The Effects of Bioinoculants Based on Mycorrhizal and Trichoderma spp. Fungi in an Apple Tree Nursery under Replantation Conditions
Abstract
:1. Introduction
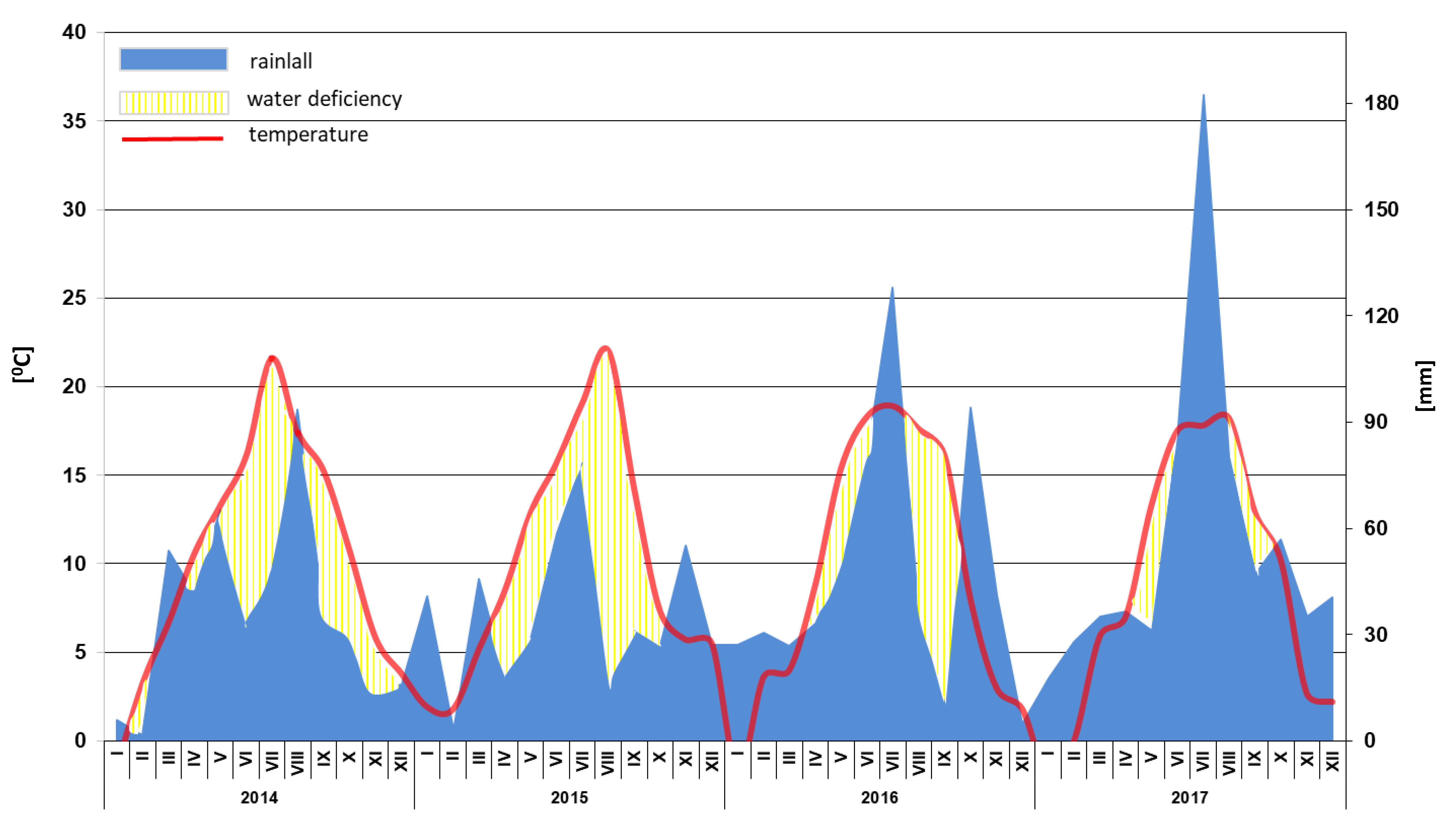
2. Materials and Methods
2.1. Experimental Design
2.2. Measurements of the Vegetative Growth of the Trees
2.3. Analysis of Biological Properties of Soil
3. Results and Discussion
3.1. The Influence of the Site on the Biological Properties of Soil
3.2. The Influence of Mycorrhizal Fungi and Trichoderma spp. on the Biological Properties of Replant Soil
3.3. Soil Enzymatic Activity in Different Vegetation Periods
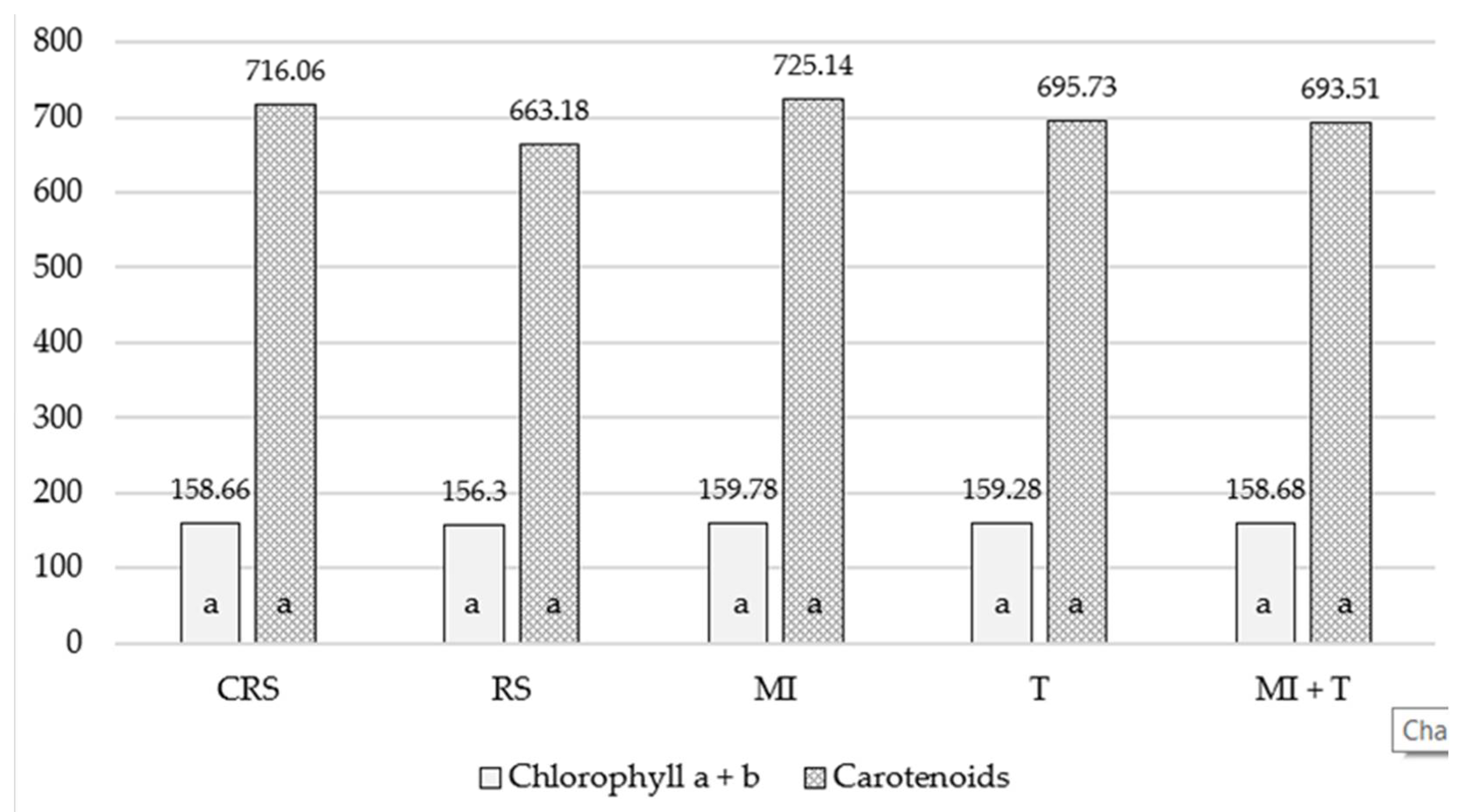
3.4. The Influence of the Site on the Growth of Apple Trees
3.5. The Influence of Soil Preparations on the Growth Power of Apple Trees
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; Mc Ferson, J.; Zhong, G.Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Laurent, A.S.; Merwin, I.A.; Thies, J.E. Long-term orchard groundcover management systems affect soil microbial communities and apple replant disease severity. Plant Soil 2008, 304, 209–225. [Google Scholar] [CrossRef]
- Atucha, A.; Emmett, B.; Bauerle, T.L. Growth rate of fine root systems influences rootstock tolerance to replant disease. Plant Soil 2014, 376, 337–346. [Google Scholar] [CrossRef]
- Yin, C.; Xiang, L.; Wang, G.; Wang, Y.; Shen, X.; Chen, X.; Zhiquan Mao, Z. How to plant apple trees to reduce replant disease in apple orchard: A study on the phenolic acid of the replanted apple orchard. PLoS ONE 2016, 11, e0167347. [Google Scholar] [CrossRef]
- Sobiczewski, P.; Treder, W.; Bryk, H.; Klamkowski, K.; Krzewińska, D.; Mikiciński, A.; Berczyński, S.; Tryngiel-Gać, A. The impact of phytosanitary treatments in the soil with signs of fatigue on the growth of apple seedlings and populations of bacteria and fungi. Pol. J. Agron. 2018, 34, 11–22. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Ann. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.T.; Wang, G.S.; Li, Y.Y.; Shen, X.; Chen, X.S.; Song, F.H.; Wu, S.J.; Che, Q.; Mao, Z.Q. Replanting affects the tree growth and fruit quality of Gala apple. J. Integr. Agric. 2014, 13, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Spath, M.; Insam, H.; Peintner, U.; Kelderer, M.; Kuhnert-Finkernagel, R.; Franke-Whittle, I.H. Linking soil biotic and abiotic factors to apple replant disease: A greenhouse approach. J. Phytophatol. 2015, 163, 287–299. [Google Scholar] [CrossRef]
- Politycka, B.; Adamska, D. Release of phenolic compounds from apple residues decomposing in soil and the influence of temperature on their degradation. Pol. J. Environ. Stud. 2003, 12, 95–98. [Google Scholar]
