Black Carbon and Its Effect on Carbon Sequestration in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples Processing
2.2. Black Carbon Content Determination
2.3. Determination of the Structure of Soil
2.4. Determination of Free Light Fraction fLFOM, Occluded Light Fraction oLFOM, and Heavy Fraction DFOM of Soil Organic Matter
2.5. Statistical Analysis
3. Results and Discussion
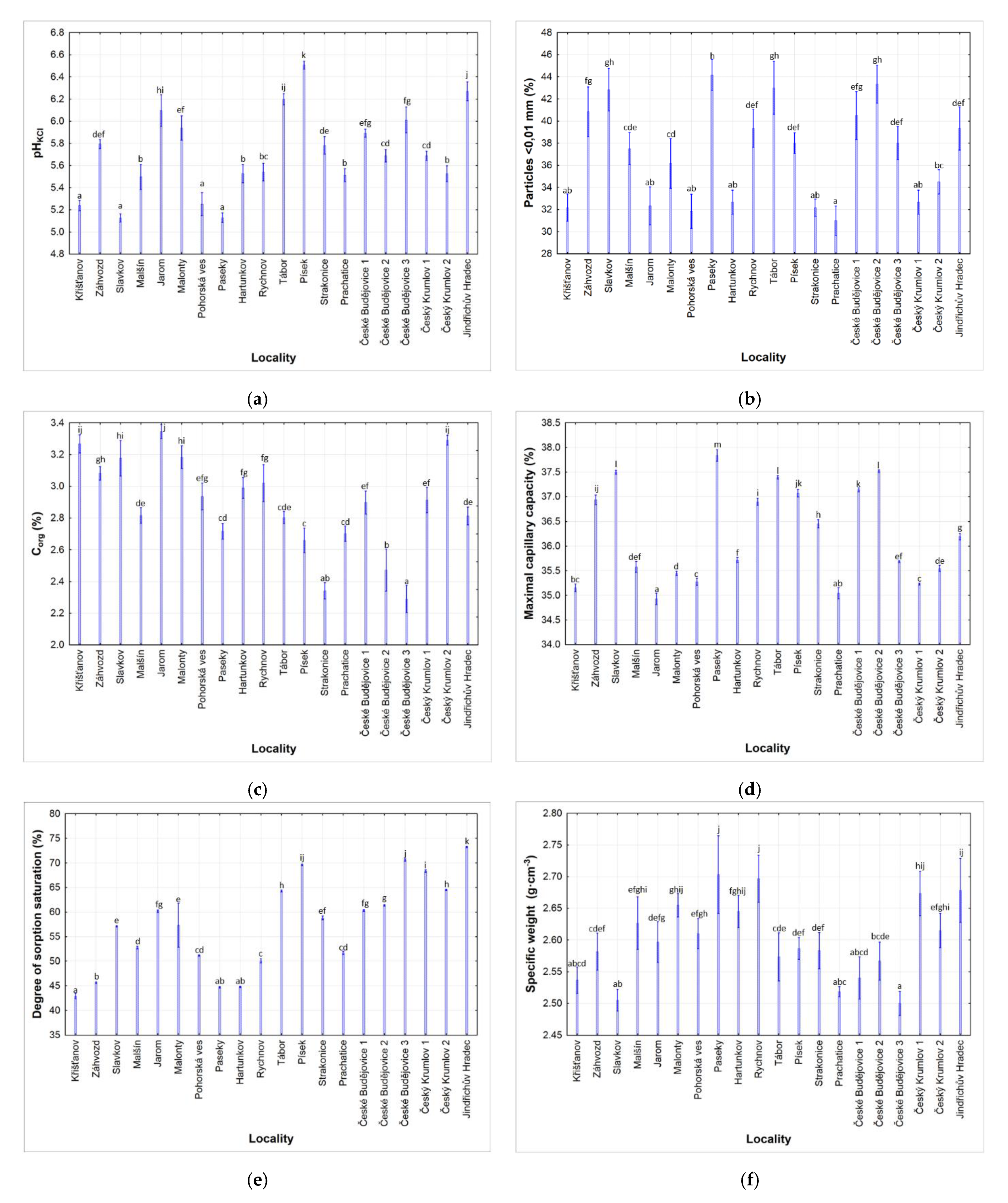
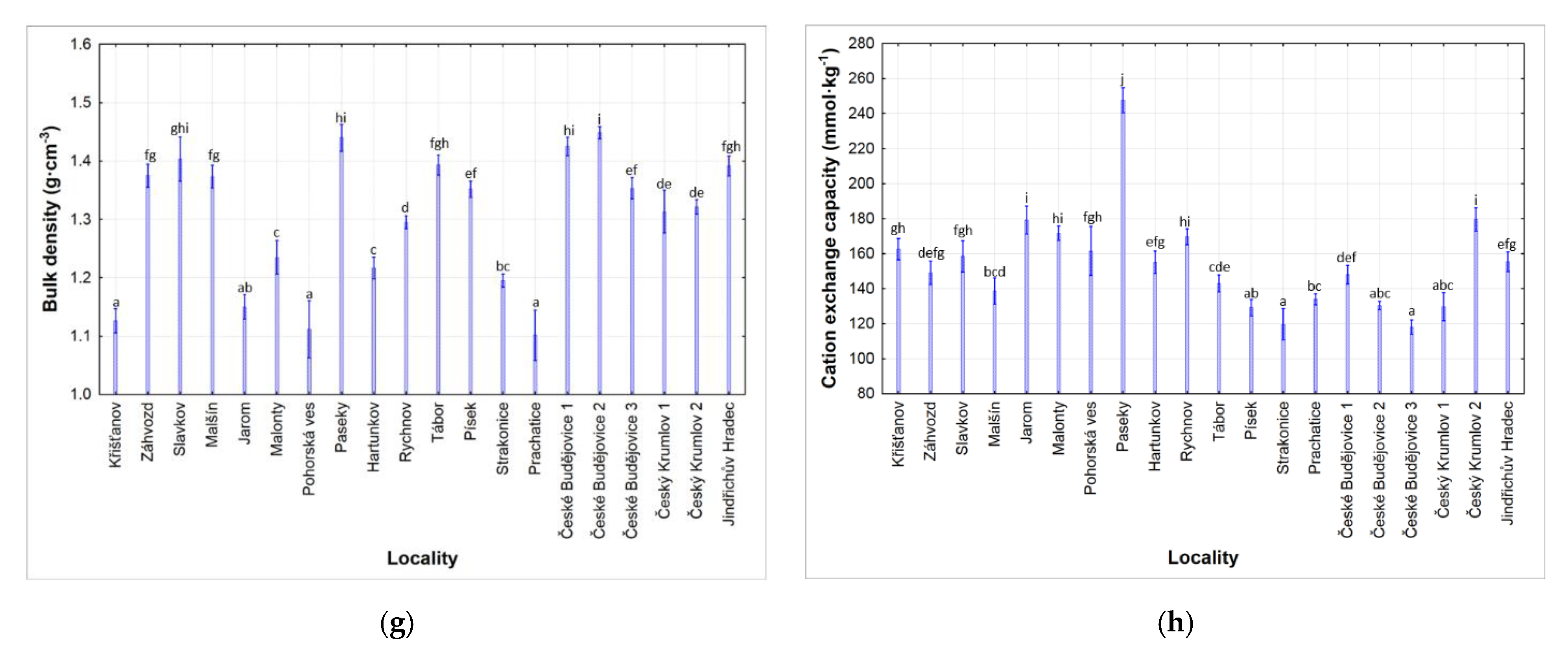
3.1. Soil Properties
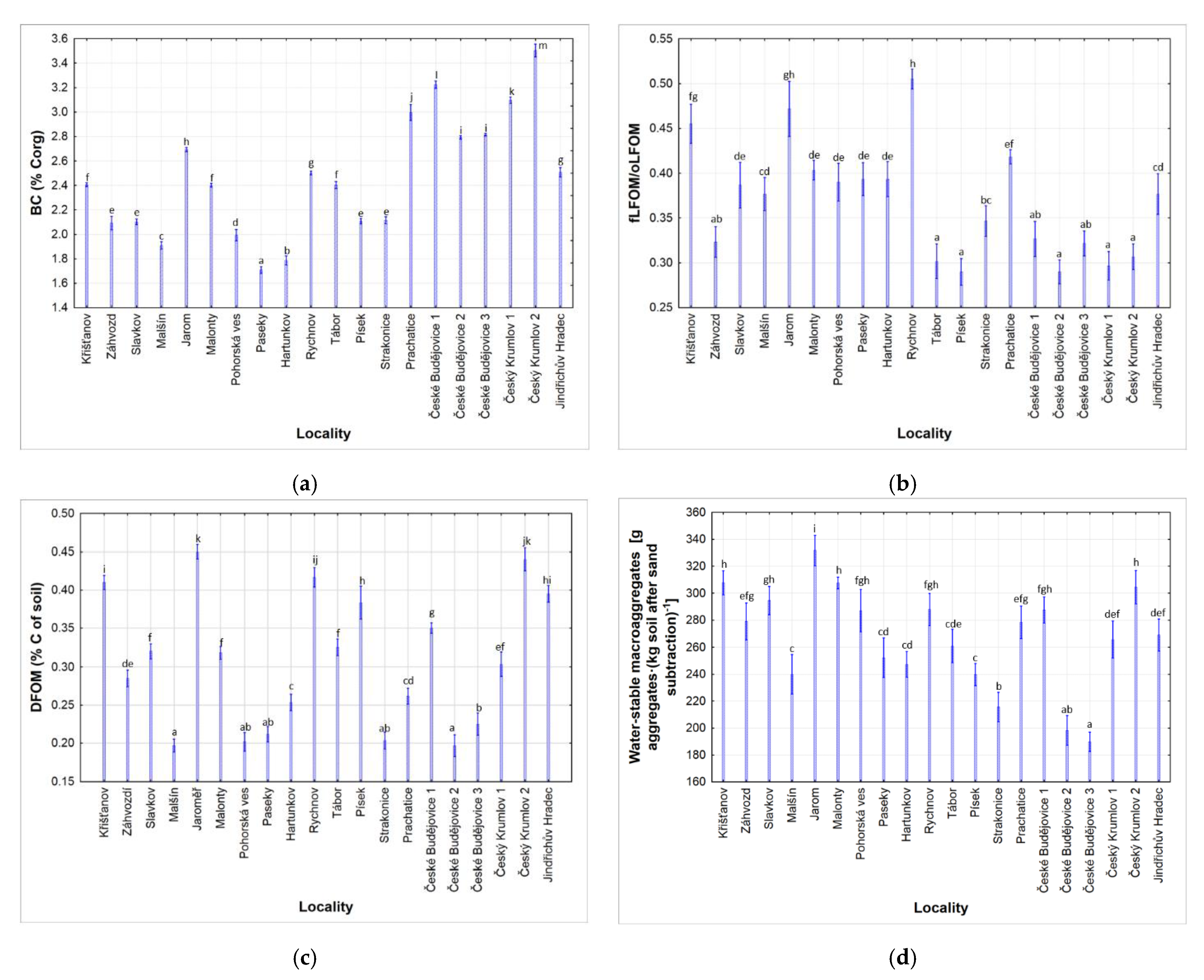
3.2. Black Carbon Content
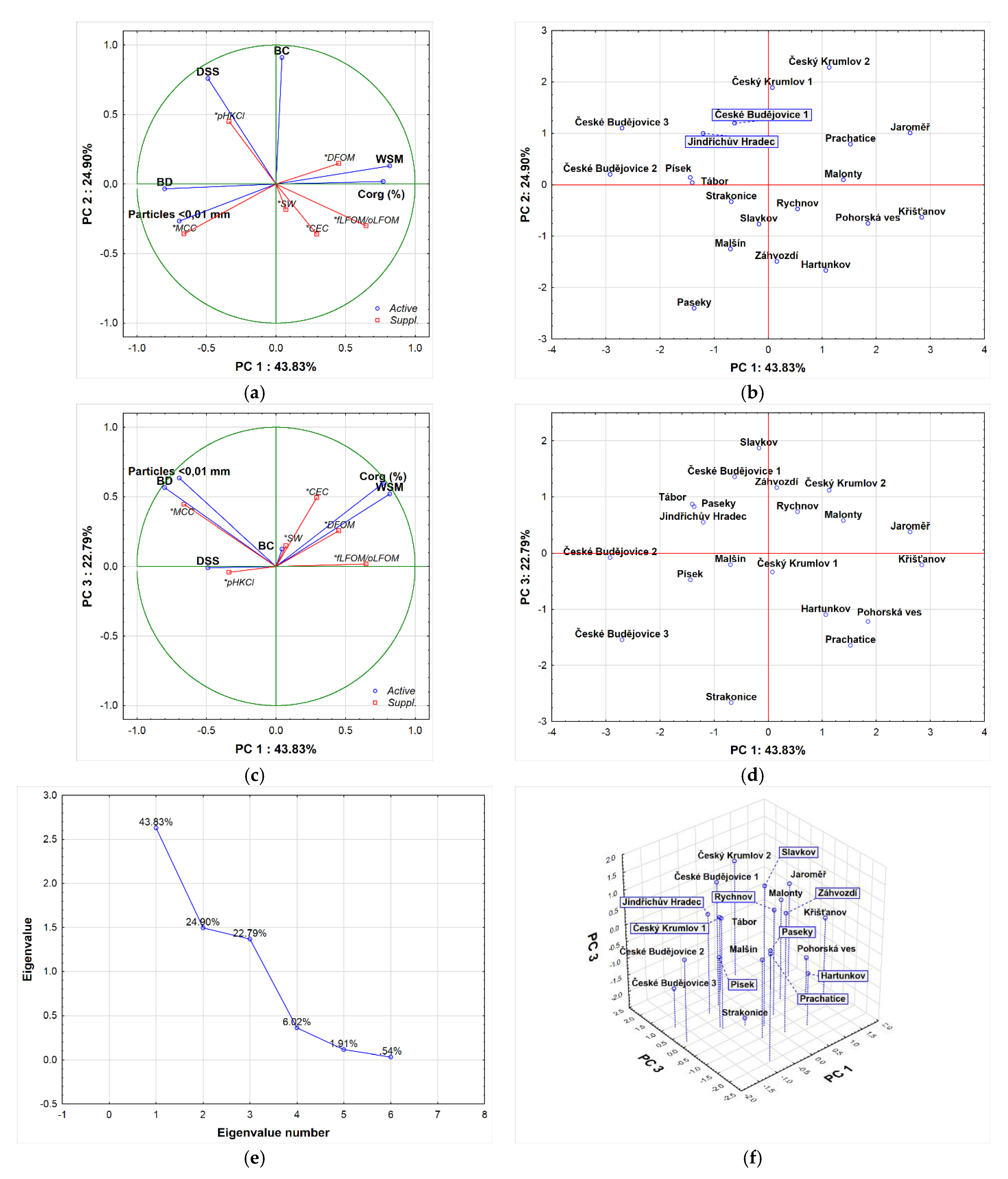
3.3. Evaluation of Results from the Point of View Principal Component Analysis and Factor Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Moudrý, J.; Bernas, J.; Kopecký, M.; Konvalina, P.; Bucur, D.; Moudrý, J.; Kolář, L.; Štěrba, Z.; Jelínková, Z. Influence of farming system on greenhouse gas emissions within cereal cultivation. Environ. Eng. Manag. J. 2018, 17, 905–914. [Google Scholar] [CrossRef]
- Janzen, H.H. Carbon cycling in earth systems—A soil science perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G.; Osmond, G. Analysis, distribution, implications, and current challenges. Global Biogeochem Cycles. 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile Organic Matter Fractions as Central Components of the Quality of Agricultural Soils: An Overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Lehmann, J. Humic