Direct and Indirect Effects of Invasive vs. Native Ant-Hemipteran Mutualism: A Meta-Analysis That Supports the Mutualism Intensity Hypothesis
Abstract
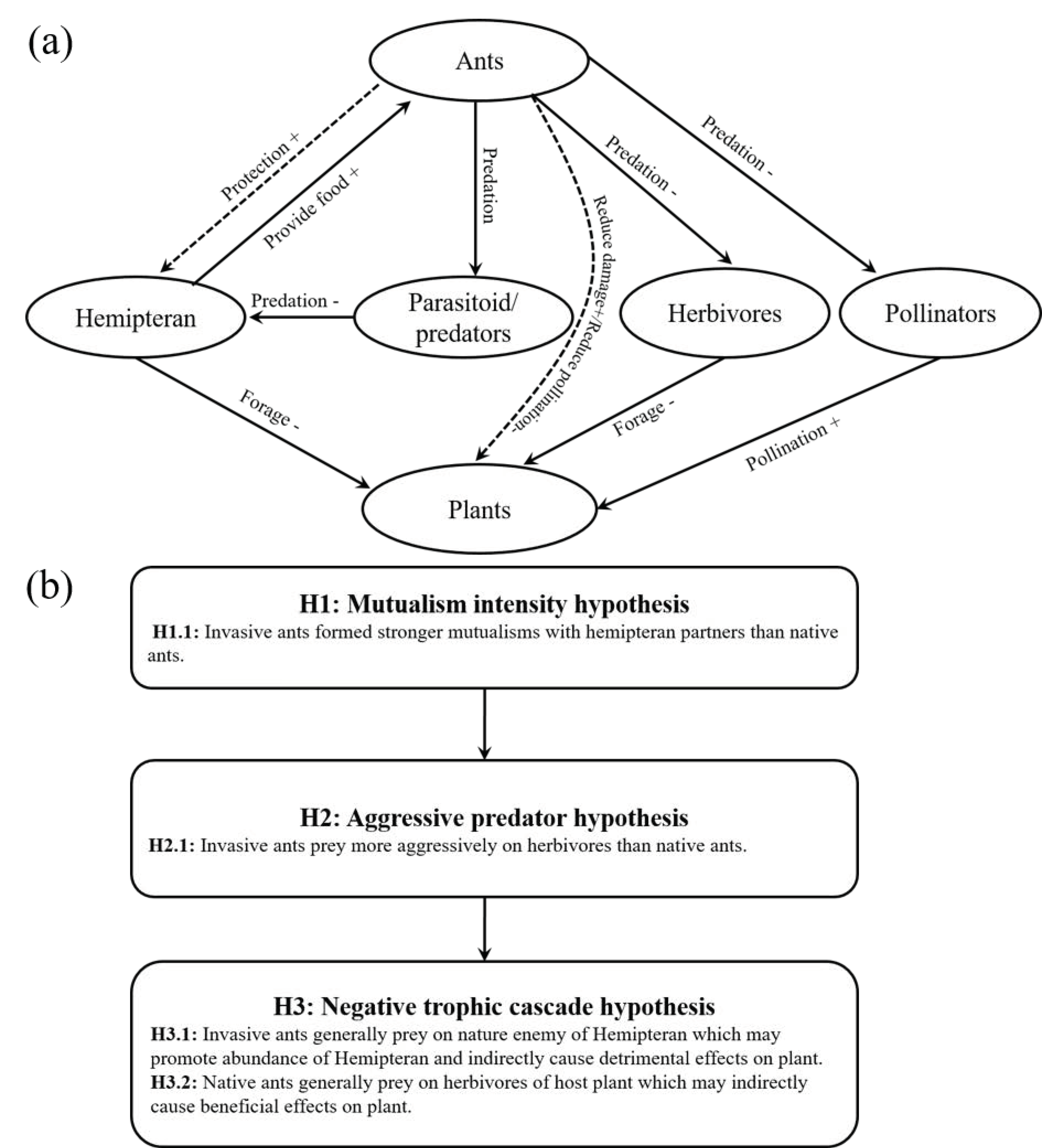
1. Introduction
2. Materials and Methods
2.1. Data Search
2.2. Criteria for Data Selection
2.3. Data Extraction and Effect Size Metrics
2.4. Categories
2.5. Data Analysis
3. Results
3.1. Prey Categories across Native and Invasive Ants
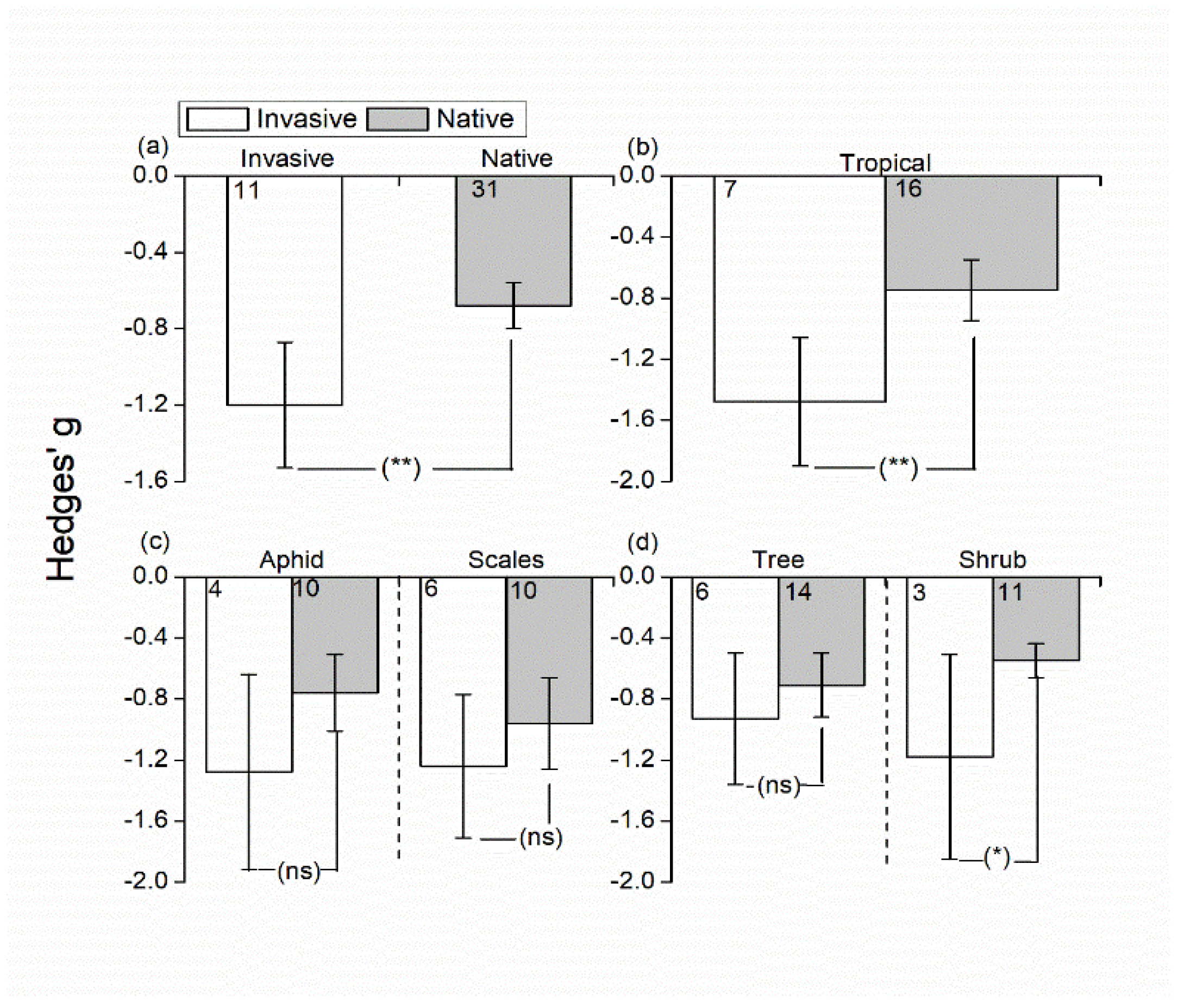
3.2. Effects of Invasive/Native Ant Exclusion on Hemipteran Abundance
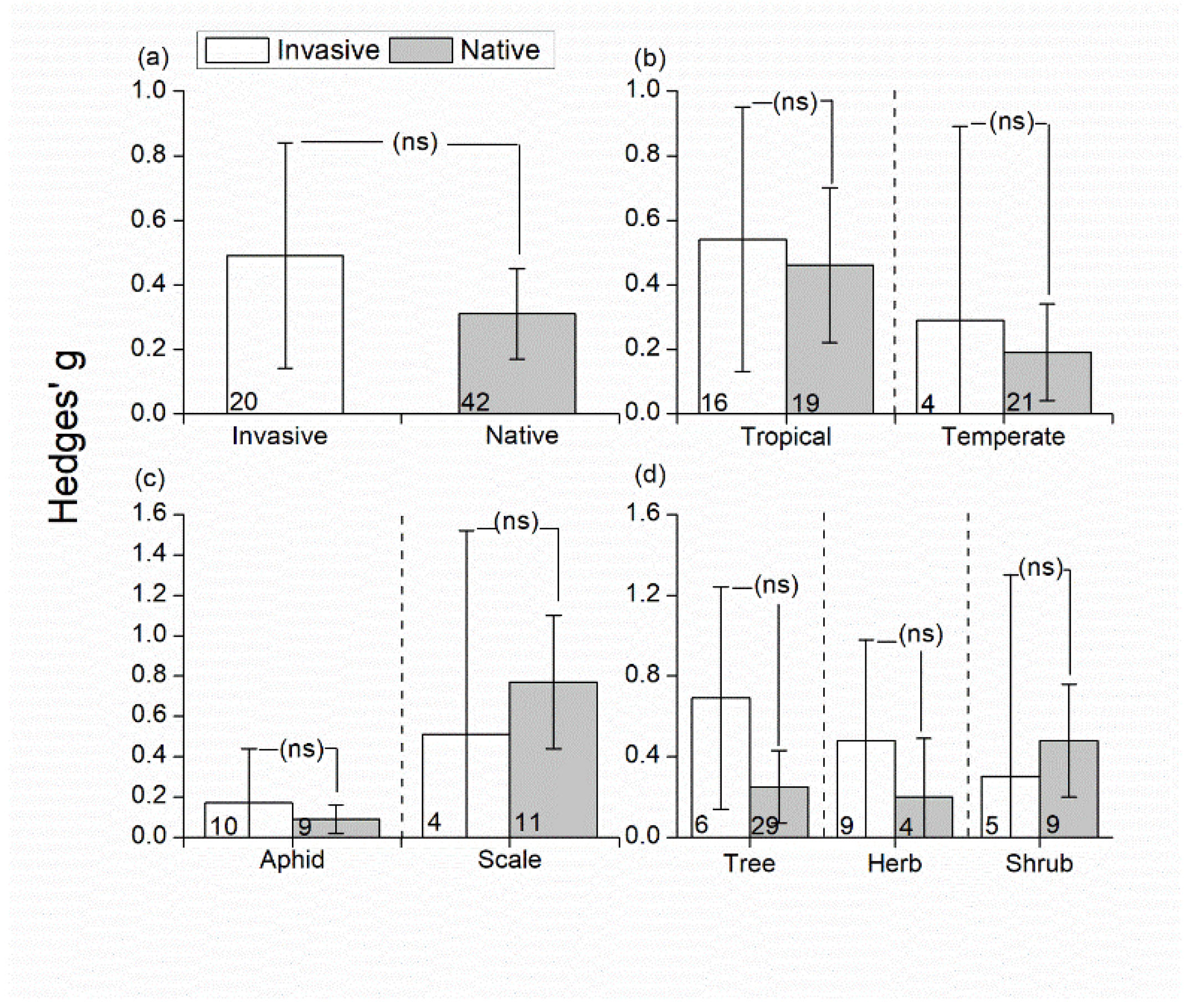
3.3. Effects of Invasive/Native Ant Exclusion on Herbivore Abundance
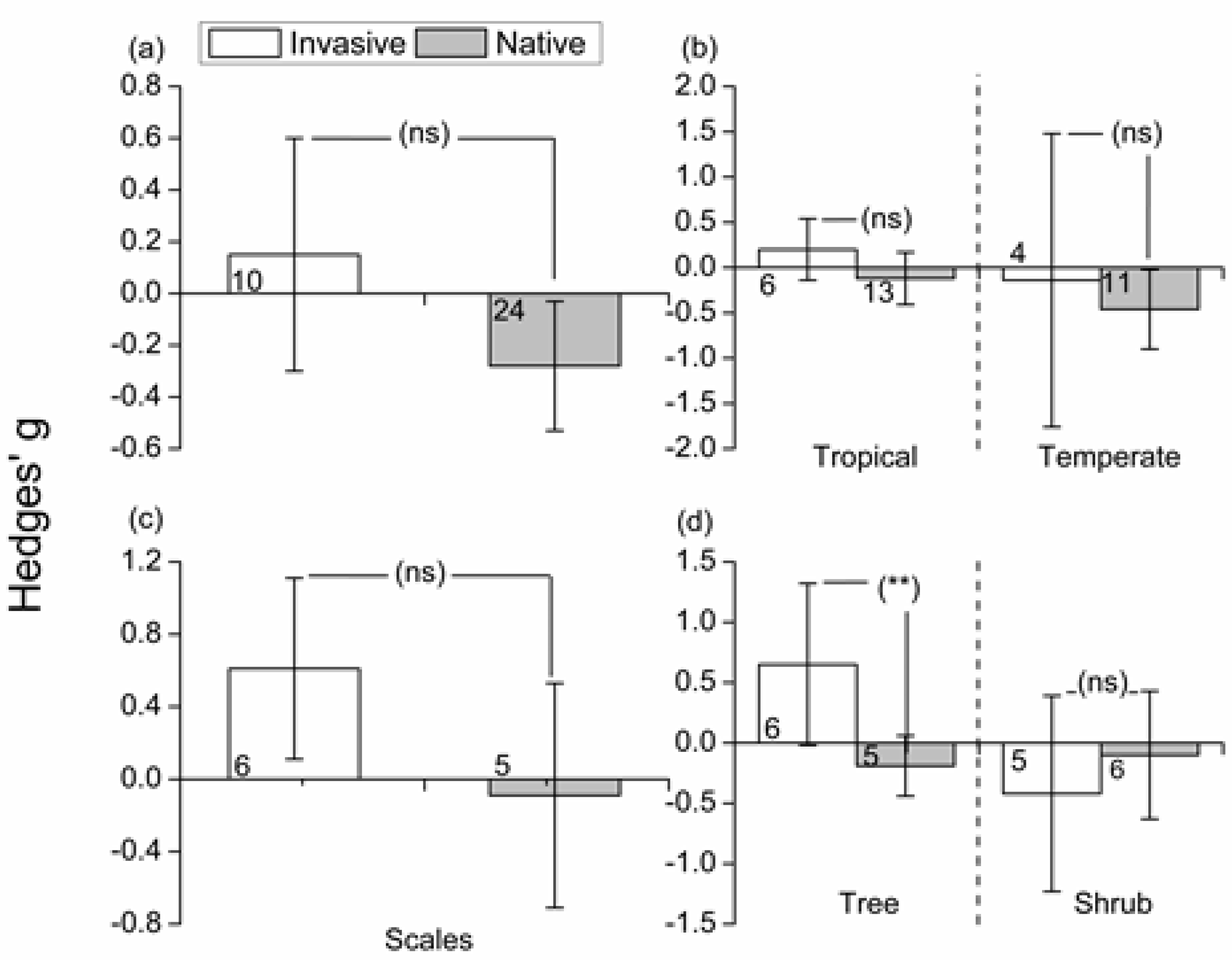
3.4. Effects of Invasive/Native Ant Exclusion on Predator/Parasitoids Abundance
3.5. Effects of Invasive/Native Ant Exclusion on Plant Fitness
4. Discussion
4.1. Effects of Invasive/Native Ant Exclusion on Hemipteran Abundance
4.2. Effects of Invasive/Native Ant Exclusion on Herbivore/Predator Abundance
4.3. Effects of Invasive/Native Ant Exclusion on Plant Fitness
4.4. Future Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P. Biodiversity as a barrier to ecological invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Traveset, A.; Richardson, D.M. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 89–113. [Google Scholar] [CrossRef]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Tena, A.; Hoddle, C.D.; Hoddle, M.S. Competition between honeydew producers in an ant-hemipteran interaction may enhance biological control of an invasive pest. Bull. Entomol. Res. 2013, 103, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, O.E.; Mathis, K.A.; Rivera, M.J. Impacts of invasive ant-hemipteran interaction, edge effects and habitat complexities on the spatial distribution of ants in citrus orchards. Agric. Ecosyst. Environ. 2021, 310, 107299. [Google Scholar] [CrossRef]
