, 382–390. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Ruiz Perez-Cacho, P.; Jabalpurwala, F. Historical Review of Citrus Flavor Research during the Past 100 Years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, R.; Li, B.; Tian, S. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Qin, G.; Tian, S. Molecular basis of 1-methylcyclopropene regulating organic acid metabolism in apple fruit during storage. Postharvest Biol. Technol. 2016, 117, 57–63. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant Growth and Fruit Quality Response of Strawberry is Improved after Exogenous Application of 24-Epibrassinolide. J. Plant. Growth Regul. 2021. [Google Scholar] [CrossRef]
- Yu, X.; Ali, M.M.; Li, B.; Fang, T.; Chen, F. Transcriptome data-based identification of candidate genes involved in metabolism and accumulation of soluble sugars during fruit development in ‘Huangguan’ plum. J. Food Biochem. 2021, 45, e13878. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, X.; Ali, M.M.; Rizwan, H.M.; Li, B.; Li, H.; Jia, K.; Yang, X.; Ma, S.; Li, S.; et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits. Int. J. Mol. Sci. 2021, 22, 5765. [Google Scholar] [CrossRef]
- Hu, W.; Wang, B.; Ali, M.M.; Chen, X.; Zhang, J.; Zheng, S.; Chen, F. Free Amino Acids Profile and Expression Analysis of Core Genes Involved in Branched-Chain Amino Acids Metabolism during Fruit Development of Longan (Dimocarpus longan Lour.) Cultivars with Different Aroma Types. Biology 2021, 10, 807. [Google Scholar] [CrossRef]
- Shi, M.; Ali, M.M.; He, Y.; Ma, S.; Rizwan, H.M.; Yang, Q.; Li, B.; Lin, Z.; Chen, F. Flavonoids Accumulation in Fruit Peel and Expression Profiling of Related Genes in Purple (Passiflora edulis f. edulis) and Yellow (Passiflora edulis f. flavicarpa) Passion Fruits. Plants 2021, 10, 2240. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Kengni, E.; Tchoundjeo, Z.; Tchouanguep, F.M.; Mbofung, C.M.F. Sensory evaluation of Dacryodes edulis fruit types. For. Trees Livelihoods 2001, 11, 57–66. [Google Scholar] [CrossRef]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of Environmental Factors on Growth and Development of Fruits. Trop. Plant. Biol. 2021, 14, 226–238. [Google Scholar] [CrossRef]
- Teixeira, R.T. Modified sucrose, starch, and ATP levels in two alloplasmic male-sterile lines of B. napus. J. Exp. Bot. 2005, 56, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant. Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar Metabolism in Stone Fruit: Source-Sink Relationships and Environmental and Agronomical Effects. Front. Plant. Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Elmardy, N.A.; Yousef, A.F.; Lin, K.; Zhang, X.; Ali, M.M.; Lamlom, S.F.; Kalaji, H.M.; Kowalczyk, K.; Xu, Y. Photosynthetic performance of rocket (Eruca sativa. Mill.) grown under different regimes of light intensity, quality, and photoperiod. PLoS ONE 2021, 16, e0257745. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Tadda, S.A.; Kalaji, H.M.; Yang, H.; Ahmed, M.A.A.; Wróbel, J.; Xu, Y.; Chen, F. Photosynthetic apparatus performance of tomato seedlings grown under various combinations of LED illumination. PLoS ONE 2021, 16, e0249373. [Google Scholar] [CrossRef]
