Characterization of Gamma-Rays-Induced Spring Wheat Mutants for Morphological and Quality Traits through Multivariate and GT Bi-Plot Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mutant Population Development
2.2. Experimental Design
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. ANOVA and Descriptive Assessment
3.2. Mean Values for Various Yield Components, Grain Yield, and Quality Traits
3.3. Mean Evaluation of Mutant Lines across Both Locations and Generations
3.4. Cluster Analysis Based upon Yield Index
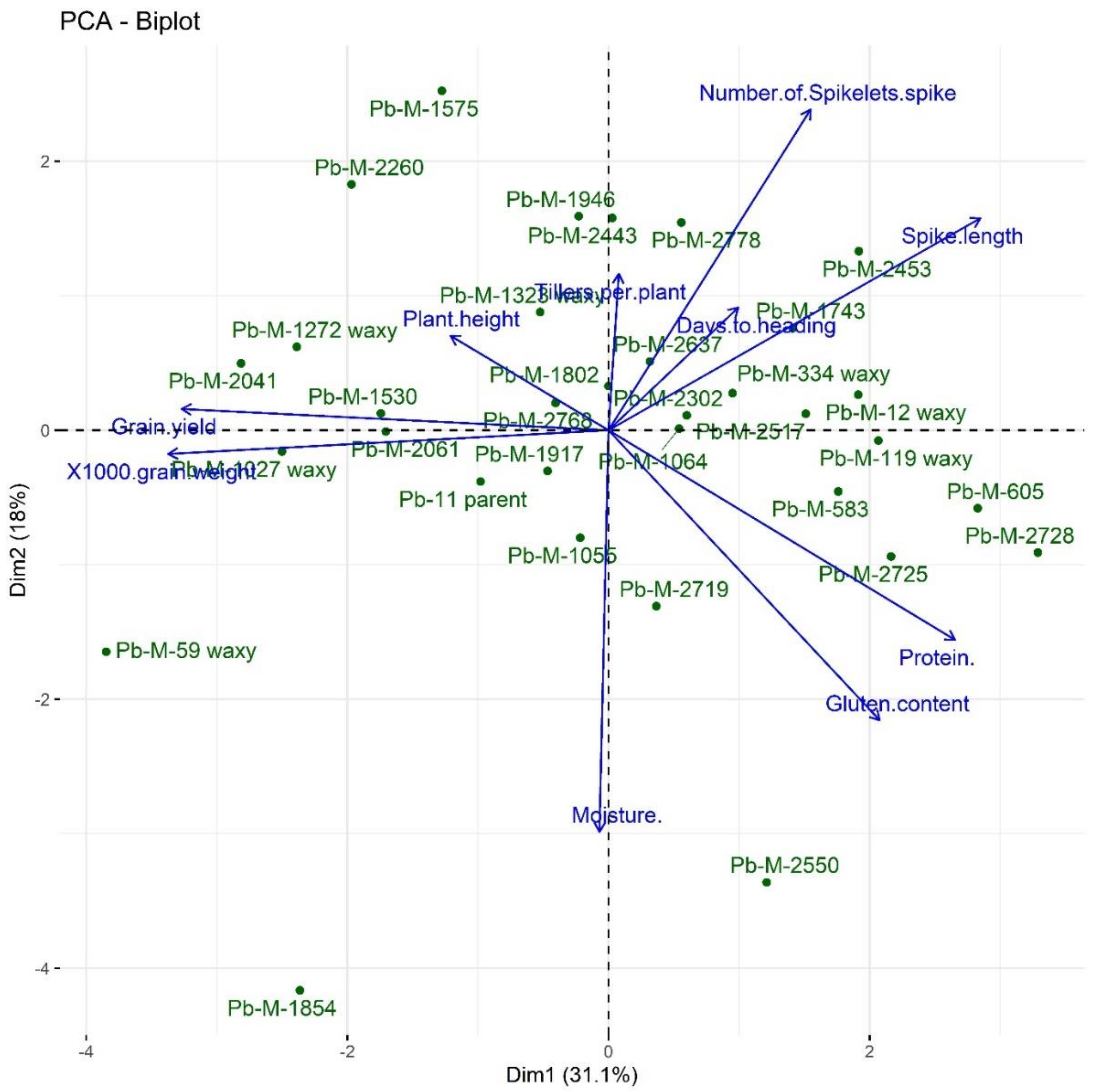
3.5. Principal Component Analysis (PCA)
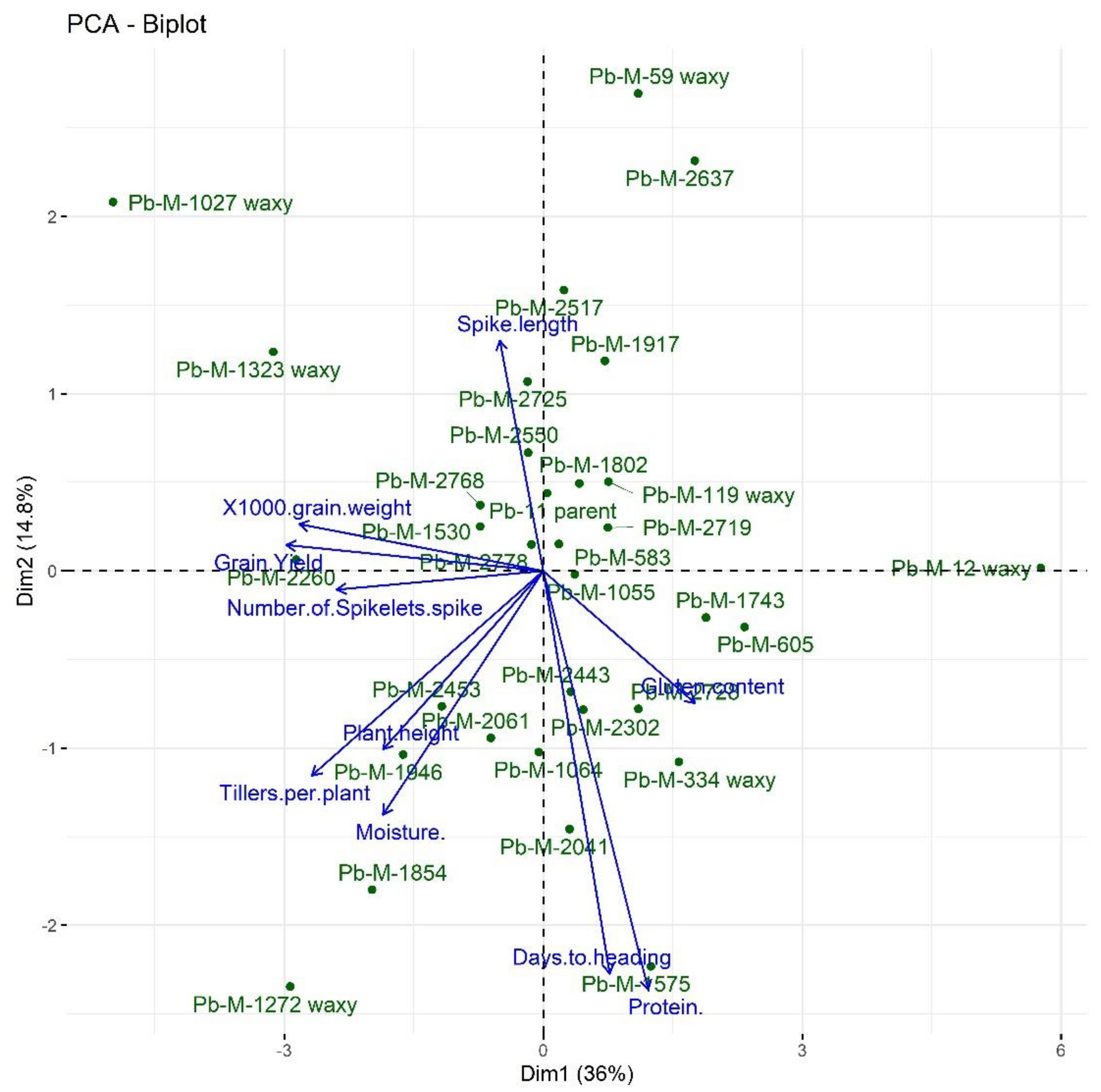
3.6. Genotype by Trait (GT) Bi-Plot Analysis
3.6.1. Interrelation among Different Traits
3.6.2. Genotype by Trait Profiles
4. Discussion
4.1. Morphological Traits
4.2. Multivariate Analysis
4.3. GT Bi-Plot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreign Agricultural Service/USDA. World agricultural production, Circular series, WAP 8-21, August 2021. 2021. Available online: https://apps.fas.usda.gov/psdonline/circulars/production (accessed on 27 August 2021).