- Milosavljević, I.; Morgan, D.J.W.; Massie, R.E.; Hoddle, M.S. Density dependent mortality, climate, and Argentine ants affect population dynamics of an invasive citrus pest, Diaphorina citri, and its specialist parasitoid, Tamarixia radiata, in Southern California, USA. Biol. Control 2021, 159, 104627. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmanek, M. Plant invasions-the role of mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.E. Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 2001, 413, 635. [Google Scholar] [CrossRef]
- Bronstein, J.L. The gift that keeps on giving: Why does biological diversity accumulate around mutualisms. In Plant-Animal Interactions; Del-Claro, K., Torezan-Silingardi, , H.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 283–306. [Google Scholar]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Ness, J.H.; Bronstein, J.L. The effects of invasive ants on prospective ant mutualists. Biol. Invasions 2004, 6, 445–461. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insect Soc. 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Miravete, V.; Roura-Pascual, N.; Dunn, R.R.; Gomez, C. How many and which ant species are being accidentally moved around the world? Biol. Lett. 2014, 10, 20140518. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R. Interactions involving plants, Homoptera, and ants. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 111–135. [Google Scholar] [CrossRef]
- Delabie, J.H.C. Trophobiosis Between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An Overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef]
- Del-Claro, K. Multitrophic relationships, conditional mutualisms, and the study of interaction biodiversity in tropical savannas. Neotrop. Entomol. 2004, 33, 665–672. [Google Scholar] [CrossRef]
- Blüthgen, N.; Verhaagh, M.; Goitía, W.; Jaffé, K.; Morawetz, W.; Barthlott, W. How plants shape the ant community in the Amazonian rainforest canopy: The key role of extrafloral nectaries and homopteran honeydew. Oecologia 2000, 125, 229–240. [Google Scholar] [CrossRef]