- Alfred, L.; Pierce, F. Sugar Composition Analysis of Commercial Citrus Juice Products. Proc. Fla. State Hort. Soc. 2016, 129, 178–180. [Google Scholar]
- Moing, A.; Rothan, C.; Svanella, L.; Just, D.; Diakou, P.; Raymond, P.; Gaudillère, J.-P.; Monet, R. Role of phospho enol pyruvate carboxylase in organic acid accumulation during peach fruit development. Physiol. Plant. 2000, 108, 1–10. [Google Scholar] [CrossRef]
- Sedaghat, S.; Rahemi, M. Enzyme Activity Regarding Sugar and Organic Acid Changes during Developmental Stages in Rainfed Fig (Ficus carica L.cv Sabz). Int. J. Fruit Sci. 2018, 18, 14–28. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Possner, D.; Brem, S.; Rast, D.M. The physiological role of malic enzyme in grape ripening. Planta 1984, 160, 444–448. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Alam, S.M.; Anwar, R.; Ali, S.; Shi, M.; Liang, D.; Lin, Z.; Chen, F. Genome-Wide Identification, Characterization and Expression Profiling of Aluminum-Activated Malate Transporters in Eriobotrya japonica Lindl. Horticulturae 2021, 7, 441. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant. Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Xie, X.; Sun, C.; Grierson, D.; Yin, X.; Chen, K. CrMYB73, a PH-like gene, contributes to citric acid accumulation in citrus fruit. Sci. Hortic. 2015, 197, 212–217. [Google Scholar] [CrossRef]
- Sawamura, M.; Shichiri, K.; Ootani, Y.; Zheng, X.H. Volatile Constituents of Several Varieties of Pummelos and Characteristics among Citrus Species. Agric. Biol. Chem. 1991, 55, 2571–2578. [Google Scholar] [CrossRef]
- Cheong, M.W.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef]
- Shaw, P.E.; Goodner, K.L.; Moshonas, M.G.; Hearn, C.J. Comparison of grapefruit hybrid fruit with parent fruit based on composition of volatile components. Sci. Hortic. 2001, 91, 71–80. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative Analysis of the Volatile Fraction of Fruit Juice from Different Citrus Species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [Green Version]
- Nour, V.; Trandafir, I.; Ionica, M.E. Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Not. Bot. Horti. Agrobot. Cluj-Napoca 2010, 38, 44–48. [Google Scholar] [CrossRef]
- Zingaretti, L.M.; Monfort, A.; Pérez-Enciso, M. Automatic Fruit Morphology Phenome and Genetic Analysis: An Application in the Octoploid Strawberry. Plant. Phenomics 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D. A classification for edible Citrus (Rutaceae). Telopea 1997, 167–172. [Google Scholar] [CrossRef]
- Fan, Z.; Xiong, H.; Luo, Y.; Wang, Y.; Zhao, H.; Li, W.; He, X.; Wang, J.; Shi, X.; Zhang, Y. Fruit Yields Depend on Biomass and Nutrient Accumulations in New Shoots of Citrus Trees. Agronomy 2020, 10, 1988. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.A.; Ramos, J.D.; da Cruz, M.D.C.M.; Pantoja, L.D.A.; dos Santos, A.S. Leaf carbohydrates in ‘Ponkan’ mandarin fruit quality under chemical thinning. Acta Sci. Agron. 2013, 35, 349–356. [Google Scholar] [CrossRef]