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Mansour, E.; Merwad, A.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; El-Sobky, E.E.A.; Oraby, H.F. Nitrogen use efficiency in spring wheat: Genotypic variation and grain yield response under sandy soil conditions. J. Agric. Sci. 2017, 155, 1407. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Khadka, K.; Raizada, M.N.; Navabi, A. Recent progress in germplasm evaluation and gene mapping to enable breeding of drought-tolerant wheat. Front. Plant Sci. 2020, 11, 1149. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Chung, G. Genetic Improvement of Cereals and Grain Legumes. Genes 2020, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kumar, A.; Pandey, D.; Prasad, B. Correlation and path coefficient analysis for yield and its attributing traits in bread wheat (Triticum aestivum L. em Thell). J. Nat. Appl. Sci. 2018, 10, 1078–1084. [Google Scholar] [CrossRef]
- Gegas, V.C.; Nazari, A.; Griffiths, S.; Simmonds, J.; Fish, L.; Orford, S.; Sayers, L.; Doonan, J.H.; Snape, J.W. A genetic framework for grain size and shape variation in wheat. Plant Cell 2010, 22, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Sonmezoglu, O.A.; Bozmaz, B.; Yildirim, A.; Kandemir, N.; Aydin, N. Genetic characterization of Turkish bread wheat landraces based on microsatellite markers and morphological characters. Turk. J. Biol. 2012, 36, 589–597. [Google Scholar]
- Kumbhar, S.D.; Kulwal, P.L.; Patil, J.V.; Sarawate, C.D.; Gaikwad, A.P.; Jadhav, A.S. Genetic diversity and population structure in landraces and improved rice varieties from India. Rice Sci. 2015, 22, 99–107. [Google Scholar] [CrossRef]
- Najaphy, A.; Parchin, R.A.; Farshadfar, E. Comparison of phenotypic and molecular characterizations of some important wheat cultivars and advanced breeding lines. Aust. J. Crop Sci. 2012, 6, 326. [Google Scholar]
- Ahmadi, M.; Farshadfar, E.; Veisi, S. Evaluation of genetic diversity in land races of bread wheat under irrigated and rainfed conditions. Int. J. Agric. Crop Sci. 2012, 4, 1627–1636. [Google Scholar]
- Mansour, E.; Moustafa, E.S.; Qabil, N.; Abdelsalam, A.; Wafa, H.A.; El Kenawy, A.; Casas, A.M.; Igartua, E. Assessing different barley growth habits under Egyptian conditions for enhancing resilience to climate change. Field Crop. Res. 2018, 224, 67–75. [Google Scholar] [CrossRef]
- Altay, F. Yield stability of some Turkish winter wheat (Triticum aestivum L.) genotypes in the Western Transtional Zone of Turkey. Turk. J. Field Crop. 2012, 17, 129–134. [Google Scholar]
- Denčić, S.R.; Mladenov, N.O.; Kobiljski, B.O. Effects of genotype and environment on breadmaking quality in wheat. Int. J. Plant Prod. 2012, 5, 71–82. [Google Scholar] [CrossRef]
- Malik, A.H.; Kuktaite, R.; Johansson, E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to bread-making quality. J. Cereal Sci. 2013, 57, 170–174. [Google Scholar] [CrossRef]
- Mikulikova, D.; Masár, S.; Horvathova, V.; Kraic, J. Stability of quality traits in winter wheat cultivars. Czech. J. Food. Sci. 2009, 27, 403–417. [Google Scholar] [CrossRef]
- Kong, L.; Si, J.; Zhang, B.; Feng, B.; Li, S.; Wang, F. Environmental modification of wheat grain protein accumulation and associated processing quality: A case study of China. Aust. J. Crop Sci. 2013, 7, 173–181. [Google Scholar]
- Miralbes, C. Discrimination of European wheat varieties using near infrared reflectance spectroscopy. Food Chem. 2008, 106, 386–389. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Pomeranz, Y. Wheat Chemistry and Technology (edited); American Association of Cereal Chemists: St. Paul, MN, USA, 1964. [Google Scholar]
- Hailegiorgis, D.; Mesfin, M.; Genet, T. Genetic divergence analysis on some bread wheat genotypes grown in Ethiopia. J. Cent. Eur. Agric. 2011, 12, 344–352. [Google Scholar] [CrossRef]
- Munjal, R. Genetic diversity in bread wheat for heat tolerance. Ekin J. Crop Breed. Genetic. 2017, 3, 60–78. [Google Scholar]
- Ahmad, H.M.; Awan, S.I.; Aziz, O.; Ali, M.A. Multivariative analysis of some metric traits in bread wheat (Triticum aestivum L.). Eur. J. Biotechnol. Biosci. 2014, 1, 22–26. [Google Scholar]
- Sahar, B.; Ahmed, B.; Naserelhaq, N.; Mohammed, J.; Hassan, O. Efficiency of selection indices in screening bread wheat lines combining drought tolerance and high yield potential. J. Plant Breed. Crop Sci. 2016, 8, 72–86. [Google Scholar] [CrossRef]