- Blüthgen, N.; Stork, N.E.; Fiedler, K. Bottom-up control and co-occurrence in complex communities: Honeydew and nectar determine a rainforest ant mosaic. Oikos 2004, 106, 344–358. [Google Scholar] [CrossRef]
- Del-Claro, K.; Byk, J.; Yugue, G.; Morato, M. Conservative benefits in an ant-hemipteran association in the Brazilian tropical savanna. Sociobiology 2006, 47, 415–422. [Google Scholar]
- Moreira, V.S.S.; Del-Claro, K. The outcomes of an ant-treehopper association on Solanum lycocarpum St. Hill: Increased membracid fecundity and reduced damage by chewing herbivores. Neotrop. Entomol. 2005, 34, 881–887. [Google Scholar] [CrossRef][Green Version]
- Messina, F.J. Plant Protection as a Consequence of an Ant-Membracid Mutualism: Interactions on Goldenrod (Solidago sp.). Ecology 1981, 62, 1433–1440. [Google Scholar] [CrossRef]
- Horvitz, C.C.; Schemske, D.W. Effects of ants and an ant-tended herbivore on seed production of a neotropical herb. Ecology 1984, 65, 1369–1378. [Google Scholar] [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B Biol. Sci. 2007, 274, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.L.; Green, P.T. Collapse of an ant-scale mutualism in a rainforest on Christmas Island. Oikos 2007, 116, 1238–1246. [Google Scholar] [CrossRef]
- McPhee, K.; Garnas, J.; Drummond, F.; Groden, E. Homopterans and an invasive red ant, Myrmica rubra (L.), in Maine. Environ. Entomol. 2012, 41, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.M.; Liang, G.W.; Zeng, L.; Lu, Y.Y.; Xu, Y.J. Interactions between ghost ants and invasive mealybugs: The case of Tapinoma melanocephalum (Hymenoptera: Formicidae) and Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Fla. Entomol. 2014, 97, 1474–1480. [Google Scholar] [CrossRef]
- Ibarra-Isassi, J.; Oliveira, P.S. Indirect effects of mutualism: Ant–treehopper associations deter pollinators and reduce reproduction in a tropical shrub. Oecologia 2018, 186, 691–701. [Google Scholar] [CrossRef]