- Li, L.J.; Hong, P.; Chen, F.; Sun, H.; Yang, Y.F.; Yu, X.; Huang, G.L.; Wu, L.M.; Ni, H. Characterization of the Aldehydes and Their Transformations Induced by UV Irradiation and Air Exposure of White Guanxi Honey Pummelo (Citrus Grandis (L.) Osbeck) Essential Oil. J. Agric. Food Chem. 2016, 64, 5000–5010. [Google Scholar] [CrossRef]
- Makkumrai, W.; Huang, Y.; Xu, Q. Comparison of Pomelo (Citrus maxima) grown in China and Thailand. Front. Agric. Sci. Eng. 2021, 8, 335–352. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Fu, X.; Chen, Q.; Wang, X. Effect of On-Tree Storage on Fruit Quality of Three Pummelo (Citrus grandis Osbeck) Cultivars. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 032052. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.K.; He, W.; Chen, Q.; Ma, J.Y.; Wang, R. Effect of bagging on fruit quality of three pummelo (Citrus grandis Osbeck) cultivars. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 29 August 2019; pp. 32–51. [Google Scholar]
- Kim, M.; Kang, S.-B.; Yun, S.K.; Kim, S.S.; Joa, J.; Park, Y. Influence of Excessively High Temperatures on the Fruit Growth and Physicochemical Properties of Shiranuhi Mandarin in Plastic-Film Greenhouse Cultivation. Plants 2021, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, M.; Kui, X.; Sun, Y.; Li, J.; Qiu, D. Comparative Studies on the Quality and Lycopene Content of Pomelo (Citrus grandis Osbeck) Cultivars. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Zheng, J.; Kallio, H.; Linderborg, K.; Yang, B. Sugars, sugar alcohols, fruit acids, and ascorbic acid in wild Chinese sea buckthorn (Hippophaë rhamnoides ssp. sinensis) with special reference to influence of latitude and altitude. Food Res. Int. 2011, 44, 2018–2026. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Trépanier, M.; Kallio, H. Effects of Genotype, Latitude, and Weather Conditions on the Composition of Sugars, Sugar Alcohols, Fruit Acids, and Ascorbic Acid in Sea Buckthorn (Hippophaë rhamnoides ssp. mongolica) Berry Juice. J. Agric. Food Chem. 2012, 60, 3180–3189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, B.; Tuomasjukka, S.; Ou, S.; Kallio, H. Effects of Latitude and Weather Conditions on Contents of Sugars, Fruit Acids, and Ascorbic Acid in Black Currant (Ribes nigrum L.) Juice. J. Agric. Food Chem. 2009, 57, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. J. Food Process. Preserv. 2017, 41, e12937. [Google Scholar] [CrossRef]
- Sáenz-Galindo, A.; López-López, L.I.; de la Cruz-Duran, F.N.; Castañeda-Facio, A.O.; Rámirez-Mendoza, L.A.; Córdova-Cisneros, K.C.; de Loera-Carrera, D. Applications of Carboxylic Acids in Organic Synthesis, Nanotechnology and Polymers. In Carboxylic Acid—Key Role in Life Sciences; InTech: London, UK, 2018. [Google Scholar]
- Kumar, V.; Sharma, A.; Bhardwaj, R.; Thukral, A.K. Analysis of organic acids of tricarboxylic acid cycle in plants using GC-MS, and system modeling. J. Anal. Sci. Technol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Khosravi, F.; Rastakhiz, N.; Iranmanesh, B.; Jafari Olia, S.S.S. Determination of Organic Acids in Fruit juices by UPLC. Int. J. Life Sci. 2015, 9, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Clements, R.L. Organic Acids in Citrus Fruits. I. Varietal Differences. J. Food Sci. 1964, 29, 276–280. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, XXI, 97–128. [Google Scholar] [CrossRef]