- Idrissi, O.; Chafika, H.; Nsarellah, N. Comparaison de lignées avancées de lentille sous stress hydrique durant la phase de floraison et formation des gousses. Nat. Technol. 2013, 8, 53A. [Google Scholar]
- Abdolshahi, R.; Nazari, M.; Safarian, A.; Sadathossini, T.S.; Salarpour, M.; Amiri, H. Integrated selection criteria for drought tolerance in wheat (Triticum aestivum L.) breeding programs using discriminant analysis. Field Crop. Res. 2015, 174, 20–29. [Google Scholar] [CrossRef]
- Eticha, F.; Grausgruber, H.; Berghoffer, E. Multivariate analysis of agronomic and quality traits of hull-less spring barley (Hordeum vulgare L.). J. Plant Breed. Crop Sci. 2010, 2, 81–95. [Google Scholar] [CrossRef]
- Marquetti, I.; Link, J.V.; Lemes, A.L.G.; dos Santos Scholz, M.B.; Valderrama, P.; Bona, E. Partial least square with discriminant analysis and near infrared spectroscopy for evaluation of geographic and genotypic origin of arabica coffee. Comput. Electron. Agric. 2016, 121, 313–319. [Google Scholar] [CrossRef]
- Das, B.; Nair, B.; Reddy, V.K.; Venkatesh, P. Evaluation of multiple linear, neural network and penalised regression models for prediction of rice yield based on weather parameters for west coast of India. Int. J. Biometeorol. 2018, 62, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sahoo, R.N.; Pargal, S.; Krishna, G.; Verma, R.; Chinnusamy, V.; Sehgal, V.K.; Gupta, V.K.; Dash, S.K.; Swain, P. Quantitative monitoring of sucrose, reducing sugar and total sugar dynamics for phenotyping of water-deficit stress tolerance in rice through spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 41–51. [Google Scholar] [CrossRef]
- Verma, S.P.; Pathak, V.N.; Verma, O.P. Interrelationship between Yield and its Contributing Traits in Wheat (Triticum aestivum L.). Int. J Curr. Microbiol. App. Sci. 2019, 8, 3209–3215. [Google Scholar] [CrossRef]
- Abbasi, Z.; Arzani, A.; Majidi, M.M. Evaluation of genetic diversity of sugar beet (Beta vulgaris L.) crossing parents using agro-morphological traits and molecular markers. J. Agric. Sci. Technol. 2014, 16, 1397–1411. [Google Scholar]
- Kavithamani, D.; Yuvaraja, A.; Selvi, B. Principal component analysis and grouping of sorghum (Sorghum bicolor L. Moench) gene pool for genetic diversity. Electron. J. Plant Breed. 2019, 10, 1426–1434. [Google Scholar] [CrossRef]
- Al-Naggar, A.M.M.; Shafik, M.M.; Musa, R.Y.M. Genetic diversity based on morphological traits of 19 maize genotypes using principal component analysis and GT biplot. Annu. Res. Rev. Biol. 2020, 68–85. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ober, E.S.; Le Bloa, M.; Clark, C.J.; Royal, A.; Jaggard, K.W.; Pidgeon, J.D. Evaluation of physiological traits as indirect selection criteria for drought tolerance in sugar beet. Field Crop. Res. 2005, 91, 231–249. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Lee, S.J.; Yan, W.; Ahn, J.K.; Chung, I.M. Effects of year, site, genotype and their interactions on various soybean isoflavones. Field Crop. Res. 2003, 81, 181–192. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Akinwale, R.O. Cultivar evaluation and trait analysis of tropical early maturing maize under Striga-infested and Striga-free environments. Field Crop. Res. 2011, 121, 186–194. [Google Scholar] [CrossRef]
- Gabriel, K.R. The biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Ilker, E.; Tonk, F.A.; Caylak, O.; Tosun, M.; Ozmen, İ. Assessment of Genotype X Environment Interactions for Grain Yield in Maize Hybrids Using Ammi and GGE Biplot Analyses. Turk. J. Field Crop. 2009, 14, 123–135. [Google Scholar]
- Ukalski, K.; Smialowski, T.; Ukalska, J. Yield and stability analysis of oat genotypes using graphical GGE method. Zywn. Nauk. Technol. J. 2010, 17, 127–140. [Google Scholar] [CrossRef]
- Ahmadi, J.; Vaezi, B.; Hossein Fotokian, M. Graphical analysis of multi-environment trials for barley yield using AMMI and GGE-biplot under rain-fed conditions. J. Plant Physiol. Breed. 2012, 2, 43–54. [Google Scholar]
- Kendal, E. Comparing durum wheat cultivars with genotype×yield×trait (GYT) and genotype× trait (GT) by biplot method. Chil. J. Agric. Res. 2019, 79, 512–522. [Google Scholar] [CrossRef]
- Mortazavian, S.M.M.; Nikkhah, H.R.; Hassani, F.A.; Sharif-al-Hosseini, M.; Taheri, M.; Mahlooji, M. GGE biplot and AMMI analysis of yield performance of barley genotypes across different environments in Iran. J. Agric. Sci. Technol. 2014, 16, 609–622. [Google Scholar]