Substances Extracted by Alkali Are Invalid Proxies for the Dynamics and Functions of Organic Matter in Terrestrial and Aquatic Ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Heymann, K.; Lehmann, J.; Solomon, D.; Liang, B.; Neves, E.; Wirick, S. Can functional group composition of alkaline isolates from black carbon-rich soils be identified on a sub-100nm scale? Geoderma 2014, 235–236, 163–169. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Llorente, M.; Glaser, B.; Turrión, M.B. Storage of organic carbon and Black carbon in density fractions of calcareous soils under different land uses. Geoderma 2010, 159, 31–38. [Google Scholar] [CrossRef]
- Lugato, E.; Morari, F.; Nardi, S.; Berti, A.; Giardini, L. Relationship between aggregate pore size distribution and organic-humic carbon in contrasting soils. Soil Tillage Res. 2009, 103, 153–157. [Google Scholar] [CrossRef]
- Von Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Haumaier, L.; Zech, W. Black carbon-possible source of highly aromatic components of soil humic acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Goldberg, E.D. Black Carbon in the Environment; John Wiley: New York, NY, USA, 1985. [Google Scholar]
- Mantell, C.H. Carbon and Graphite Handbook; Interscience: New York, NY, USA, 1968; p. 446. [Google Scholar]
- Mattson, J.S.; Mark, H.B. Activated Carbon: Surface Chemistry and Adsorption from Solution; M. Dekker: New York, NY, USA, 1971. [Google Scholar]