- Luro, F.; Gatto, J.; Costantino, G.; Pailly, O. Analysis of genetic diversity in Citrus. Plant. Genet. Resour. 2011, 9, 218–221. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, Q.; Zhou, G.; Liu, X.; Ma, Z.; Gu, Q. Identification of genes associated with soluble sugar and organic acid accumulation in ‘Huapi’ kumquat (Fortunella crassifolia Swingle) via transcriptome analysis. J. Sci. Food Agric. 2021, 101, 4321–4331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Q.; Yin, X.-R.; Lin, Q.; Chen, J.-Y.; Allan, A.C.; Xu, C.-J.; Chen, K.-S. Effect of hot air treatment on organic acid- and sugar-metabolism in Ponkan (Citrus reticulata) fruit. Sci. Hortic. 2012, 147, 118–125. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-Metabolizing Enzymes in Transport Tissues and Adjacent Sink Structures in Developing Citrus Fruit. Plant. Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, Y.; Shen, S.; Petreikov, M.; Schaffer, A.A. The contribution of sucrose to total sugar content in melons. Acta Hortic. 2000, 479–486. [Google Scholar] [CrossRef]
- Moscatello, S.; Famiani, F.; Proietti, S.; Farinelli, D.; Battistelli, A. Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (Actinidia deliciosa, cv Hayward). Sci. Hortic. 2011, 128, 197–205. [Google Scholar] [CrossRef]

| Cultivars | Location | Harvesting Time |
|---|---|---|
| Liuyuezao | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Early August |
| Hongzuanmi | Pinghe, Fujian (24°17′25″ N 117°16′35″ E 400 m) | Early December |
| Zhangyimi | Pinghe, Fujian (24°17′25″ N 117°16′35″ E 400 m) | Early December |
| Minihong | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Early November |
| Guanximi1 | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Hongroumi1 | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Hongmianmi1 | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Sanhongmi1 | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Jinjumi | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Huangjinmi1 | Pinghe, Fujian (24°16′57″ N 117°15′52″ E 200 m) | Mid October |
| Hongmianmi2 | Yongchun, Fujian (25°20′18″ N 118°15′54″ E 200 m) | Early October |
| Sanhongmi2 | Yongchun, Fujian (25°20′18″ N 118°15′54″ E 150 m) | Early October |
| Guanximi2 | Pujiang, Sichuan (25°20′18″ N 118°15′54″ E 500 m) | Late October |
| Hongroumi2 | Pujiang, Sichuan (25°20′18″ N 118°15′54″ E 500 m) | Late October |
| Hongmianmi3 | Pujiang, Sichuan (25°20′18″ N 118°15′54″ E 500 m) | Late October |
| Sanhongmi3 | Pujiang, Sichuan (25°20′18″ N 118°15′54″ E 500 m) | Mid November |
| Huangjinmi2 | Pujiang, Sichuan (25°20′18″ N 118°15′54″ E 500 m) | Mid November |
| Sanhongmi4 | Qingyuan, Guangdong (23°45′04″ N 113°04′02″ E 100 m) | Early November |
| Wendan | Xianyou, Fujian (25°24′23″ N 118°44′06″ E 100 m) | Late October |