- Sayar, M.S.; Han, Y. Determination of seed yield and yield components of grass pea (Lathyrus sativus L.) lines and evaluations using GGE Biplot analysis method. Tarım. Bilimleri. Dergisi. 2015, 21, 78–92. [Google Scholar] [CrossRef][Green Version]
- Kendal, E.; Dogan, Y. Stability of a candidate and cultivars (Hordeum vulgare L.) by GGE biplot analysis of multi environment yield trials in spring barley. Agric. For. 2015, 61, 307–318. [Google Scholar] [CrossRef]
- Akcura, M.; Kokten, K.; Akcacik, A.G.; Aydogan, S. Pattern analysis of Turkish bread wheat landraces and cultivars for grain and flour quality. Turk. J. Field Crop. 2016, 21, 120–130. [Google Scholar] [CrossRef]
- Oral, E.; Kendal, E.; Dogan, Y. Selection the best barley genotypes to multi and special environments by AMMI and GGE biplot models. Fresen. Environ. Bulletin. 2018, 27, 5179–5187. [Google Scholar]
- Kumar, B.; Ruchi, G.M.L.; Upadhyay, A. Genetic variability, diversity and association of quantitative traits with grain yield in bread wheat (Triticum aestivum L.). Asian J. Agric. Res. 2009, 1, 4–6. [Google Scholar]
- Bilsborrow, P.; Cooper, J.; Tetard-Jones, C.; Średnicka-Tober, D.; Barański, M.; Eyre, M.; Schmidt, C.; Shotton, P.; Volakakis, N.; Cakmak, I.; et al. The effect of organic and conventional management on the yield and quality of wheat grown in a long-term field trial. Eur. J. Agron. 2013, 51, 71–80. [Google Scholar] [CrossRef]
- Steel, R.G. Principles and Procedures of Statistics a Biometrical Approach (No. 519.5 S8); McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Ullah, I.; Ashraf, M.; Zafar, Y. Genotypic variation for drought tolerance in cotton (Gossypium hirsutum L.): Leaf gas exchange and productivity. Flora-Morphology, Distribution. Funct. Ecol. Plants. 2008, 203, 105–115. [Google Scholar] [CrossRef]
- Hintze, J. NCSS and PASS number crucher statistical systems. NCSS Statistical Software: Kaysville, UT, USA, 2001. Available online: http://www.NCSS.com (accessed on 27 August 2021).
- Frutos, E.; Galindo, M.P.; Leiva, V. An interactive biplot implementation in R for modeling genotype-by-environment interaction. Stochastic Environ. Res. Risk Assess. 2014, 28, 1629–1641. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2003; p. 213. [Google Scholar]
- Osekita, O.S.; Ajayi, A.T. Character expression and selection differential for yield and its components in soybean (Glycine max (L.) Merrill). Acad. J. Agric. Res. 2013, 1, 167–171. [Google Scholar]
- Drezner, G.; Dvojkovic, K.; Horvat, D.; Novoselovic, D.; Lalic, A. Environmental impacts on wheat agronomic and quality traits. Cereal Res. Commun. 2007, 35, 357–360. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Kettlewell, P.S.; Poulton, P.R.; Hollins, P.D. Grain quality in the Broadbalk wheat experiment and the winter North Atlantic oscillation. J. Agric. Sci. 2008, 146, 541. [Google Scholar] [CrossRef]
- Xie, Q. Physiological and Genetic Determination of Yield and Yield Components in a Bread Wheat× Spelt Mapping Population. Doctoral Dissertation, University of Nottingham, Nottingham, UK, 2015. [Google Scholar]
- ALIU, S.A.; Fetahu, S. Determination on genetic variation for morphological traits and yield components of new winter wheat (Triticum aestivum L.) lines. Not. Sci. Biol. 2010, 2, 121–124. [Google Scholar] [CrossRef][Green Version]
- Shinwari, Z.K.; Rehman, H.I.N.A.; Rabbani, M.A. SDS-Page based genetic divergence in safflower (Carthamus Tinctorius L.). Pak. J. Bot. 2014, 46, 811–815. [Google Scholar]
- Singh, M.K.; Sharma, P.K.; Tyagi, B.S.; Singh, G. Genetic analysis for morphological traits and protein content in bread wheat (Triticum aestivum L.) under normal and heat stress environments. Indian J. Genet. Plant Breed. 2013, 73, 320–324. [Google Scholar] [CrossRef]
- Singh, G.; Kulshreshtha, N.; Singh, B.N.; Setter, T.L.; Singh, M.K.; Saharan, M.S.; Tyagi, B.S.; Verma, A.; Sharma, I. Germplasm characterization, association and clustering for salinity and waterlogging tolerance in bread wheat (Triticum aestivum). Indian J. Agric. Sci. 2014, 84, 1102–1110. [Google Scholar]
- Sabaghnia, N.; Janmohammadi, M.; Bashiri, A.; Asghari-Shirghan, R. Genetic variation of several bread wheat (Triticum aestivum L.) genotypes based on some morphological traits. Agron. Sci. 2014, 69, 44–54. [Google Scholar]
- Jaiswal, J.P. Genetic Diversity Analysis in Exotic Germplasm Accessions of Bread Wheat (Triticum aestivum L.) by Cluster Analysis. Electron. J. Plant Breed. 2015, 6, 1111–1117. [Google Scholar]