- Medalia, A.I.; Rivin, D. Particulate carbon and other components of soot and carbon black. Carbon N. Y. 1982, 20, 481–492. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J.; Crutzen, P.J. Toward a global estimate of black carbon in residues of vegetation fires representing a sink of atmospheric CO2 and a source of O2. Glob. Biogeochem. Cycles 1995, 9, 491–501. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819. [Google Scholar] [CrossRef]
- Seiler, W.; Crutzen, P. Estimates of Gross and Net Fluxes of Carbon Between. Clim. Chang. 1980, 2, 207–247. [Google Scholar] [CrossRef]
- Oades, J.M. An overview of processes affecting the cycling of organic carbon in soils. In The Role of Non-Living Organic Matter in the Earth’s Carbon Cycle; Zepp, R.G., Sonntag, C.H., Eds.; Wiley: New York, NY, USA, 1995; pp. 293–303. [Google Scholar]
- Kuhlbusch, T.A.J.; Andreae, M.O.; Cachier, H.; Goldammer, J.G.; Lacaux, J.P.; Shea, R.; Crutzen, P.J. Black carbon formation by savanna fires: Measurements and implications for the global carbon cycle. J. Geophys. Res. Atmos. 1996, 101, 23651–23665. [Google Scholar] [CrossRef]
- Kopecký, M.; Kolář, L.; Konvalina, P.; Strunecký, O.; Teoderescu, F.; Mráz, P.; Peterka, J.; Váchalová, R.; Bernas, J.; Bartoš, P.; et al. Modified Biochar—A Tool for Wastewater Treatment. Energies 2020, 13, 5270. [Google Scholar] [CrossRef]