- Hofmann, A.; Wittenmayer, L.; Arnold, G.; Schieber, A.; Merbach, W. Root exudation of phloridzin by apple seedlings (Malus x domestica Borkh.) with symptoms of apple replant disease. J. Appl. Bot. Food Qual. 2012, 82, 193–198. [Google Scholar]
- Utkhede, R.S. Soil sickness, replant problem or replant disease and its integrated control. Allelopath. J. 2006, 18, 23–38. [Google Scholar]
- Tewoldemedhin, Y.T.; Mazzola, M.; Labuschagne, I.; McLeod, A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol. Biochem. 2011, 43, 1917–1927. [Google Scholar] [CrossRef]
- Kelderer, M.M.; Manici, L.; Caputo, F.; Thalheimer, M. Planting in the ‘inter-row’ to overcome replant disease in apple orchards: A study on the effectiveness of the practice based on microbial indicators. Plant Soil 2012, 357, 381–393. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Mazzola, M. Auxin-mediated relationships between apple plants and root inhabiting fungi: Impact on root pathogens and potentialities of growth-promoting populations. Plant Pathol. 2015, 64, 843–851. [Google Scholar] [CrossRef]
- Kobi, H.B.; Martins, M.C.; Silva, P.I.; Souza, J.L.; Carneiro, J.C.S.; Heleno, F.; Queiroz, M.E.L.R.; Costa, N.M. Organic and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Gąstoł, M.; Domagała-Swiątkiewicz, I. Mycorrhizal inoculation of apple in replant soils—Enhanced tree growth and mineral status. Acta Sci. Pol. Hortorum Cultus 2015, 14, 17–37. [Google Scholar]
- Poveda, J. Trichoderma parareesei favors the tolerance of rapeseed (Brassica napus L.) to salinity and sought due to a chorismate Mutase. Agronomy 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Khoshmanzar, E.; Aliasgharzad, N.; Neyshabouri, M.R.; Khoshru, B.; Arzanlou, M.; Lajayer, B.A. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int. J. Environ. Sci. Technol. 2019, 17, 869–878. [Google Scholar] [CrossRef]
- Rubio, M.B.; Hermosa, R.; Vicente, R.; Gómez-Acosta, F.A.; Morcuende, R.; Monte, E.; Bettiol, W. The combination of Trichoderma harzianum and chemical fertilization leads to the deregulation of phytohormone networking, preventing the adaptive responses of tomato plants to salt stress. Front. Plant Sci. 2017, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Hyakumachi, M. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae induce systemic resistance against Cucumber mosaic virus in cucumber plants. Plant Soil 2012, 361, 397–409. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucini, L.; Colla, G.; Miras Morebo, M.B.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride deferentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, A.S.; TariqJaveed, M. Effects of different inoculum densities of Trichoderma harzianum and Trichoderma viride against Meloidogyne javanica on tomato. Saudi J. Biol. Sci. 2016, 23, 288–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, A.; Lubraco, G. Mycorrhizal inoculation enhances growth and nutrient uptake of micropropagated apple rootstocks during weaning in commercial substrates of high nutrient availability. Appl. Soil Ecol. 2000, 15, 113–118. [Google Scholar] [CrossRef]