- Kumar, P.; Singh, G.; Kumar, S.; Kumar, A.; Ojha, A. Genetic analysis of grain yield and its contributing traits for their implications in improvement of bread wheat cultivars. J. Nat. Appl. Sci. 2016, 8, 350–357. [Google Scholar] [CrossRef]
- Jan, S.A.; Shinwari, Z.K.; Rabbani, M.A. Morpho-biochemical evaluation of Brassica rapa sub-species for salt tolerance. Genetika 2016, 48, 323–338. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Behtash, F.; Janmohammadi, M. Graphic analysis of trait relations of spinach (Spinacia oleracea L.) landraces using the biplot method. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2015, 63, 1187–1194. [Google Scholar] [CrossRef]
- Verma, P.N.; Singh, B.N.; Singh, G.; Singh, M.K.; Setter, T.L. Genetic diversity analysis for yield and other agronomic traits in bread wheat under water logged sodic soil condition. J. Wheat Res. 2014, 6, 51–58. [Google Scholar]
- Vora, Z.N.; Patel, J.B.; Pansuriya, A.G.; Yusufzai, S.A. Genetic divergence analysis in bread wheat (Triticum aestivum L.). Res. Environ. Life Sci. 2017, 10, 291–294. [Google Scholar]
- Sheykhi, A.; Pirdashti, H.; Abbasian, A.; Niknejhad, Y. Segregation of some wheat (Triticum aestivum L.) genotypes using cluster analysis procedure. Int. J. Farm Alli. Sci. 2014, 3, 225–229. [Google Scholar]
- Gharib, M.A.A.H.; Qabil, N.; Salem, A.H.; Ali, M.M.A.; Awaad, H.A.; Mansour, E. Characterization of wheat landraces and commercial cultivars based on morpho-phenological and agronomic traits. Cereal Res. Commun. 2021, 49, 149–159. [Google Scholar] [CrossRef]
- Spanic, V.; Cosic, J.; Zdunic, Z.; Drezner, G. Characterization of Agronomical and Quality Traits of Winter Wheat (Triticum aestivum L.) for Fusarium Head Blight Pressure in Different Environments. Agronomy 2021, 11, 213. [Google Scholar] [CrossRef]
- Meena, N.; Mishra, V.K.; Baranwal, D.K.; Singh, A.K.; Rai, V.P.; Prasad, R.; Arun, B.; Chand, R. Genetic evaluation of spring wheat (Triticum aestivum L.) recombinant inbred lines for spot blotch (Bipolaris Sorokiniana) resistance and yield components under natural conditions for South Asia. J. Agric. Sci. Technol. 2014, 16, 1429–1440. [Google Scholar]
- Bhanupriya, B.; Satyanarayana, N.; Mukherjee, S.; Sarkar, K. Genetic diversity of wheat genotypes based on principal component analysis in Gangetic alluvial soil of West Bengal. J. Crop Weed. 2014, 10, 104–107. [Google Scholar]
- Ali, M.A.; Zulkiffal, M.; Anwar, J.; Hussain, M.; Farooq, J.; Khan, S.H. Morpho-physiological diversity in advanced lines of bread wheat under drought conditions at post-anthesis stage. J. Anim. Plant Sci. 2015, 25, 431–441. [Google Scholar]
- Yan, W.; Tinker, N.A. An integrated system of biplot analysis for displaying, interpreting, and exploring genotype–by-environment interactions. Crop Sci. 2005, 45, 1004–1016. [Google Scholar] [CrossRef]
- Hagos, H.G.; Abay, F. AMMI and GGE biplot analysis of bread wheat genotypes in the northern part of Ethiopia. J. Plant Breed. Genet. 2013, 1, 12–18. [Google Scholar]
- Stanisavljević, D.; Mitrović, B.; Mirosavljević, M.; Ćirić, M.; Čanak, P.; Stojaković, M.; Ivanović, M. Identification of the most desirable maize testing environments in northern Serbia. Ratar. I Povrt. /Field Veg. Crop. Res. 2013, 50, 28–35. [Google Scholar]
- Adjabi, A.; Bouzerzour, A.; Benmahammed, A. Stability analysis of durum wheat (Triticum durum Desf.) grain yield. J. Agron. 2014, 13, 131–139. [Google Scholar] [CrossRef]
- Dogan, Y.; Kendal, E.; Oral, E. Identifying of relationship between traits and grain yield in spring barley by GGE Biplot analysis. Agri. For./Poljopr. I Sumar. 2016, 62, 239–252. [Google Scholar] [CrossRef]
- Karami, E.; Arshadi, A.; Sartip, A.; Zare, M.; Rezabakhsh, P. Genotypes performance in relation to drought tolerance in barley using multi-environment trials. Agron. Res. 2018, 16, 5–21. [Google Scholar]
- Oury, F.X.; Godlin, C. Yield and grain protein concentration in bread wheat: How to use the negative relationship between the two characters to identify favorable genotypes? Euphytica 2007, 157, 45–57. [Google Scholar] [CrossRef]
- Tayyar, S. Variation in grain yield and quality of Romanian bread wheat varieties compared to local varieties in northwestern Turkey. Rom. Biotechnol. Lett. 2010, 15, 5189–5196. [Google Scholar]
- Peterson, D.M.; Wesenberg, D.M.; Burrup, D.E.; Erickson, C.A. Relationships among agronomic traits and grain composition in oat genotypes grown in different environments. Crop Sci. 2005, 45, 249–255. [Google Scholar] [CrossRef]