- Masiello, C.A. New directions in black carbon organic geochemistry. Mar. Chem. 2004, 92, 201–213. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Skjemstad, J.O.; Gehrt, E.; Kögel-Knabner, I. Charred organic carbon in German chernozemic soils. Eur. J. Soil Sci. 1999, 50, 351–365. [Google Scholar] [CrossRef]
- Braida, W.J.; Pignatello, J.J.; Lu, Y.; Ravikovitch, P.I.; Neimark, A.V.; Xing, B. Sorption hysteresis of benzene in charcoal particles. Environ. Sci. Technol. 2003, 37, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Skjemstad, J.O.; Jäger, C. Carbon isotope geochemistry and nanomorphology of soil black carbon: Black chernozemic soils in central Europe originate from ancient biomass burning. Glob. Biogeochem. Cycles 2002, 16, 70-1–70-8. [Google Scholar] [CrossRef] [Green Version]
- Trompowsky, P.M.; De Melo Benites, V.; Madari, B.E.; Pimenta, A.S.; Hockaday, W.C.; Hatcher, P.G. Characterization of humic like substances obtained by chemical oxidation of eucalyptus charcoal. Org. Geochem. 2005, 36, 1480–1489. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The “Terra Preta” phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef]
- Lehmann, J.; Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching an an archaeological Anthrosol and a Ferralsol. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Lehmann, J.; Liang, B.; Solomon, D.; Lerotic, M.; Luizão, F.; Kinyangi, J.; Schäfer, T.; Wirick, S.; Jacobsen, C. Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy for mapping nano-scale distribution of organic carbon forms in soil: Application to black carbon particles. Glob. Biogeochem. Cycles 2005, 19, 1–12. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Wolbach, W.S.; Anders, E. Elemental carbon in sediments: Determination and isotopic analysis in the presence of kerogen. Geochim. Cosmochim. Acta 1989, 53, 1637–1647. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Clarke, P.; Taylor, J.A.; Oades, J.M.; McClure, S.G. The chemistry and nature of protected carbon in soil. Aust. J. Soil Res. 1996, 34, 251–271. [Google Scholar] [CrossRef]
- Hayatsu, R.; Scott, R.G.; Winans, R.E. Oxidation of coal. In Oxidation in Organic Chemistry; Trahanovsky, W.S., Ed.; Academic Press: New York, NY, USA, 1982; pp. 279–354. [Google Scholar]
- Shafizadeh, F.; Sekiguchi, Y. Development of aromaticity in cellulosic chars. Carbon N. Y. 1983, 21, 511–516. [Google Scholar] [CrossRef]
- Schnitzer, M. The chemistry and reactions of humic substances. In Ecology and Coal Resource Development; Based on the International Congress for Energy and the Ecosystem, Held at the University of North Dakota, Grand Forks, North Dakota, 12–16 June 1978; Pergamon Press: Oxford, UK, 1979; pp. 807–819. [Google Scholar] [CrossRef]
- Hedges, J.I.; Eglinton, G.; Hatcher, P.G.; Kirchman, D.L.; Arnosti, C.; Derenne, S.; Evershed, R.P.; Kögel-Knabner, I.; De Leeuw, J.W.; Littke, R.; et al. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000, 31, 945–958. [Google Scholar] [CrossRef]