- Villamil, N.; Boege, K.; Stone, G.N. Ant-pollinator conflict results in pollinator deterrence but no nectar trade-offs. Front. Plant Sci. 2018, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.A.; Del-Claro, K. Effects of different ant species on the attendance of neighbouring hemipteran colonies and the outcomes for the host plant. J. Nat. Hist. 2018, 52, 415–428. [Google Scholar] [CrossRef]
- Trager, M.D.; Bhotika, S.; Hostetler, J.A.; Andrade, G.V.; Rodriguez-Cabal, M.A.; McKeon, C.S.; Osenberg, C.W.; Bolker, B.M. Benefits for plants in ant-plant protective mutualisms: A meta-analysis. PLoS ONE 2010, 5, e14308. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.A.; Holland, J.N. Quantitative synthesis of context dependency in ant–plant protection mutualisms. Ecology 2009, 90, 2384–2392. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Abbott, J.; Gilbert, L.E. Imported crazy ant displaces imported fire ant, reduces and homogenizes grassland ant and arthropod assemblages. Biol. Invasions 2013, 15, 2429–2442. [Google Scholar] [CrossRef]
- Hu, G.Y.; Frank, J.H. Effect of the red imported fire ant (Hymenoptera: Formicidae) on dung-inhabiting arthropods in florida. Environ. Entomol. 1996, 25, 1290–1296. [Google Scholar] [CrossRef]
- Cole, F.R.; Medeiros, A.C.; Loope, L.L.; Zuehlke, W.W. Effects of the Argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 1992, 73, 1313–1322. [Google Scholar] [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. A facultative mutualism between aphids and an invasive ant increases plant reproduction. Ecol. Entomol. 2010, 35, 190–199. [Google Scholar] [CrossRef]
- Blancafort, X.; Gómez, C. Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol. Int. J. Ecol. 2005, 28, 49–55. [Google Scholar] [CrossRef]
- Schemske, D.W.; Mittelbach, G.G.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef]
- Rosumek, F.B.; Silveira, F.A.O.; de Neves, F.S.; de Barbosa, N.P.U.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Ma, K. The ecological effects of the ant-hemipteran mutualism: A meta-analysis. Basic Appl. Ecol. 2012, 13, 116–124. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Effect size based on means. In Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Cornwall, UK, 2009; pp. 21–32. [Google Scholar]