- Kendal, E.; Sayar, M.S. The stability of some spring triticale genotypes using biplot analysis. J. Anim. Plant Sci. 2016, 26, 754–765. [Google Scholar]
- Yan, W.; Frégeau-Reid, J. Genotype by Yield* Trait (GYT) Biplot: A Novel Approach for Genotype Selection based on Multiple Traits. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Baljani, R.; Shekari, F.; Sabaghnia, N. Biplot analysis of trait relations of some safflower (Carthamus tinctorius L.) genotypes in Iran. Crop Res. 2015, 50, 63–73. [Google Scholar]
- Paramesh, M.; Reddy, D.M.; Priya, M.S.; Sudhakar, P.S.P.; Reddy, K.H.P. GT biplot analysis for yield and drought related traits in mung bean (Vigna radiata L. Wilczek). Electron. J. Plant Breed. 2016, 7, 538–543. [Google Scholar] [CrossRef]
- Kendal, E. Evaluation of some barley genotypes with genotype by yield* trait (GYT) biplot method. Agri. For./Poljopr. I Sumar. 2020, 6, 137–150. [Google Scholar]
- Xu, N.; Fok, M.; Li, J.; Yang, X.; Yan, W. Optimization of cotton variety registration criteria aided with a genotype-by-trait biplot analysis. Sci. Rep. 2017, 7, 17237. [Google Scholar] [CrossRef]
- González, A.M.; Monteagudo, A.B.; Casquero, P.A.; De Ron, A.M.; Santalla, M. Genetic variation and environmental effects on agronomical and commercial quality traits in the main European market classes of dry bean. Field Crop. Res. 2006, 95, 336–347. [Google Scholar] [CrossRef]
- Mohammadi, R.; Amri, A. Graphic analysis of trait relations and genotype evaluation in durum wheat. J. Crop Improv. 2011, 25, 680–696. [Google Scholar] [CrossRef]
- Dolatabad, S.S.; Choukan, R.; Hervan, E.M.; Dehghani, H. Multi-environment analysis of traits relation and hybrids comparison of maize based on the genotype by trait biplot. Am. J. Agric. Biol. Sci. 2010, 5, 107–113. [Google Scholar] [CrossRef][Green Version]
- Samonte, S.O.P.; Tabien, R.E.; Wilson, L.T. Parental selection in rice cultivar improvement. Rice Sci. 2013, 20, 45–51. [Google Scholar] [CrossRef]



| Generations | Trait | Genotypes | Error | Total |
|---|---|---|---|---|
| Df | 64 | 32 | 98 | |
| M7 | Days to heading | 19.05 ** | 2.79 | 24.11 |
| Plant height | 99.65 ** | 6.61 | 107.30 | |
| Tillers per plant | 1.56 ** | 0.39 | 4.50 | |
| Spike length | 4.41 ** | 42.95 | 47.73 | |
| Number of spikelets per spike | 4.79 ** | 2.06 | 7.62 | |
| 1000-grain weight | 21.13 ** | 2.51 | 44.95 | |
| Grain yield | 510,874 ** | 30,347 | 614,024 | |
| Df | 32 | 33 | 65 | |
| Protein content | 1.45 ** | 0.01 | 1.45 | |
| Moisture content | 0.07 ** | 0.01 | 0.15 | |
| Gluten content | 3.15 ** | 0.35 | 7.88 | |
| Df | 64 | 32 | 98 | |
| M8 | Days to heading | 11.95 ** | 0.03 | 12.07 |
| Plant height | 53.72 ** | 1.90 | 58.65 | |
| Tillers per plant | 0.96 ** | 0.63 | 2.17 | |
| Spike length | 1.86 ** | 1.34 | 3.41 | |
| Number of spikelets per spike | 2.06 ** | 1.44 | 5.48 | |
| 1000-grain weight | 35.34 ** | 0.35 | 36.31 | |
| Grain yield | 958,408 ** | 10,662 | 969,070 | |
| Df | 32 | 33 | 65 | |
| Protein content | 2.69 ** | 1.25 | 3.95 | |
| Moisture content | 0.50 ** | 0.33 | 0.83 | |
| Gluten content | 21.05 ** | 7.59 | 28.61 |
| Generations | Trait | Genotypes | Error | Total |
|---|---|---|---|---|
| M7 | Df | 32 | 64 | 98 |
| Days to heading | 20.04 ** | 5.85 | 59.36 | |
| Plant height | 34.19 ** | 6.65 | 69.37 | |
| Tillers per plant | 0.54 ** | 0.34 | 0.89 | |
| Spike length | 1.02 ** | 0.75 | 10.40 | |
| Number of spikelets per spike | 2.68 ** | 1.89 | 14.75 | |
| 1000-grain weight | 34.14 ** | 2.03 | 46.33 | |
| Grain yield | 585,260 ** | 27,621 | 624,181 | |
| Df | 32 | 33 | 65 | |
| Protein content | 1.30 ** | 0.03 | 1.33 | |
| Moisture content | 0.087 ** | 0.01 | 0.10 | |
| Gluten content | 2.57 ** | 0.20 | 2.76 | |
| Df | 64 | 32 | 98 | |
| M8 | Days to heading | 10.81 ** | 0.52 | 14.29 |
| Plant height | 49.14 ** | 1.51 | 54.85 | |
| Tillers per plant | 0.35 ** | 0.32 | 1.05 | |
| Spike length | 4.55 ** | 0.35 | 5.09 | |
| Number of Spikelets per spike | 2.15 ** | 1.41 | 5.14 | |
| 1000-grain weight | 67.35 ** | 0.30 | 68.04 | |
| Grain yield | 2,111,692 ** | 1977 | 2,113,669 | |
| Df | 32 | 33 | 65 | |
| Protein content | 0.44 ** | 0.02 | 0.46 | |
| Moisture content | 0.18 ** | 0.01 | 0.19 | |
| Gluten content | 1.85 ** | 0.52 | 2.36 |
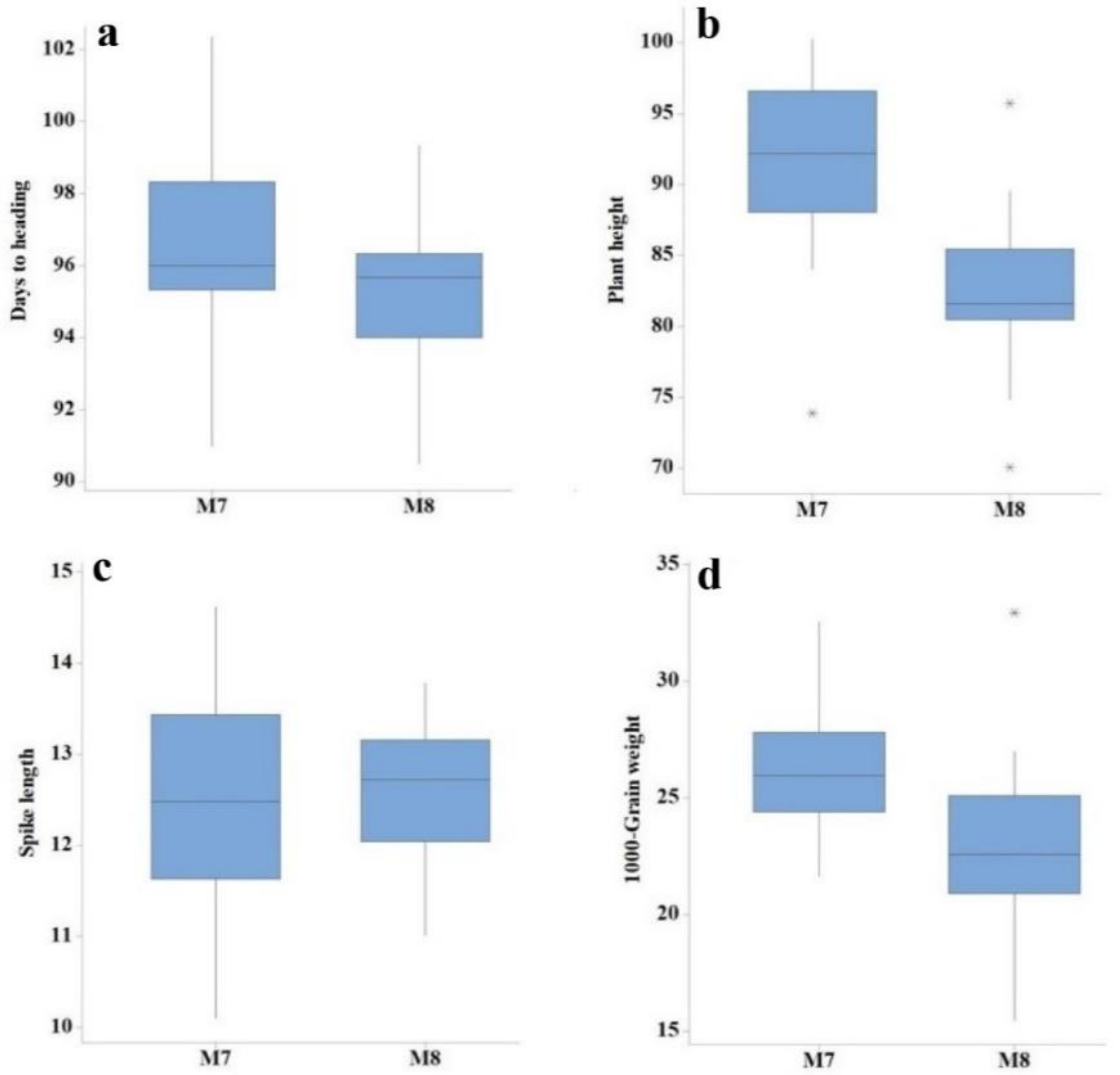
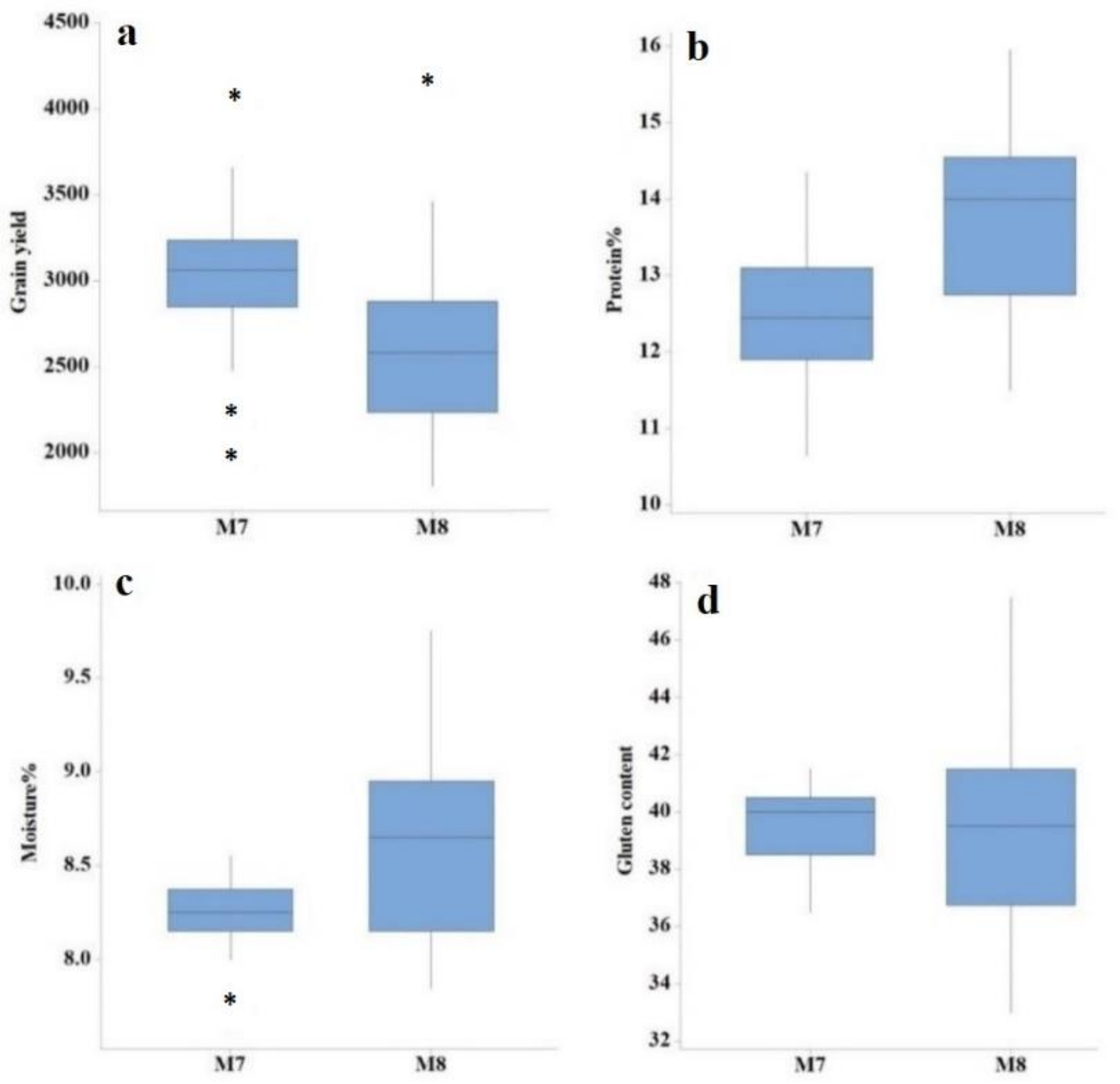