- Gélinas, Y.; Prentice, K.M.; Baldock, J.A.; Hedges, J.I. An improved thermal oxidation method for the quantification of soot/graphitic black carbon in sediments and soils. Environ. Sci. Technol. 2001, 35, 3519–3525. [Google Scholar] [CrossRef]
- Eliasl, V.O.; Simoneit, B.R.T.; Cordeiro, R.C.; Turcq, B. Evaluating levoglucosan as an indicator of biomass burning in Carajás, Amazônia: A comparison to the charcoal record. Geochim. Cosmochim. Acta 2001, 65, 267–272. [Google Scholar] [CrossRef]
- Yamashita, T.; Flessa, H.; John, B.; Helfrich, M.; Ludwig, B. Organic matter in density fractions of water-stable aggregates in silty soils: Effect of land use. Soil Biol. Biochem. 2006, 38, 3222–3234. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Kopecký, M.; Peterka, J.; Kolář, L.; Konvalina, P.; Maroušek, J.; Váchalová, R.; Herout, M.; Strunecký, O.; Batt, J.; Tran, D.K. Influence of selected maize cultivation technologies on changes in the labile fraction of soil organic matter sandy-loam cambisol soil structure. Soil Tillage Res. 2021, 207. [Google Scholar] [CrossRef]
- Sohi, S.P.; Mahieu, N.; Arah, J.R.M.; Powlson, D.S.; Madari, B.; Gaunt, J.L. A Procedure for Isolating Soil Organic Matter Fractions Suitable for Modeling. Soil Sci. Soc. Am. J. 2001, 65, 1121–1128. [Google Scholar] [CrossRef]