- Grubbs, F.E. Sample criteria for testing outlying observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Vidal, M.C.; Murphy, S.M. Bottom-up vs. top-down effects on terrestrial insect herbivores: A meta-analysis. Ecol. Lett. 2018, 21, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Vevea, J.L. Fixed-and random-effects models in meta-analysis. Psychol. Methods 1998, 3, 486–504. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Small-study effects in meta-analysis. In Meta-Analysis with R; Gentleman, R., Hornik, K., Parmigiani, G., Eds.; Springer: Cham, Switzerland, 2015; pp. 107–141. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. An introduction to meta-analysis in R. In Meta-Analysis with R; Gentleman, R., Hornik, K., Parmigiani, G., Eds.; Springer: Cham, Switzerland, 2015; pp. 3–17. [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species-a global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Del-Claro, K.; Marquis, R.J. Ant species identity has a greater effect than fire on the outcome of an ant protection system in Brazilian Cerrado. Biotropica 2015, 47, 459–467. [Google Scholar] [CrossRef]
- Ovadia, O.; Schmitz, O.J. Weather variation and trophic interaction strength: Sorting the signal from the noise. Oecologia 2004, 140, 398–406. [Google Scholar] [CrossRef]
- Piovia-Scott, J.; Yang, L.H.; Wright, A.N. Trophic cascades in time: The causes and consequences of temporal variation in the strength of top-down effects. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 281–300. [Google Scholar] [CrossRef]
- Baum, J.K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Gaigher, R.; Samways, M.J.; Henwood, J.; Jolliffe, K. Impact of a mutualism between an invasive ant and honeydew-producing insects on a functionally important tree on a tropical island. Biol. Invasions 2011, 13, 1717–1721. [Google Scholar] [CrossRef]