| Generation | Trait | Min | Max | Mean | SD | CV (%) | LSD |
|---|---|---|---|---|---|---|---|
| M7 | Days to heading | 91.00 | 102.00 | 96.87 | 2.51 | 1.73 | 2.73 |
| Plant height (cm) | 73.90 | 100.64 | 91.99 | 5.76 | 2.79 | 4.19 | |
| Tillers per plant | 4.00 | 7.00 | 5.30 | 0.73 | 12.25 | 1.02 | |
| Spike length (cm) | 10.10 | 14.62 | 12.49 | 1.21 | 6.56 | 1.34 | |
| Number of spikelets per spike | 15.00 | 21.00 | 18.52 | 1.73 | 7.74 | 2.34 | |
| 1000-grain weight | 21.65 | 32.52 | 26.23 | 2.65 | 6.05 | 1.29 | |
| Grain yield (kg/ha) | 2026.30 | 4098.40 | 3041.30 | 412.66 | 5.73 | 284.15 | |
| Protein content (%) | 10.65 | 14.35 | 12.46 | 0.84 | 0.95 | 0.24 | |
| Moisture content (%) | 7.65 | 8.55 | 8.25 | 0.19 | 1.02 | 0.17 | |
| Gluten content | 37.00 | 42.00 | 39.87 | 1.32 | 1.49 | 1.20 | |
| M8 | Days to heading | 91.00 | 99.00 | 95.24 | 1.95 | 0.18 | 0.27 |
| Plant height (cm) | 70.04 | 89.52 | 81.92 | 4.23 | 1.68 | 2.25 | |
| Tillers per plant | 5.00 | 7.00 | 6.06 | 0.61 | 12.92 | 1.30 | |
| Spike length (cm) | 11.01 | 13.78 | 12.53 | 0.79 | 9.23 | 1.89 | |
| Number of spikelets per spike | 15.00 | 19.00 | 17.85 | 1.12 | 6.82 | 1.96 | |
| 1000-grain weight | 15.49 | 32.93 | 22.86 | 3.43 | 2.59 | 0.96 | |
| Grain yield (kg/ha) | 1806.30 | 4183.30 | 2592.50 | 534.91 | 4.66 | 168.43 | |
| Protein content (%) | 11.50 | 15.95 | 13.79 | 1.16 | 8.12 | 2.28 | |
| Moisture content (%) | 7.85 | 9.75 | 8.61 | 0.49 | 6.86 | 1.16 | |
| Gluten content | 33.00 | 48.00 | 39.61 | 3.30 | 7.00 | 5.61 |
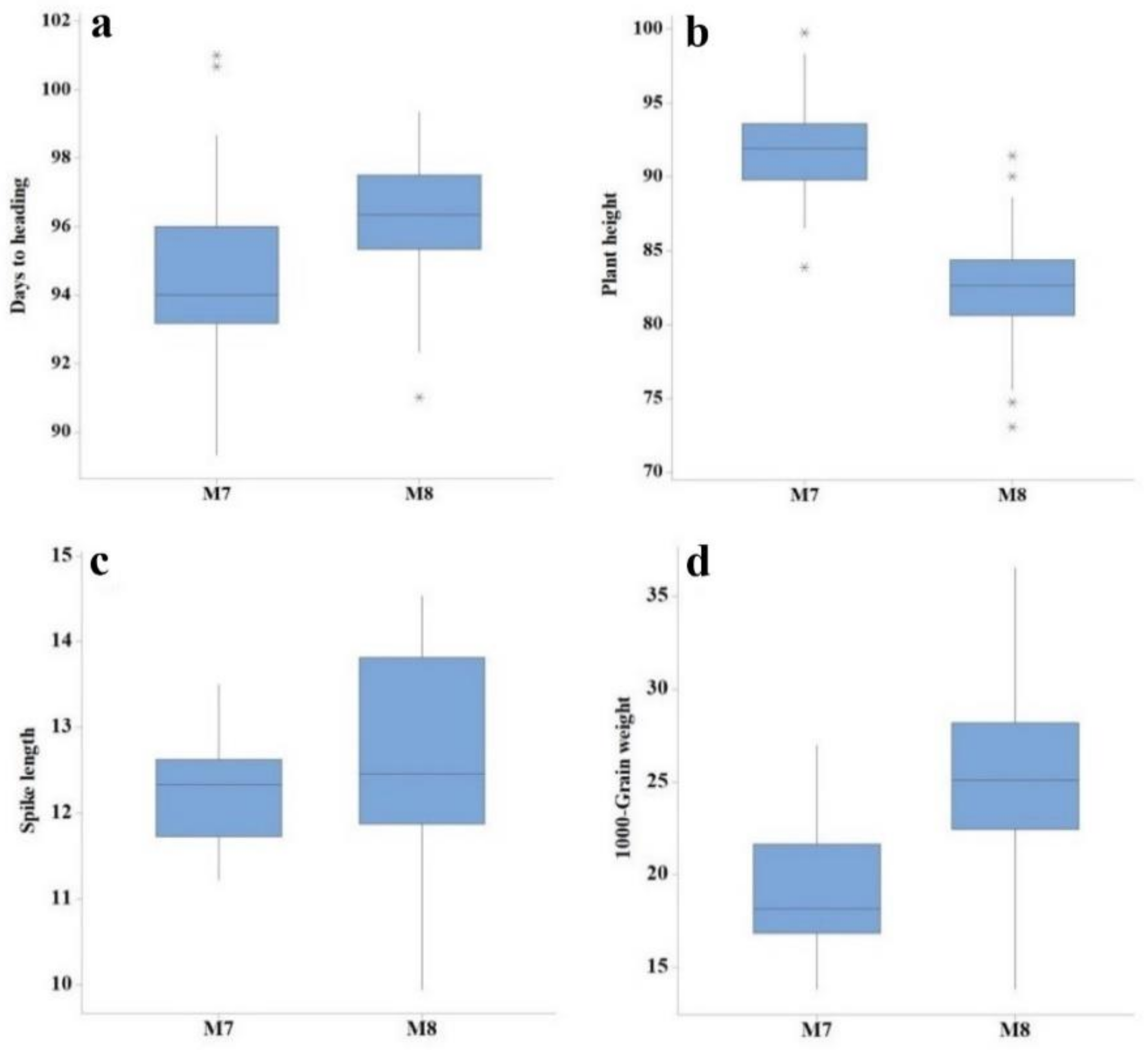
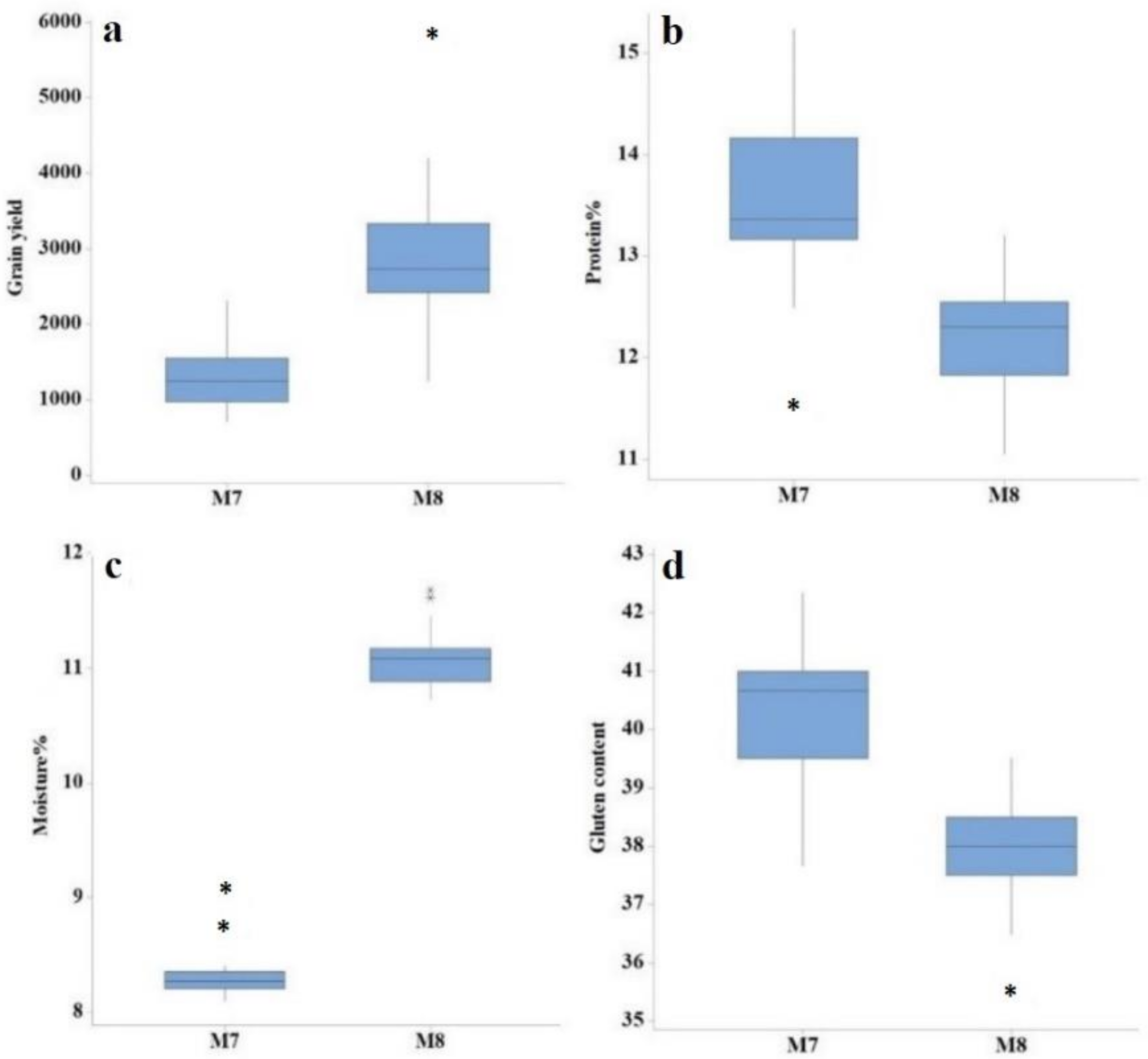
| Generation | Trait | Min | Max | Mean | SD | CV (%) | LSD |
|---|---|---|---|---|---|---|---|
| M7 | Days to heading | 89.00 | 101.00 | 94.52 | 2.65 | 2.56 | 2.73 |
| Plant height (cm) | 83.87 | 99.74 | 91.67 | 3.38 | 2.81 | 4.19 | |
| Tillers per plant | 4.00 | 6.00 | 4.64 | 0.60 | 12.50 | 1.02 | |
| Spike length (cm) | 11.22 | 13.50 | 12.27 | 0.58 | 7.04 | 1.34 | |
| Number of spikelets per spike | 17.00 | 21.00 | 19.00 | 1.50 | 7.38 | 2.34 | |
| 1000-grain weight | 13.83 | 26.95 | 19.08 | 3.27 | 7.47 | 2.59 | |
| Grain yield (kg/ha) | 717.86 | 2309.40 | 1343.60 | 441.69 | 12.37 | 284.15 | |
| Protein content (%) | 11.53 | 15.23 | 13.57 | 0.81 | 1.21 | 0.33 | |
| Moisture content (%) | 8.10 | 8.87 | 8.32 | 0.18 | 1.08 | 0.18 | |
| Gluten content | 37.67 | 42.33 | 40.29 | 1.02 | 1.10 | 0.90 | |
| M8 | Days to heading | 91.00 | 99.00 | 96.09 | 1.89 | 0.75 | 1.18 |
| Plant height (cm) | 73.02 | 91.44 | 82.89 | 4.05 | 1.58 | 2.00 | |
| Tillers per plant | 6.00 | 7.00 | 6.61 | 0.50 | 8.68 | 0.93 | |
| Spike length (cm) | 9.94 | 14.54 | 12.68 | 1.23 | 4.69 | 0.97 | |
| Number of spikelets per spike | 17.00 | 21.00 | 19.30 | 1.42 | 6.33 | 1.94 | |
| 1000-grain weight | 13.85 | 36.55 | 25.47 | 4.74 | 2.14 | 0.89 | |
| Grain yield (kg/ha) | 1244.50 | 5819.10 | 2951.20 | 838.39 | 5.49 | 72.52 | |
| Protein content (%) | 11.05 | 13.20 | 12.26 | 0.47 | 1.10 | 0.27 | |
| Moisture content (%) | 10.73 | 11.68 | 11.07 | 0.24 | 1.10 | 0.23 | |
| Gluten content | 36.00 | 40.00 | 38.36 | 0.96 | 1.89 | 1.46 |
| Sr. # | Genotypes | DTH | Plant Height | TPP | Spike Length | Number of Spikelets Per Spike | TGW | Grain Yield | Protein Content | Moisture Content | Gluten Content |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pb-11 parent | 96.25 | 88.00 | 5.57 | 12.35 | 18.00 | 22.43 | 2433.71 | 12.22 | 9.35 | 40.25 |
| 2 | Pb-M-1027 waxy | 90.46 | 87.91 | 6.33 | 12.48 | 19.00 | 28.04 | 3110.04 | 12.19 | 9.15 | 37.88 |
| 3 | Pb-M-1055 | 95.88 | 88.28 | 5.28 | 12.55 | 18.50 | 22.95 | 2088.02 | 12.85 | 9.11 | 39.67 |
| 4 | Pb-M-1064 | 94.83 | 91.62 | 5.86 | 13.27 | 18.50 | 24.33 | 2442.28 | 13.65 | 9.18 | 40.13 |
| 5 | Pb-M-119 waxy | 96.61 | 81.20 | 5.38 | 12.25 | 18.50 | 21.00 | 2091.18 | 13.10 | 9.10 | 38.83 |
| 6 | Pb-M-12 waxy | 98.23 | 76.37 | 4.90 | 11.47 | 18.00 | 18.13 | 1648.42 | 13.29 | 8.78 | 40.96 |
| 7 | Pb-M-1272 waxy | 97.46 | 89.61 | 5.86 | 11.72 | 19.00 | 27.08 | 3265.75 | 12.94 | 9.36 | 38.71 |
| 8 | Pb-M-1323 waxy | 93.75 | 86.28 | 5.76 | 12.42 | 20.00 | 28.42 | 3082.38 | 12.86 | 8.96 | 39.29 |
| 9 | Pb-M-1530 | 96.75 | 87.15 | 5.67 | 12.91 | 18.50 | 25.19 | 2806.99 | 12.74 | 9.21 | 38.17 |
| 10 | Pb-M-1575 | 98.25 | 91.66 | 5.68 | 13.20 | 18.00 | 22.27 | 2373.52 | 13.45 | 9.23 | 39.11 |
| 11 | Pb-M-1743 | 96.79 | 84.93 | 5.33 | 12.53 | 19.00 | 21.33 | 1917.99 | 12.96 | 8.87 | 39.21 |
| 12 | Pb-M-1802 | 97.55 | 87.89 | 5.69 | 12.24 | 18.00 | 24.15 | 2408.46 | 12.73 | 8.94 | 38.92 |