- John, B.; Yamashita, T.; Ludwig, B.; Flessa, H. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 2005, 128, 63–79. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.; de Moraes Sa, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils-Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef] [Green Version]
- Gulde, S.; Chung, H.; Amelung, W.; Chang, C.; Six, J. Soil Carbon Saturation Controls Labile and Stable Carbon Pool Dynamics. Soil Sci. Soc. Am. J. 2008, 72, 605–612. [Google Scholar] [CrossRef]
- Sequeira, C.H.; Alley, M.M.; Jones, B.P. Evaluation of potentially labile soil organic carbon and nitrogen fractionation procedures. Soil Biol. Biochem. 2011, 43, 438–444. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- DIN ISO 11277:2020. Soil quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- Balesdent, J.; Pétraud, J.-P.; Feller, C. Effets des ultrasons sur la distribution granulométrique des matières organiques des sols. Sci. Sol 1991, 29, 95–106. [Google Scholar]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Study of free and occluded particulate organic matter in soils by solid state 13c cp/mas nmr spectroscopy and scanning electron microscopy. Aust. J. Soil Res. 1994, 32, 285–309. [Google Scholar] [CrossRef]
- Meloun, M.; Militký, J. Statistical Data Analysis, A Practical Guide with 1250 Exercises and Answer Key on CD; Woodhead Publishing India: New Delhi, India, 2011. [Google Scholar]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
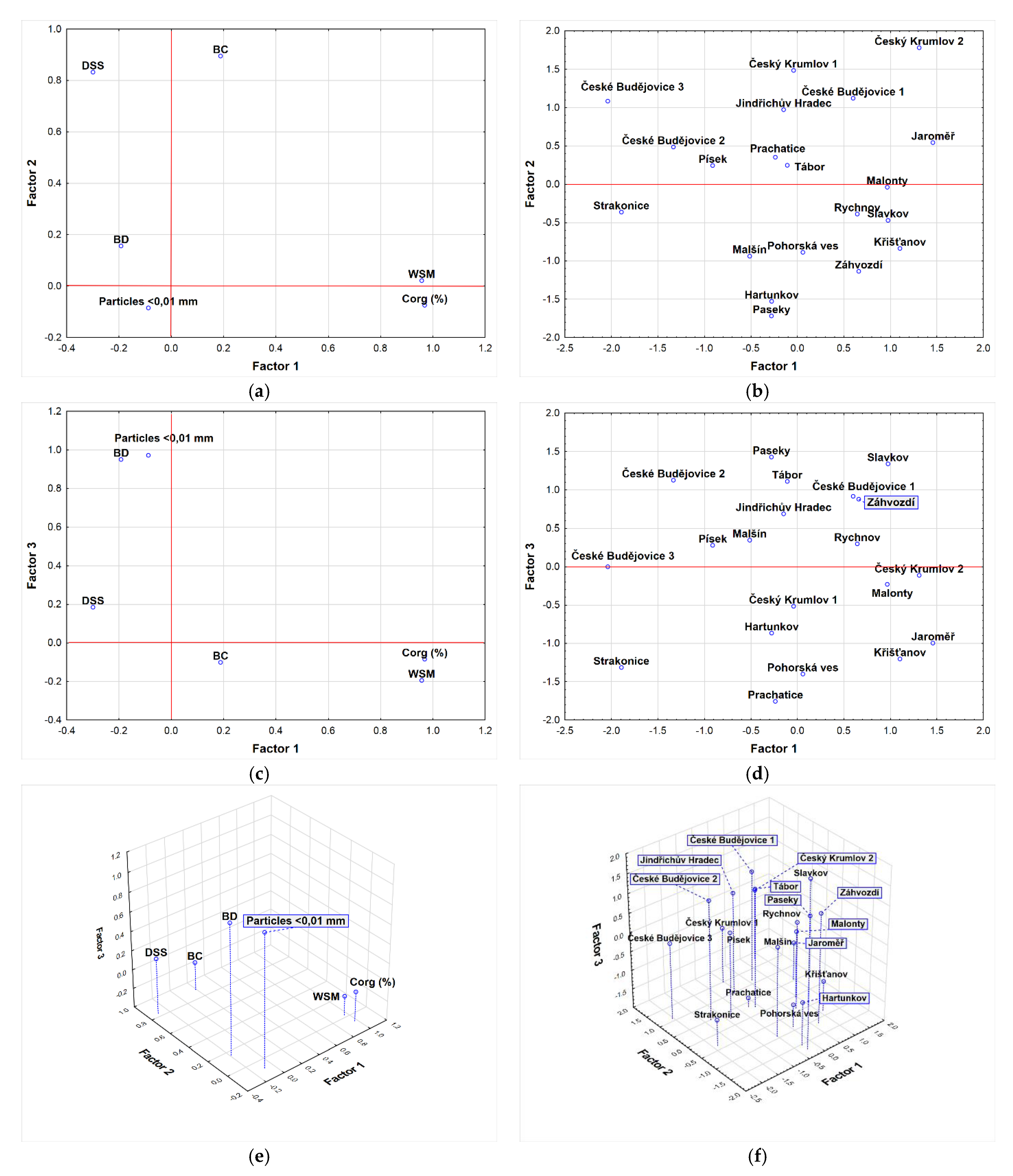