| Siji | Fuding, Fujian (27°18′13″ N 120°19′18″ E 200 m) | Mid November |
| Shuijing | Fuqing, Fujian (25°44′50″ N 119°12′00″ E 100 m) | Late November |
| Wubu | Banan, Chongqing (29°20′54″ N 106°30′47″ E 250 m) | Early December |
| Hangwanmi | Shanghang, Fujian (24°56′45″ N 116°31′17″ E 200 m) | Early December |
| Shatian | Rong, Guangxi (22°46′45″ N 110°36′37″ E 400 m) | Mid December |
| Cultivar | Fruit Weight (g) | Fruit Width (mm) | Fruit Length (mm) | Fruit Shape Index |
|---|---|---|---|---|
| Liuyuezao | 1243.27 eh | 14 efg | 14.87 def | 1.06 a–d |
| Hongzuanmi | 1553.17 bcd | 16.63 bc | 15 b–f | 0.9 ghi |
| Zhangyimi | 1608.28 bc | 16.56 bc | 16.88 bc | 1.01 a–f |
| Minihong | 264.63 k | 8.6 h | 7.4 i | 0.86 ij |
| Guanximi1 | 1268.9 d–g | 14.27 d–g | 14.33 e–h | 1 b–f |
| Hongroumi1 | 1109.99 fi | 13.64 efg | 13.6 fgh | 0.99 b–g |
| Hongmianmi1 | 961.92 hij | 12.92 g | 12.82 gh | 0.99 b–g |
| Sanhongmi1 | 1442.06 cde | 16.73 bc | 16.99 b | 1.01 a–f |
| Jinjumi | 1036.04 f–j | 14.52 def | 16.02 b–e | 1.1 a |
| Huangjinmi1 | 899.05 ij | 14.42 def | 14.81 efg | 1.02 a–f |
| Hongmianmi2 | 1105.52 fi | 13.97 efg | 15.25 b–f | 1.09 ab |
| Sanhongmi2 | 1223.4 e–h | 14.55 de | 15.68 b–e | 1.07 abc |
| Guanximi2 | 1852.92 ab | 17.03 b | 16.83 bcd | 0.98 c–h |
| Hongroumi2 | 1730.98 abc | 18.49 a | 19.4 a | 1.05 a–e |
| Hongmianmi3 | 1936.14 a | 18.7 a | 20.7 a | 1.1 a |
| Sanhongmi3 | 1945.85 a | 19.56 a | 19.63 a | 1 b–f |
| Huangjinmi2 | 1290.44 def | 14.36 def | 15.56 b–f | 1.08 ab |
| Sanhongmi4 | 1103.97 f–i | 14.63 de | 15.53 b–f | 1.06 a–d |
| Wendan | 827.66 ij | 14.86 de | 14.05 eh | 0.94 fi |
| Siji | 765.09 j | 13.13 fg | 12.76 h | 0.97 d–h |
| Shuijing | 1304.11 def | 16.3 bc | 14.43 e–h | 0.88 hij |
| Wubu | 1496.53 cde | 16.96 bc | 13.6 fgh | 0.8 j |
| Hangwanmi | 1669.23 abc | 15.6 cd | 14.93 c–f | 0.95 e–i |
| Shatian | 968.11 g–j | 14.66 de | 14.9 c–f | 1.01 a–f |
| LSD (p ≤ 0.05) | 301.640 | 1.392 | 2.003 | 0.099 |
| Cultivar | Peel Thickness | Segments (No.) | Pulp Weight (g) | Pulp Color | Soluble Solids (Brix°) | Vitamin C (mg/100 g) |
|---|---|---|---|---|---|---|
| Liuyuezao | 1.1 k | 13.88 def | 880.58 e–h | White | 10.27 h–k | 41.93 d–g |
| Hongzuanmi | 1.51 g–k | 15 cde | 1154.41 a–d | Light yellow | 13.51 b | 51.86 bc |
| Zhangyimi | 2.05 c–f | 13.88 def | 1078.61 b–e | Yellow | 11.03 e–j | 48.71 cde |
| Minihong | 0.46 l | 11 g | 210.25 m | Red | 12.42 bcd | 36.53 fg |
| Guanximi1 | 1.51 g–k | 14 def | 981.67 def | White | 10.62 f–k | 35.95 fg |
| Hongroumi1 | 1.37 jk | 14.66 cde | 869.66 fgh | Red | 13.02 bc | 34.79 g |
| Hongmianmi1 | 1.26 jk | 15.22 cd | 699.05 hij | White | 11.3 d–h | 41 d–g |
| Sanhongmi1 | 1.42 h–k | 12 fg | 1009.34 c–f | Red | 11.73 c–g | 41.31 d–g |
| Jinjumi | 1.63 f–k | 15 cde | 708.83 hij | Orange | 11.18 d–i | 47.55 cde |
| Huangjinmi1 | 1.42 ijk | 15.22 cd | 628.88 ijk | Orange | 12.07 cde | 48.44 cde |
| Hongmianmi2 | 2.19 b–e | 14.22 cde | 701.57 hij | White | 10.91 e–j | 46.57 cde |
| Sanhongmi2 | 2.66 b | 14.44 cde | 738.22 hij | Red | 10.84 e–j | 44.2 c–g |
| Guanximi2 | 1.94 c–i | 14.66 cde | 1351.66 a | White | 9.94 ijk | 42.08 d–g |