| 13 | Pb-M-1854 | 96.00 | 87.75 | 5.92 | 10.86 | 18.00 | 27.15 | 3001.59 | 13.12 | 9.39 | 39.17 |
| 14 | Pb-M-1917 | 97.42 | 88.55 | 5.10 | 12.73 | 18.00 | 23.91 | 2384.68 | 12.94 | 8.91 | 38.42 |
| 15 | Pb-M-1946 | 96.08 | 88.73 | 5.90 | 12.82 | 20.00 | 23.02 | 2757.61 | 12.76 | 9.12 | 39.38 |
| 16 | Pb-M-2041 | 97.79 | 90.69 | 5.39 | 11.73 | 18.00 | 25.16 | 2911.46 | 12.96 | 9.04 | 38.04 |
| 17 | Pb-M-2061 | 94.75 | 89.87 | 5.60 | 12.16 | 19.00 | 24.50 | 2670.98 | 12.81 | 9.38 | 38.87 |
| 18 | Pb-M-2260 | 96.34 | 86.77 | 6.00 | 12.21 | 20.00 | 26.93 | 3219.03 | 12.40 | 9.08 | 37.67 |
| 19 | Pb-M-2302 | 97.50 | 86.88 | 5.46 | 11.90 | 18.50 | 23.08 | 2466.15 | 12.95 | 8.93 | 39.88 |
| 20 | Pb-M-2443 | 95.75 | 87.14 | 5.50 | 12.45 | 19.00 | 21.63 | 2417.59 | 13.08 | 8.99 | 39.75 |
| 21 | Pb-M-2453 | 95.08 | 89.47 | 6.32 | 13.50 | 20.00 | 23.55 | 2192.27 | 13.23 | 9.16 | 39.96 |
| 22 | Pb-M-2517 | 95.25 | 86.44 | 5.29 | 13.31 | 18.50 | 23.17 | 2572.64 | 12.90 | 9.00 | 40.19 |
| 23 | Pb-M-2550 | 92.92 | 88.75 | 5.29 | 12.39 | 18.50 | 21.43 | 2175.05 | 13.68 | 9.19 | 39.08 |
| 24 | Pb-M-2637 | 94.49 | 84.21 | 5.18 | 12.83 | 18.50 | 21.26 | 2145.48 | 12.38 | 9.02 | 39.45 |
| 25 | Pb-M-2719 | 94.17 | 83.85 | 5.34 | 11.66 | 18.50 | 22.48 | 2240.87 | 13.28 | 8.84 | 40.00 |
| 26 | Pb-M-2725 | 96.50 | 87.18 | 5.71 | 13.04 | 19.00 | 21.91 | 2261.81 | 13.21 | 8.95 | 39.55 |
| 27 | Pb-M-2728 | 96.67 | 86.46 | 5.90 | 12.49 | 18.50 | 17.60 | 1721.88 | 13.50 | 9.43 | 39.25 |
| 28 | Pb-M-2768 | 94.42 | 87.55 | 5.63 | 12.80 | 18.50 | 24.94 | 2801.25 | 13.16 | 8.79 | 38.42 |
| 29 | Pb-M-2778 | 95.33 | 88.72 | 5.71 | 13.29 | 19.00 | 22.61 | 2600.04 | 12.82 | 8.98 | 39.92 |
| 30 | Pb-M-334 waxy | 95.58 | 86.78 | 5.44 | 12.08 | 18.50 | 21.97 | 2118.48 | 13.55 | 8.90 | 40.04 |
| 31 | Pb-M-583 | 94.71 | 87.06 | 5.49 | 13.37 | 18.00 | 22.83 | 2342.93 | 13.42 | 9.13 | 39.50 |
| 32 | Pb-M-59 waxy | 92.97 | 84.38 | 5.24 | 12.00 | 17.50 | 27.42 | 3269.98 | 12.84 | 8.81 | 38.58 |
| 33 | Pb-M-605 | 95.17 | 86.71 | 5.35 | 13.05 | 19.50 | 20.55 | 1970.30 | 13.65 | 9.05 | 40.97 |
| Traits | Principal Component (PC) | Eigen Value | Percent Variance | Cumulative Percentage |
|---|---|---|---|---|
| Days to heading | PC1 | 3.5986 | 35.99 | 35.99 |
| Plant height | PC2 | 1.8355 | 18.36 | 54.34 |
| Tillers per plant | PC3 | 1.2496 | 12.5 | 66.84 |
| Spike length | PC4 | 0.8840 | 8.84 | 75.68 |
| Number of spikelets per spike | PC5 | 0.8125 | 8.13 | 83.8 |
| 1000-grain weight | PC6 | 0.6463 | 6.46 | 90.27 |
| Grain Yield | PC7 | 0.4393 | 4.39 | 94.66 |
| Protein content | PC8 | 0.2842 | 2.84 | 97.5 |
| Moisture content | PC9 | 0.1825 | 1.83 | 99.33 |
| Gluten content | PC10 | 0.0671 | 0.67 | 100 |
| PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|
| Grain yield | Spike length | Moisture content | Number of spikelets per spike | Days to heading |
| 1000-grain weight | Number of spikelets per spike | Days to heading | __ | __ |
| Tillers per plant | Plant height | Number of spikelets per spike | __ | __ |
| Gluten content | Tillers per plant | __ | __ | __ |
| Plant height | __ | __ | __ | __ |
| Protein content | __ | __ | __ | __ |
| Moisture content | __ | __ | __ | __ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, S.; Ishfaq, S.; Ikram, M.; Nawaz, M.A.; Rahman, M.-u.-. Characterization of Gamma-Rays-Induced Spring Wheat Mutants for Morphological and Quality Traits through Multivariate and GT Bi-Plot Analysis. Agronomy 2021, 11, 2288. https://doi.org/10.3390/agronomy11112288
Zulfiqar S, Ishfaq S, Ikram M, Nawaz MA, Rahman M-u-. Characterization of Gamma-Rays-Induced Spring Wheat Mutants for Morphological and Quality Traits through Multivariate and GT Bi-Plot Analysis. Agronomy. 2021; 11(11):2288. https://doi.org/10.3390/agronomy11112288
Chicago/Turabian StyleZulfiqar, Sana, Shumila Ishfaq, Muhammad Ikram, Muhammad Amjad Nawaz, and Mehboob-ur- Rahman. 2021. "Characterization of Gamma-Rays-Induced Spring Wheat Mutants for Morphological and Quality Traits through Multivariate and GT Bi-Plot Analysis" Agronomy 11, no. 11: 2288. https://doi.org/10.3390/agronomy11112288
APA StyleZulfiqar, S., Ishfaq, S., Ikram, M., Nawaz, M. A., & Rahman, M.-u.-. (2021). Characterization of Gamma-Rays-Induced Spring Wheat Mutants for Morphological and Quality Traits through Multivariate and GT Bi-Plot Analysis. Agronomy, 11(11), 2288. https://doi.org/10.3390/agronomy11112288