- Ridgway, H.J.; Kandula, D.R.W.; Stewart, A. Arbuscular mycorrhiza improve apple rootstock growth in soil conductive to specific replant disease. N. Z. Plant Prot. 2008, 61, 48–53. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1978, 5, 1332–1334. [Google Scholar]
- Ladd, N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Thalmann, A. Zur methodik der bestimmung der dehydrogenaseaktivität im boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirdschaft Forsch. 1968, 21, 249–258. [Google Scholar]
- Gołębiowska, J.; Pędziwilk, Z. CO2 release as on index of biological activity of cultivated soils. Acta Microbiol. Pol. 1984, 33, 249–256. [Google Scholar]
- Allen, M.F.; Moore, T.S.; Christensen, M.; Stanton, N. Growth of vesicular-arbuscular mycorrhizal and nonmycorrhizal Bouteloua gracilis in a defined medium. Mycologia 1979, 71, 666–669. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P. The effect of microbiological products on soil properties in the conditions of replant disease. Zemdirb. Agric. 2013, 100, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Wolińska, A.; Stępniewska, Z.; Szymańska, E. Dehydrogenase activity of soil microorganisms and total DNA level in soil of different use. J. Agric. Sci. Technol. B 2013, 3, 613–621. [Google Scholar]
- Furtak, K.; Gajda, A.M. Activity of dehydrogenases as an indicator of soil environment quality. Pol. J. Soil Sci. 2017, 50, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, C.; Manquel, D.; Cornejo, P.; Soto, J.; Sampedro, I.; Ocampo, J. Effects of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. J. Soil Sci. Plant Nutr. 2012, 12, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in west Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Natywa, M.; Selwet, M. Respiratory and dehydrogenase activities in the soils under maize growth in the conditions of irrigated and nonirrigated fields. Agricultura 2011, 10, 93–100. [Google Scholar]
- Järvan, M.; Edesi, L.; Adamson, A.; Võsa, T. Soil microbial communities and dehydrogenases activity depending on farming systems. Plant Soil Environ. 2014, 60, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Yu, J.; Nong, M.; Kang, S.; Zhang, J. Partial root-zone irrigation enhanced soil enzyme activities and water use of maize under different ratios of inorganic to organic nitrogen fertilizers. Agric. Water Manag. 2010, 97, 925–934. [Google Scholar] [CrossRef]