| Parameter | Factor Weight | Contributions of a Given Factor to the Communality | |||||
|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Communalities | |
| BC | 0.1885 | 0.8944 | −0.1017 | 0.0355 | 0.8355 | 0.8458 | 0.4384 |
| BD | −0.1917 | 0.1561 | 0.9496 | 0.0368 | 0.0611 | 0.9628 | 0.9087 |
| DSS | −0.2992 | 0.8325 | 0.1843 | 0.0895 | 0.7827 | 0.8166 | 0.5265 |
| Corg | 0.9692 | −0.0750 | −0.0854 | 0.9393 | 0.9449 | 0.9522 | 0.8948 |
| WSM | 0.9578 | 0.0212 | −0.1946 | 0.9173 | 0.9178 | 0.9556 | 0.9084 |
| Particles < 0.01 mm | −0.0876 | −0.0849 | 0.9715 | 0.0077 | 0.0149 | 0.9586 | 0.8845 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopecký, M.; Kolář, L.; Váchalová, R.; Konvalina, P.; Batt, J.; Mráz, P.; Menšík, L.; Hoang, T.N.; Dumbrovský, M. Black Carbon and Its Effect on Carbon Sequestration in Soil. Agronomy 2021, 11, 2261. https://doi.org/10.3390/agronomy11112261
Kopecký M, Kolář L, Váchalová R, Konvalina P, Batt J, Mráz P, Menšík L, Hoang TN, Dumbrovský M. Black Carbon and Its Effect on Carbon Sequestration in Soil. Agronomy. 2021; 11(11):2261. https://doi.org/10.3390/agronomy11112261
Chicago/Turabian StyleKopecký, Marek, Ladislav Kolář, Radka Váchalová, Petr Konvalina, Jana Batt, Petr Mráz, Ladislav Menšík, Trong Nghia Hoang, and Miroslav Dumbrovský. 2021. "Black Carbon and Its Effect on Carbon Sequestration in Soil" Agronomy 11, no. 11: 2261. https://doi.org/10.3390/agronomy11112261