| Hongroumi2 | 3.33 a | 15 cde | 1042.95 c–f | Red | 10.43 h–k | 39.39 efg |
| Hongmianmi3 | 2.3 bcd | 13 efg | 1192.52 abc | White | 10.25 h–k | 48.53 cde |
| Sanhongmi3 | 2.43 bc | 16.33 bc | 1257.27 ab | Red | 10.54 g–k | 47.62 cde |
| Huangjinmi2 | 1.95 c–h | 14 def | 868.08 fgh | Orange | 11.18 d–i | 49.16 cd |
| Sanhongmi4 | 1.76 d–j | 15.66 cd | 776.07 ghi | Red | 10.9 e–j | 45.3 c–f |
| Wendan | 2.05 c–f | 15 cde | 403.55 lm | White | 11.83 c–f | 42.36 d–g |
| Siji | 1.97 c–g | 14 def | 438.43 kl | White | 9.4 kl | 34.99 g |
| Shuijing | 1.74 e–j | 13 efg | 878.48 e–h | White | 8.59 l | 37.12 fg |
| Wubu | 1.99 c–g | 18.66 a | 957.86 d–g | White | 9.82 jkl | 60.89 b |
| Hangwanmi | 2.3 bc | 18 ab | 995.75 c–f | White | 11.76 c–g | 48.56 cde |
| Shatian | 1.73 e–j | 11.66 g | 559.54 jkl | White | 15.14 a | 84.58 a |
| LSD (p ≤ 0.05) | 0.538 | 2.189 | 203.69 | 1.285 | 9.441 |
| Cultivar | Fructose (mg/g) | Glucose (mg/g) | Sucrose (mg/g) | Total sugars (mg/g) |
|---|---|---|---|---|
| Liuyuezao | 20.68 bc | 21 b | 21.16 k | 62.84 mn |
| Hongzuanmi | 21.62 b | 21.19 b | 38.91 b–e | 81.72 c |
| Zhangyimi | 17.08 jk | 16.86 ij | 34.92 fgh | 68.87 i–l |
| Minihong | 24.24 a | 24.22 a | 19.9 k | 68.37 jkl |
| Guanximi1 | 18.16 g–j | 17.9 f–i | 39.49 bcd | 75.57 efg |
| Hongroumi1 | 19.88 cde | 19.3 cde | 55.28 a | 94.47 a |
| Hongmianmi1 | 20.44 bcd | 20.17 bc | 40.48 bc | 81.1 cd |
| Sanhongmi1 | 18.71 e–i | 18.33 d–h | 40.91 b | 77.96 cde |
| Jinjumi | 18.56 f–i | 18.39 d–h | 35.95 efg | 72.91 ghi |
| Huangjinmi1 | 19.6 c–f | 19.39 cde | 37.26 def | 76.26efg |
| Hongmianmi2 | 17.8 ij | 17.65 ghi | 29.19 j | 64.66 lmn |
| Sanhongmi2 | 17.99 hij | 17.2 hij | 37.85 b–f | 73.06 f–i |
| Guanximi2 | 18.53 f–i | 18.13 e–i | 29.95 j | 66.62 klm |
| Hongroumi2 | 18.49 f–i | 18.28 d–h | 38.01 b–f | 74.79 e–h |
| Hongmianmi3 | 19.11 e–h | 18.65 d–g | 31.35 ij | 69.12 ijk |
| Sanhongmi3 | 18.43 f–i | 18.14 e–i | 31.87 hij | 68.45 jkl |
| Huangjinmi2 | 19.02 e–i | 18.59 d–g | 33.51 ghi | 71.13 hij |
| Sanhongmi4 | 19.3 d–g | 18.76 d–g | 37.6 c–f | 75.67 efg |
| Wendan | 18.16 g–j | 18.04 e–i | 40.31 bcd | 76.51 efg |
| Siji | 18.59 f–i | 18.49 d–h | 29.81 j | 66.9 j–m |
| Shuijing | 16.25 k | 16.17 j | 28.96 j | 61.39 n |
| Wubu | 19.33 d–g | 19.26 c–f | 38.75 b–e | 77.35 def |
| Hangwanmi | 19.23 d–h | 19.61 cd | 37.21 def | 76.05 efg |
| Shatian | 17.85 ij | 17.73 ghi | 54.04 a | 89.62 b |
| LSD (p ≤ 0.05) | 1.249 | 1.365 | 3.117 | 4.292 |
| Cultivar | Pyruvate (mg/g) | Fumaric Acid (mg/g) | Succinic Acid (mg/g) | Tartaric Acid (mg/g) | Quinic Acid (mg/g) | Citric Acid (mg/g) | Malic Acid (mg/g) | Cis-Aconitic Acid (mg/g) | Total Acids (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Liuyuezao | 1.12 cde | 0.04 de | 0.03 b–e | 0.02 bcd | 0.14 a | 9.08 bc | 1.41 a | 0.13 ab | 12 b |
| Hongzuanmi | 1.76 a | 0.02 e | 0.02 c–f | 0.02 cd | 0.01 efg | 11.65 a | 1.29 ab | 0.03 gh | 14.83 a |
| Zhangyimi | 1.23 bcd | 0.03 de | 0.02 b–f | 0.02 bcd | 0.05 cd | 7.91 cde | 1.02 bc | 0.03 gh | 10.36 cd |
| Minihong | 1.84 a | 0.04 de | 0.02 c–f | 0.02 cd | 0.05 cd | 11.85 a | 0.51 e–i | 0.16 a | 14.52 a |