- Sumorok, B.; Sas Paszt, L.; Głuszek, S.; Derkowska, E.; Żurawicz, E. The effect of mycorrhization and mulching of apple trees ‘Gold Milenium’ and blackcurrant bushes ‘Tiben’ on the occurrence of arbuscular mycorrhizal fungi. J. Fruit Ornam. Plant Res. 2011, 19, 35–49. [Google Scholar]
- Mikiciuk, L.; Sas-Paszt, L.; Mikiciuk, M.; Derkowska, E.; Trzciński, P.; Głuszek, A.; Rudnicka, J. Mycorrhizal frequency, physiological parameters, and yield of strawberry plants inoculated with endomycorrhizal fungi and rhizosphere bacteria. Mycorrhiza 2019, 29, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Sheng, M.; Chen, X.; Zhang, X.; Hamel, C.; Cui, X.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2017, 599–600, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Horwath, W.; Scow, K. Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity. Pedobiologia 2011, 54, 71–78. [Google Scholar] [CrossRef]
- Weaver, M.; Zabłotowicz, R.; Krutz, L.; Bryson, C.; Locke, M. Microbial and vegetative changes associated with development of a constructed wetland. Ecol. Indic. 2012, 13, 37–45. [Google Scholar] [CrossRef]
- Yuan, B.; Yue, D. Soil microbial and enzymatic activities across a chronosequence of chinese pine plantation development on the Loess Plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Ogaya, R. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. Forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Weiß, S.; Winkelmann, T. Transcriptome profiling in leaves representing aboveground parts of apple replant disease affected Malus domestica ‘M26’ plants. Sci. Hortic. 2017, 222, 111–125. [Google Scholar] [CrossRef]
- Raj, H.; Sharma, S.D. Integration of soil solarization and chemical sterilization with beneficial microorganisms for the control of white root and growth of nursery apple. Sci. Hortic. 2009, 119, 126–131. [Google Scholar] [CrossRef]
- Świerczyński, S.; Stachowiak, A.; Golcz-Polaszewska, M. Maiden pear trees growth in replant soil after inoculation of rootstocks with mycorrhizal inoculum. Nauka Przyr. Technol. 2015, 9, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Aka-Kacar, Y.; Akpinar, C.; Agar, A.; Yalcin-Mendi, Y.; Serce, S.; Ortas, I. The effect of mycorrhiza in nutrient uptake and biomass of cherry rootstocks during acclimatization. Rom. Biotech. Lett. 2010, 15, 5246–5252. [Google Scholar]
- Monticelli, S.; Puppi, G.; Damiano, C. Effects of in vivo mycorrhization on micropropagated fruit tree rootstocks. Appl. Soil Ecol. 2010, 15, 105–111. [Google Scholar] [CrossRef]
- Mehta, P.; Bharat, N.K. Effect of indigenous arbuscular- mycorrhiza (Glomus spp) on apple (Malus domestica) seedlings grown in replant diseased soil. Ind. J. Agric. Sci. 2013, 83, 1173–1178. [Google Scholar]
