Changes in Ultrastructure and Oxidation Resistance of Peel of Pear Cultivars during Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pear Fruit Samples
2.2. Experimental Design and Methods
2.2.1. Experimental Design
2.2.2. Determination Method
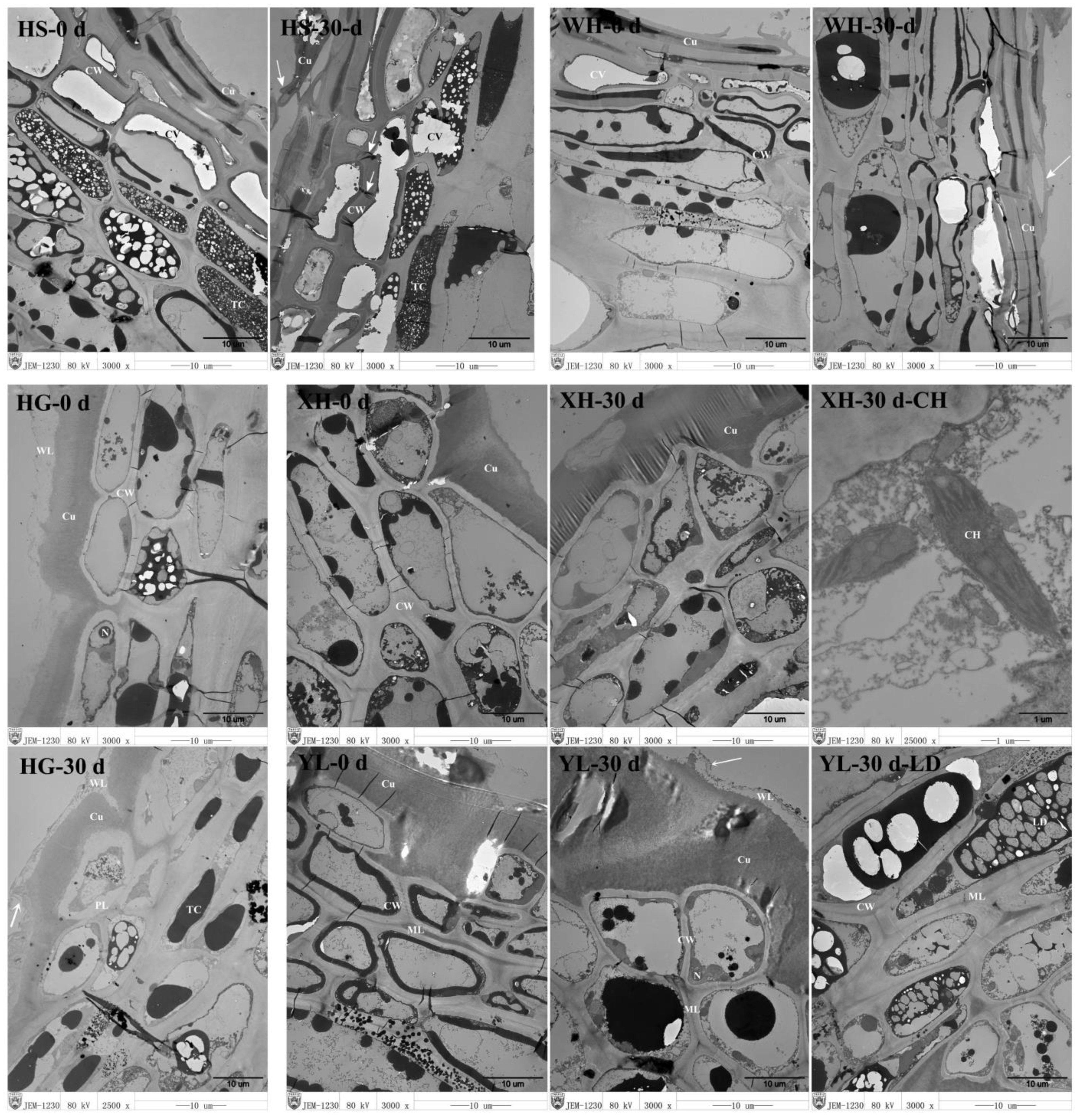
Peel Ultrastructure
Determination of the Antioxidant Enzyme Activity and Total Polyphenol and Total Flavonoids Contents
Total Antioxidant Capacity (T-AOC)
2.3. Statistical Analysis of the Data
3. Results
3.1. Peel Ultrastructure of the Samples
3.2. The Changes in Total Polyphenol and Total Flavonoids Content
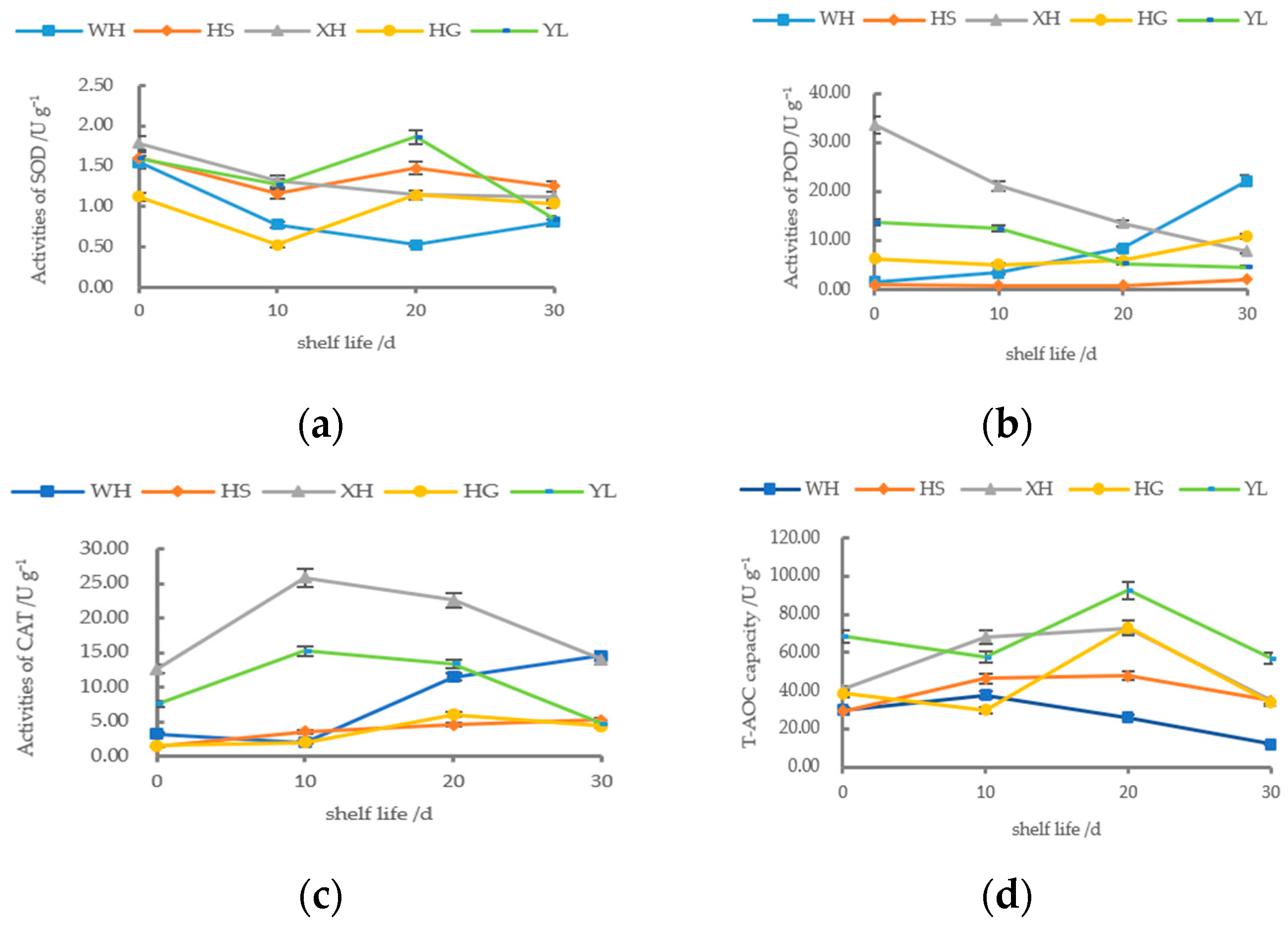
3.3. The Changes in Activity of the Antioxidant Enzyme
3.4. Analysis of the Total Antioxidant Capacity
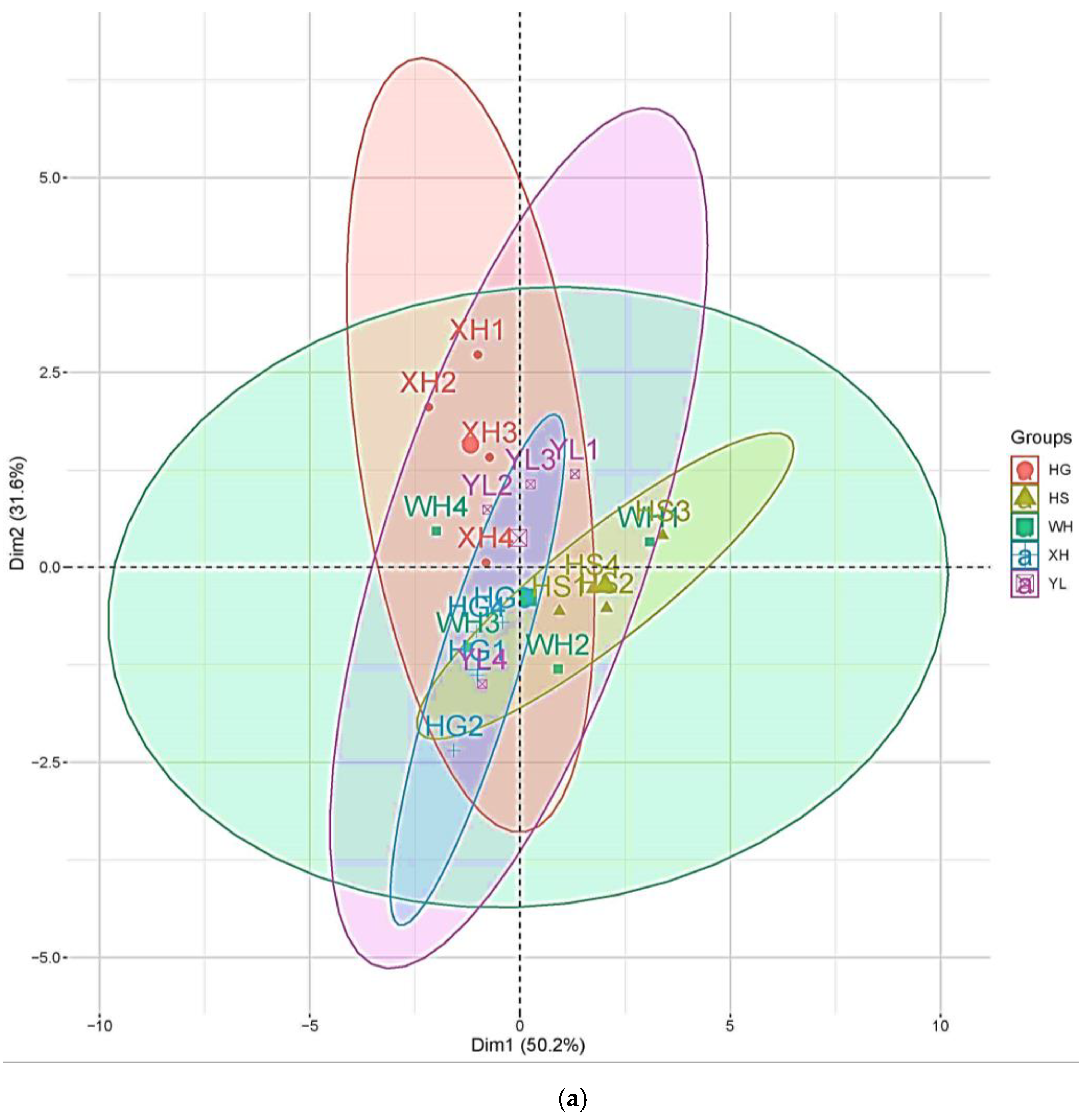
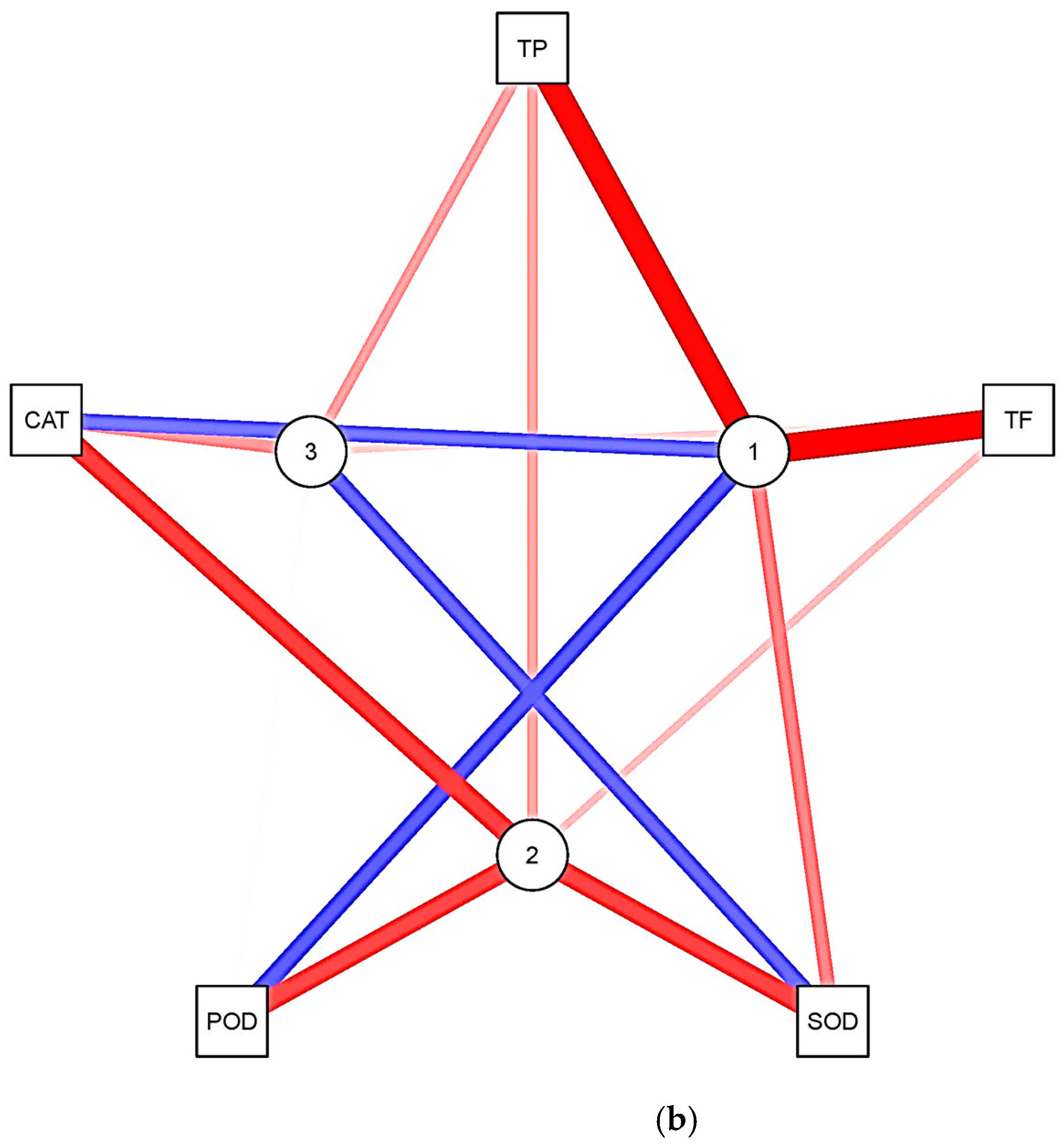
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.F.; Zhang, Y.; Huo, H.L.; Tian, L.M.; Zhang, S.M. Analysis of leaf morphological diversity of pear (Pyrus L.) preserved in China. Acta Hortic. 2021, 1303, 55–62. [Google Scholar] [CrossRef]