- Helms, K.R.; Vinson, S.B. Plant resources and colony growth in an invasive ant: The importance of honeydew-producing hemiptera in carbohydrate transfer across trophic levels. Environ. Entomol. 2008, 37, 487–493. [Google Scholar] [CrossRef]
- Zhou, A.; Lu, Y.; Zeng, L.; Xu, Y.; Liang, G. Does mutualism drive the invasion of two alien species? The case of Solenopsis invicta and Phenacoccus solenopsis. PLoS ONE 2012, 7, e41856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, B.; Lu, M.; Cook, J.M.; Yang, D.-R.; Dunn, D.W.; Wang, R.-W. Chemical camouflage: A key process in shaping an ant-treehopper and fig-fig wasp mutualistic network. Sci. Rep. 2018, 8, 1833. [Google Scholar] [CrossRef]
- Frago, E.; Godfray, H.C.J. Avoidance of intraguild predation leads to a long-term positive trait-mediated indirect effect in an insect community. Oecologia 2014, 174, 943–952. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C. Trait-mediated trophic interactions: Is foraging theory keeping up? Trends Ecol. Evol. 2013, 28, 119–125. [Google Scholar] [CrossRef]
- Finke, D.L.; Denno, R.F. Predator diversity and the functioning of ecosystems: The role of intraguild predation in dampening trophic cascades. Ecol. Lett. 2005, 8, 1299–1306. [Google Scholar] [CrossRef]
- Neves, F.D.; Fagundes, M.; Sperber, C.F.; Fernandes, G.W. Tri-trophic level interactions affect host plant development and abundance of insect herbivores. Arthropod-Plant Interact. 2011, 5, 351–357. [Google Scholar] [CrossRef]
- Wimp, G.M.; Whitham, T.G. Biodiversity Consequences of Predation and Host Plant Hybridization on an Aphid-Ant Mutualism. Ecology 2001, 82, 440–452. [Google Scholar] [CrossRef]
- Fowler, S.; MacGarvin, M. The impact of hairy wood ants, Formica lugubris, on the guild structure of herbivorous insects on birch, Betula pubescens. J. Anim. Ecol. 1985, 54, 847–855. [Google Scholar] [CrossRef]