- Grzyb, Z.S.; Sas Paszt, L.; Piotrowski, W.; Malusa, E. The influence of mycorrhizal fungi on the growth of apple and sour cherry maidens fertilized with different bioproducts in the organic nursery. J. Life Sci. 2015, 9, 221–228. [Google Scholar]
- Druzic-Orlic, J.; Redzepovic, S. Influence of arbuscular mycorrhizal fungi on fruit rootstocks. Acta Hortic. 2008, 767, 393–396. [Google Scholar] [CrossRef]
- Boyer, L.R.; Brain, P.; Xu, X.-M.; Jeffries, P. Inoculation of drought stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, H.; Yildirim, E.; Turan, M.; Pehluvan, M.; Donmez, F. Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria x ananassa). Hort Sci. 2013, 48, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Zhao, P.; Wang, M.; Ahammed, G.J. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedling. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morenoa, M.J.; Moreno-Márqueza, M.C.; Moreno-Alíasb, I.; Rapoportb, H.; Fernández-Escobar, R. Interaction between mycorrhization with Glomus intraradices and phosphorus in nursery olive plants. Sci. Hortic. 2018, 233, 249–255. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Vafadar, F.; Amooaghaie, R.; Otroshy, M. Effects of plant-growth promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J. Plant Interact. 2014, 9, 128–136. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, S.; Mo, X.; Li, Y.; Fu, Y.; Liu, Z. Effects of plant growth promoting rhizobacteria and N source on plant growth and N and P uptake by tomato grown on calcareous soil. Pedossphere 2017, 27, 1027–1036. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Tullio, M.; Rivera, C.M.; Rea, E. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol. Fert. Soils 2008, 44, 501–509. [Google Scholar] [CrossRef]



| Treatment | >150 µm | >75 µm | >50 µm | Total in 100 g Air-dm |
|---|---|---|---|---|
| CRS | 35 | 455 | 510 | 1000 |
| RS | 30 | 340 | 385 | 755 |
| MI | 50 | 870 | 890 | 1810 |
| Treatment | Spring | Summer | Autumn | Average for Treatment |
|---|---|---|---|---|
| CRS | 1.49 g (0.15) | 1.18 de (0.03) | 2.86 h (0.49) | 1.84 b |
| RS | 0.24 a (0.02) | 0.85 c (0.04) | 0.48 b (0.14) | 0.52 a |
| MI | 0.80 c (0.03) | 0.33 ab (0.03) | 1.24 ef (0.06) | 0.79 a |
| T | 0.87 c (0.04) | 0.96 cd (0.02) | 1.55 g (0.06) | 1.13 ab |
| MI + T | 0.95 cd (0.04) | 0.41 ab (0.05) | 1.43 fg (0.07) | 0.93 ab |
| Average for term | 0.87 b | 0.74 a | 1.51 c |
| Treatment | Spring | Summer | Autumn | Average for Treatment |
|---|---|---|---|---|
| CRS | 6.38 de (0.97) | 3.26 ab (0.69) | 4.09 bc (0.57) | 4.58 b |
| RS | 2.01 a (0.43) | 1.90 a (0.75) | 3.35 ab (0.47) | 2.42 a |
| MI | 7.03 e (1.40) | 3.77 b (0.74) | 6.19 de (1.79) | 5.66 b |