- Li, X.G. Review on the development of pear industry in China in 30 years after the reform and opening-up policy. Fruit Yantai 2008, 4, 4–6. [Google Scholar]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Zhang, Y.X. Theory of Fruit Tree Cultivation (Northern Edition); Chinese Agriculture Press: Beijing, China, 2003; pp. 56–60. [Google Scholar]
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta 2003, 1620, 1–7. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epid ermal surfaces. J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y. Effect of Cuticular Wax of Pingguoli Pear from Developing and Storage on Alternaria Alternata Pre-Penetration Process. Master’s Dissertation, Gansu Agricultural University, Lanzhou, China, 2016; pp. 1–78. [Google Scholar]
- Wang, J.Z. Xinpingli, a new late Chinese pear cultivar with long keep quality. J. Fruit Sci. 2004, 21, 391–392. [Google Scholar]
- Fric, F.; Wolf, G. Hydrolytic enzymes of ungerminated and germinated conidia of Erysiphe graminis DC. f. sp. Hordei Marchal. J. Phytopathol. 1994, 140, 1–10. [Google Scholar] [CrossRef]
- Fauth, M. Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol. 1998, 117, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.L.; Guo, R.Z.; Li, X.Y.; Liu, C.L.; Kou, X.H. Effects of different coating treatments on antioxidant ability in postharvest pear. Food Sci. 2012, 33, 261–267. [Google Scholar]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Galvis Sánchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef]
- Peng, F.; Yang, Y.D.; Cheng, C.H.; Xie, Y.; Liu, Y.L. Optimization of microwave-assisted extraction of total flavonoids from“An” pear (Pyrus ussuriensis Maxim) and components analysis. Food Sci. Technol. 2015, 40, 196–203. [Google Scholar]
- Zou, Q. Guidance of Plant Physiology and Biochemistry Experiment; China Agriculture Press: Beijing, China, 1995; pp. 59–99. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–76. [Google Scholar] [CrossRef]
- Rajeswari, V.; Paliwal, K. Peroxidase and catalase changes during in vitro adventitious shoot organogenesis from hypocotyls of Albizia odoratissima L.f. (Benth). Acta Physiol. Plant. 2008, 30, 825–832. [Google Scholar] [CrossRef]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef]
- Peng, Y.B.; Zhang, D.P. Ultrastructure of Epidermis and Flesh of the Developing Apple Fruit. Acta Bot. Sin. 2000, 42, 794–802. [Google Scholar]