| Guanximi1 | 0.74 g–j | 0.15 cde | 0.01 c–f | 0.01 d | 0.01 efg | 6.57 efg | 0.3 hi | 0.07 cde | 7.89 ghi |
| Hongroumi1 | 1.35 bc | 0.04 de | 0 f | 0.01 d | 0.06 c | 6.59 d–g | 0.37 f–i | 0.09 cd | 8.55 fgh |
| Hongmianmi1 | 1.45 b | 0.03 de | 0.02 c–f | 0.02 cd | 0.03 d–g | 6.76 d–g | 0.29 i | 0.1 bc | 8.72 e–h |
| Sanhongmi1 | 0.61 i–l | 0.15 cde | 0.01 c–f | 0.01 d | 0.02 efg | 5.72 fgh | 0.33 ghi | 0.06 d–g | 6.95 ijk |
| Jinjumi | 0.74 g–j | 0.18 bcd | 0.04 ab | 0.04 bc | 0.06 cd | 6.41 fg | 0.48 f–i | 0.08 cde | 8.06 ghi |
| Huangjinmi1 | 0.67 h–l | 0.19 bcd | 0.05 a | 0.04 b | 0.06 cd | 5.94 fgh | 0.53 d–i | 0.07 c–f | 7.59 hij |
| Hongmianmi2 | 0.9 e–h | 0.15 cde | 0.02 b–f | 0.02 cd | 0.04 cde | 7.79 cde | 0.3 hi | 0.07 c–g | 9.32 d–g |
| Sanhongmi2 | 0.64 i–l | 0.17 b–e | 0.03 abc | 0.02 bcd | 0.05 cd | 5.57 f–i | 0.72 c–f | 0.06 d–g | 7.32 h–k |
| Guanximi2 | 1.01 def | 0.03 de | 0.02 b–f | 0.01 d | 0.02 efg | 6.43 fg | 0.46 f–i | 0.13 b | 8.15 ghi |
| Hongroumi2 | 0.96 efg | 0.15 b–e | 0.02 b–f | 0.1 a | 0.03 c–g | 8.22 c | 0.51 e–i | 0.02 h | 10.06 c–f |
| Hongmianmi3 | 0.72 h–k | 0.14 cde | 0.02 c–f | 0.01 d | 0.01 g | 6.79 def | 0.18 i | 0.03 gh | 7.93 ghi |
| Sanhongmi3 | 1 ef | 0.15 b–e | 0.02 b–f | 0.01 d | 0.01 efg | 8.65 bc | 0.23 i | 0.05 e–h | 10.16 cde |
| Huangjinmi2 | 0.89 e–h | 0.03 de | 0.02 c–f | 0.01 d | 0.04 c–f | 9.67 b | 0.67 c–h | 0.02 h | 11.38 bc |
| Sanhongmi4 | 0.48 l | 0.25 bc | 0.01 def | 0.01 d | 0 g | 4.31 ij | 0.88 cde | 0.02 h | 5.99 kl |
| Wendan | 1.01 def | 0.15 cde | 0.01 ef | 0.01 d | 0.05 cd | 8.84 bc | 0.89 cd | 0.02 h | 11.01 bc |
| Siji | 0.52 jkl | 0.17 b–e | 0.02 b–f | 0.02 cd | 0.1 b | 4.87 hi | 0.41 f–i | 0.03 gh | 6.17 jk |
| Shuijing | 0.5 kl | 0.05 de | 0.03 abc | 0.02 d | 0.01 efg | 3.01 j | 0.95 bc | 0.01 h | 4.62 l |
| Wubu | 1.28 bc | 0.04 de | 0.03 abc | 0.02 cd | 0.02 efg | 7.93 cd | 0.69 c–g | 0.04 fgh | 10.08 c–f |
| Hangwanmi | 1.77 a | 0.45 a | 0.03 bcd | 0.02 cd | 0.05 cd | 11.08 a | 1.42 a | 0.03 gh | 14.89 a |
| Shatian | 0.83 f–i | 0.31 ab | 0.04 ab | 0.02 cd | 0.01 fg | 5.43 ghi | 0.94 bc | 0.09 cd | 7.7 hij |
| LSD (p ≤ 0.05) | 0.234 | 0.155 | 0.021 | 0.022 | 0.031 | 1.348 | 0.373 | 0.035 | 1.551 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.; Ali, M.M.; Gong, J.; She, W.; Pan, D.; Guo, Z.; Yu, Y.; Chen, F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy 2021, 11, 2393. https://doi.org/10.3390/agronomy11122393
Pan T, Ali MM, Gong J, She W, Pan D, Guo Z, Yu Y, Chen F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy. 2021; 11(12):2393. https://doi.org/10.3390/agronomy11122393
Chicago/Turabian StylePan, Tengfei, Muhammad Moaaz Ali, Jiangmei Gong, Wenqin She, Dongming Pan, Zhixiong Guo, Yuan Yu, and Faxing Chen. 2021. "Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China" Agronomy 11, no. 12: 2393. https://doi.org/10.3390/agronomy11122393
APA StylePan, T., Ali, M. M., Gong, J., She, W., Pan, D., Guo, Z., Yu, Y., & Chen, F. (2021). Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy, 11(12), 2393. https://doi.org/10.3390/agronomy11122393