- LeVan, K.E.; Holway, D.A. Ant–aphid interactions increase ant floral visitation and reduce plant reproduction via decreased pollinator visitation. Ecology 2015, 96, 1620–1630. [Google Scholar] [CrossRef]
- Ness, J.H. A mutualism’s indirect costs: The most aggressive plant bodyguards also deter pollinators. Oikos 2006, 113, 506–514. [Google Scholar] [CrossRef]
- LeVan, K.E.; Hung, K.-L.J.; McCann, K.R.; Ludka, J.T.; Holway, D.A. Floral visitation by the Argentine ant reduces pollinator visitation and seed set in the coast barrel cactus, Ferocactus viridescens. Oecologia 2014, 174, 163–171. [Google Scholar] [CrossRef]
- De Vries, J.; Evers, J.B.; Poelman, E.H. Dynamic plant–plant–herbivore interactions govern plant growth–defence integration. Trends Plant Sci. 2017, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Barbosa, D. Integrating studies on plant–pollinator and plant–herbivore interactions. Trends Plant Sci. 2016, 21, 125–133. [Google Scholar] [CrossRef]
- Dotseth, E.J.; Larsen, K.J.; Staehle, L.M. Tending ants (Hymenoptera: Formicidae) regulate Dalbulus quinquenotatus (Homoptera: Cicadellidae) population dynamics. Environ. Entomol. 2001, 30, 757–762. [Google Scholar] [CrossRef]
- O’Dowd, D.J.; Green, P.T.; Lake, P.S. Invasional ‘meltdown’ on an oceanic island. Ecol. Lett. 2003, 6, 812–817. [Google Scholar] [CrossRef]
- Perilla-Henao, L.M.; Casteel, C.L. Vector-borne bacterial plant pathogens: Interactions with hemipteran insects and plants. Front. Plant Sci. 2016, 7, 1163. [Google Scholar] [CrossRef] [PubMed]
- Pitan, O.O.R.; Mwansat, G.; Akinyemi, S.O.S.; Adebayo, O.S.; Akinlosotu, T.A. Effect of mango mealybug and sooty mould attack on mango and the impact of the released Gyranusoidea tebygi Noyes on yield. Fruits 2002, 57, 105–113. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Alberola, J.S.; Garcia-Marí, F.; Pekas, A. Attract and distract: Manipulation of a food-mediated protective mutualism enhances natural pest control. Agric. Ecosyst. Environ. 2017, 246, 168–174. [Google Scholar] [CrossRef]