- Benzie, I.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, H.; Zhou, H.; Cheng, J.; Yang, H. Mspectraai: A powerful platform for deciphering proteome profiling of multi-tumor mass spectrometry data by using deep neural networks. BMC Bioinform. 2020, 21, 439. [Google Scholar] [CrossRef]
- Schreiber, L.; Skrabs, M.; Hartmann, K.D.; Diamantopoulos, P.; Simanova, E.; Santrucek, J. Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 2001, 214, 274–282. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid. Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Curry, E. Ultrastructure of epicuticular wax aggregates during fruit development in apple (Malus domestica borkh.). J. Pomol. Hortic. Sci. 2005, 80, 668–676. [Google Scholar]
- Jeffree, C.E. The fine structure of the plant cuticle. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 11–125. [Google Scholar]
- Segado, P.; Domã Nguez, E.; Heredia, A. Ultrastructure of the epidermal cell wall and cuticle of tomato fruit (Solanum lycopersicum L.) during development. Plant Physiol. 2016, 170, 935–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riederer, M.; Schönherr, J. Development of plant cuticles: Fine structure and cutin composition of Clivia miniata Reg leaves. Planta 1988, 174, 127–138. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zheng, Y.C.; Cao, Y.F.; Tian, L.M.; Dong, X.G.; Zhang, Y.; Qi, D.; Huo, H.L. The composition and content of polyphenols in 16 parts of ‘Zaosu’ and ‘Nanguoli’. Sci. Agric. Sin. 2017, 50, 545–555. [Google Scholar]
- Zeng, S.M.; Yang, J.; Wang, L.; Wang, S.K.; Li, X.G. Study on phenolics content and its antioxidant activity in the fruit of pear species. J. Fruit Sci. 2014, 31, 39–44. [Google Scholar]
- Stinco, C.M.; Baroni, M.V.; Naranjo, R.D.D.P.; Wunderlin, D.A.; Heredia, F.J.; Meléndez-Martínez, A.J. Hydrophilic antioxidant compounds in orange juice from different fruit cultivars: Composition and antioxidant activity evaluated by chemical and cellular based (saccharomyces cerevisiae) assays. J. Food Compos. Anal. 2015, 37, 1–10. [Google Scholar] [CrossRef]


| Cultivars | Total Polyphenol mg kg−1 | Total Flavonoids mg kg−1 | SOD U g−1 | POD U g−1 | CAT U g−1 | T-AOC U g−1 |
|---|---|---|---|---|---|---|
| HS | 2085.82 b | 1362.34 b | 1.38 a | 1.12 a | 3.68 a | 39.48 ab |
| WH | 1800.12 b | 1039.76 ab | 0.91 a | 8.86 ab | 7.82 a | 26.37 a |