| T | 4.64 b–d (1.10) | 5.69 c–e (0.67) | 6.72 e (0.53) | 5.68 b |
| MI + T | 7.48 e (1.12) | 13.52 f (1.12) | 5.74 c–e (1.52) | 8.91 c |
| Average for term | 5.51 a | 5.63 a | 5.22 a |
| Treatment | Spring | Summer | Autumn | Average for Treatment |
|---|---|---|---|---|
| CRS | 57.50 h (7.40) | 30.81 d–f (5.24) | 32.63 ef (5.14) | 40.31 c |
| RS | 16.58 ab (1.72) | 9.24 a (2.04) | 11.63 a (1.80) | 12.48 a |
| MI | 53.79 h (5.89) | 27.27 c–e (3.62) | 20.54 bc (3.37) | 33.87 b |
| T | 52.10 h (6.24) | 32.30 d–f (4.23) | 24.05 c–d (2.94) | 36.15 bc |
| MI + T | 42.03 g (7.51) | 38.32 fg (5.62) | 26.32 c–e (3.24) | 35.56 b |
| Average for term | 44.4 c | 27.59 b | 23.03 a |
| Enzyme | 2015 | 2016 |
|---|---|---|
| Dehydrogenases | 0.88 a | 1.21 b |
| Proteases | 4.77 a | 6.13 b |
| Treatment | Height of Trees (cm) | Number of Side Shoots | Total Length of Side Shoots (cm) |
|---|---|---|---|
| CRS | 177.8 b (9.15) | 10 ab (2.58) | 86.16 bc (8.41) |
| RS | 147.0 a (13.54) | 7.0 a (1.34) | 40.23 a (10.24) |
| MI | 176.7 b (11.02) | 10 ab (1.84) | 48.33 ab (3.38) |
| T | 175.8 b (9.85) | 10 ab (1.53) | 87.09 bc (8.24) |
| MI + T | 190.5 c (11.74) | 12 b (1.76) | 128.09 c (9.85) |
| Treatment | Weight of Leaves (g) | Width of Leaves (cm) | Length of Leaves (cm) | Surface Area of Leaves (cm2) |
|---|---|---|---|---|
| CRS | 14.62 b (1.75) | 2.32 c (0.02) | 3.13 b (0.03) | 42.47 b (1.84) |
| RS | 7.56 a (1.10) | 1.72 a (0.87) | 2.56 a (0.21) | 25.88 a (2.85) |
| MI | 14.33 b (1.90) | 2.02 b (0.03) | 3.02 b (0.03) | 46.11 c (1.53) |
| T | 16.06 c (1.40) | 2.16 bc (0.02) | 3.49 c (0.02) | 45.83 c (1.62) |
| MI + T | 17.52 d (1.20) | 1.87 ab (0.02) | 2.98 b (0.03) | 48.94 d (1.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zydlik, Z.; Zydlik, P.; Wieczorek, R. The Effects of Bioinoculants Based on Mycorrhizal and Trichoderma spp. Fungi in an Apple Tree Nursery under Replantation Conditions. Agronomy 2021, 11, 2355. https://doi.org/10.3390/agronomy11112355
Zydlik Z, Zydlik P, Wieczorek R. The Effects of Bioinoculants Based on Mycorrhizal and Trichoderma spp. Fungi in an Apple Tree Nursery under Replantation Conditions. Agronomy. 2021; 11(11):2355. https://doi.org/10.3390/agronomy11112355
Chicago/Turabian StyleZydlik, Zofia, Piotr Zydlik, and Robert Wieczorek. 2021. "The Effects of Bioinoculants Based on Mycorrhizal and Trichoderma spp. Fungi in an Apple Tree Nursery under Replantation Conditions" Agronomy 11, no. 11: 2355. https://doi.org/10.3390/agronomy11112355
APA StyleZydlik, Z., Zydlik, P., & Wieczorek, R. (2021). The Effects of Bioinoculants Based on Mycorrhizal and Trichoderma spp. Fungi in an Apple Tree Nursery under Replantation Conditions. Agronomy, 11(11), 2355. https://doi.org/10.3390/agronomy11112355