- Tay, J.-W.; Hoddle, M.S.; Mulchandani, A.; Choe, D.-H. Development of an alginate hydrogel to deliver aqueous bait for pest ant management. Pest Manag. Sci. 2017, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- McCalla, K.A.; Tay, J.-W.; Mulchandani, A.; Choe, D.-H.; Hoddle, M.S. Biodegradable alginate hydrogel bait delivery system effectively controls high-density populations of Argentine ant in commercial citrus. J. Pest Sci. 2020, 93, 1031–1042. [Google Scholar] [CrossRef]
- Tay, J.-W.; Choe, D.-H.; Mulchandani, A.; Rust, M.K. Hydrogels: From controlled release to a new bait delivery for insect pest management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; Hoddle, M.S. Laboratory screening of selected synthetic and organic insecticides for efficacy against Argentine ants when incorporated into alginate hydrogel beads, 2021. Arthropod Manag. Tests 2021, 46, tsab072. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Lu, M.; Peng, Y.-Q.; Segar, S.T. Direct and Indirect Effects of Invasive vs. Native Ant-Hemipteran Mutualism: A Meta-Analysis That Supports the Mutualism Intensity Hypothesis. Agronomy 2021, 11, 2323. https://doi.org/10.3390/agronomy11112323
Wang B, Lu M, Peng Y-Q, Segar ST. Direct and Indirect Effects of Invasive vs. Native Ant-Hemipteran Mutualism: A Meta-Analysis That Supports the Mutualism Intensity Hypothesis. Agronomy. 2021; 11(11):2323. https://doi.org/10.3390/agronomy11112323
Chicago/Turabian StyleWang, Bo, Min Lu, Yan-Qiong Peng, and Simon T. Segar. 2021. "Direct and Indirect Effects of Invasive vs. Native Ant-Hemipteran Mutualism: A Meta-Analysis That Supports the Mutualism Intensity Hypothesis" Agronomy 11, no. 11: 2323. https://doi.org/10.3390/agronomy11112323
APA StyleWang, B., Lu, M., Peng, Y.-Q., & Segar, S. T. (2021). Direct and Indirect Effects of Invasive vs. Native Ant-Hemipteran Mutualism: A Meta-Analysis That Supports the Mutualism Intensity Hypothesis. Agronomy, 11(11), 2323. https://doi.org/10.3390/agronomy11112323