| XH | 1485.23 ab | 842.07 a | 1.34 a | 18.98 b | 18.77 b | 54.02 bc |
| HG | 1025.01 a | 712.70 a | 0.96 a | 7.03 a | 3.52 a | 43.81 ab |
| YL | 1556.53 ab | 939.52 a | 1.40 a | 8.97 ab | 10.23 a | 68.76 c |
| F-value | 3.614 | 3.878 | 1.893 | 3.430 | 7.045 | 4.085 |
| p-value of Cultivars | 0.030 | 0.023 | 0.164 | 0.035 | 0.002 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, H.; Zhou, Z.; Xu, J.; Tian, L.; Dong, X.; Zhang, Y.; Qi, D.; Liu, C.; Cao, Y. Changes in Ultrastructure and Oxidation Resistance of Peel of Pear Cultivars during Shelf Life. Agronomy 2021, 11, 2274. https://doi.org/10.3390/agronomy11112274
Huo H, Zhou Z, Xu J, Tian L, Dong X, Zhang Y, Qi D, Liu C, Cao Y. Changes in Ultrastructure and Oxidation Resistance of Peel of Pear Cultivars during Shelf Life. Agronomy. 2021; 11(11):2274. https://doi.org/10.3390/agronomy11112274
Chicago/Turabian StyleHuo, Hongliang, Zhiqin Zhou, Jiayu Xu, Luming Tian, Xingguang Dong, Ying Zhang, Dan Qi, Chao Liu, and Yufen Cao. 2021. "Changes in Ultrastructure and Oxidation Resistance of Peel of Pear Cultivars during Shelf Life" Agronomy 11, no. 11: 2274. https://doi.org/10.3390/agronomy11112274






