Comparative Physiological, Biochemical, and Proteomic Responses of Photooxidation-Prone Rice Mutant 812HS under High Light Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Chlorophyll Content
2.3. Net Photosynthetic Content and Chlorophyll Fluorescence Determination
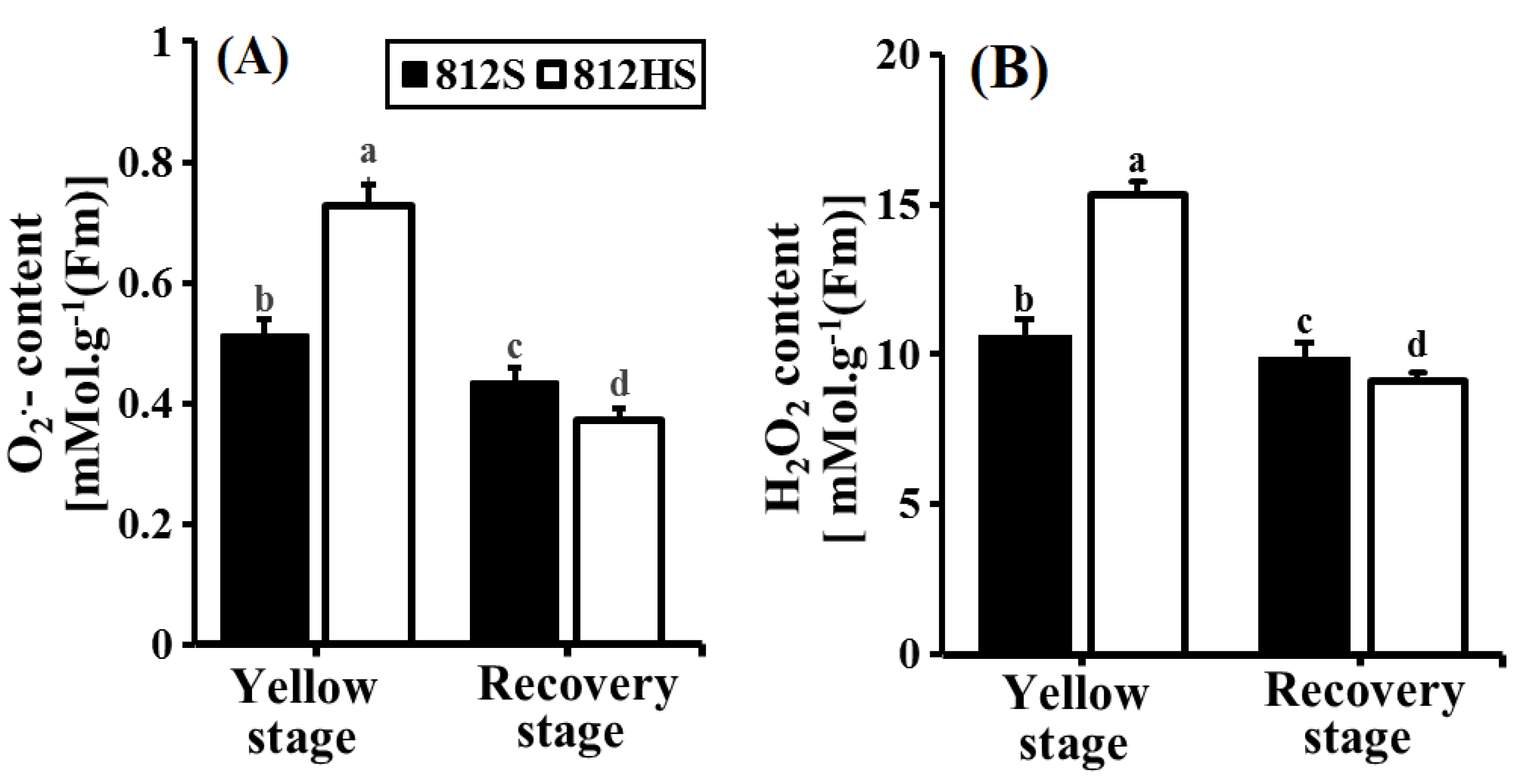
2.4. O2•– and H2O2 Estimation
2.5. Ca2+-ATPase, ATP Content, and Mg2+-ATPase Activities
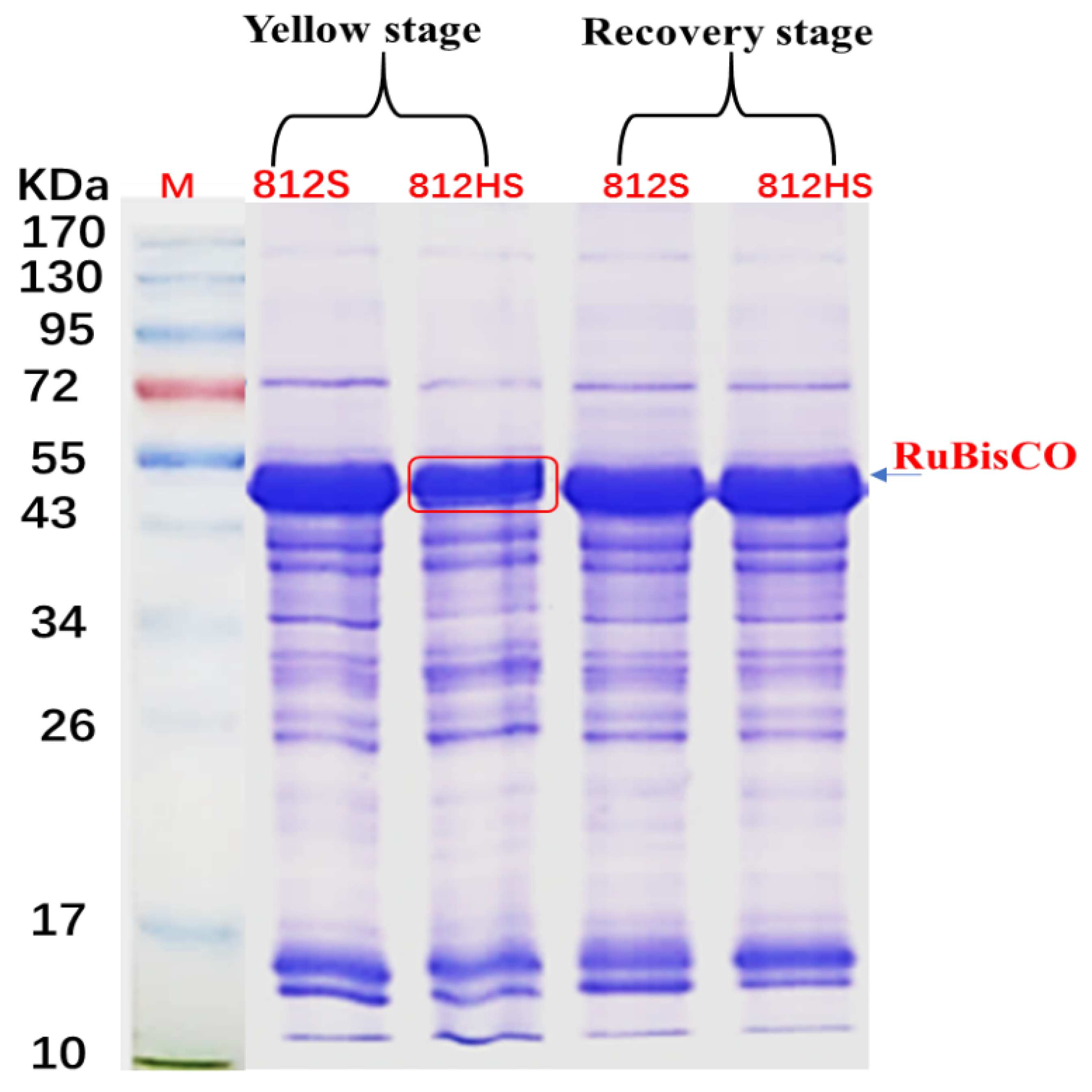
2.6. SDS-PAGE
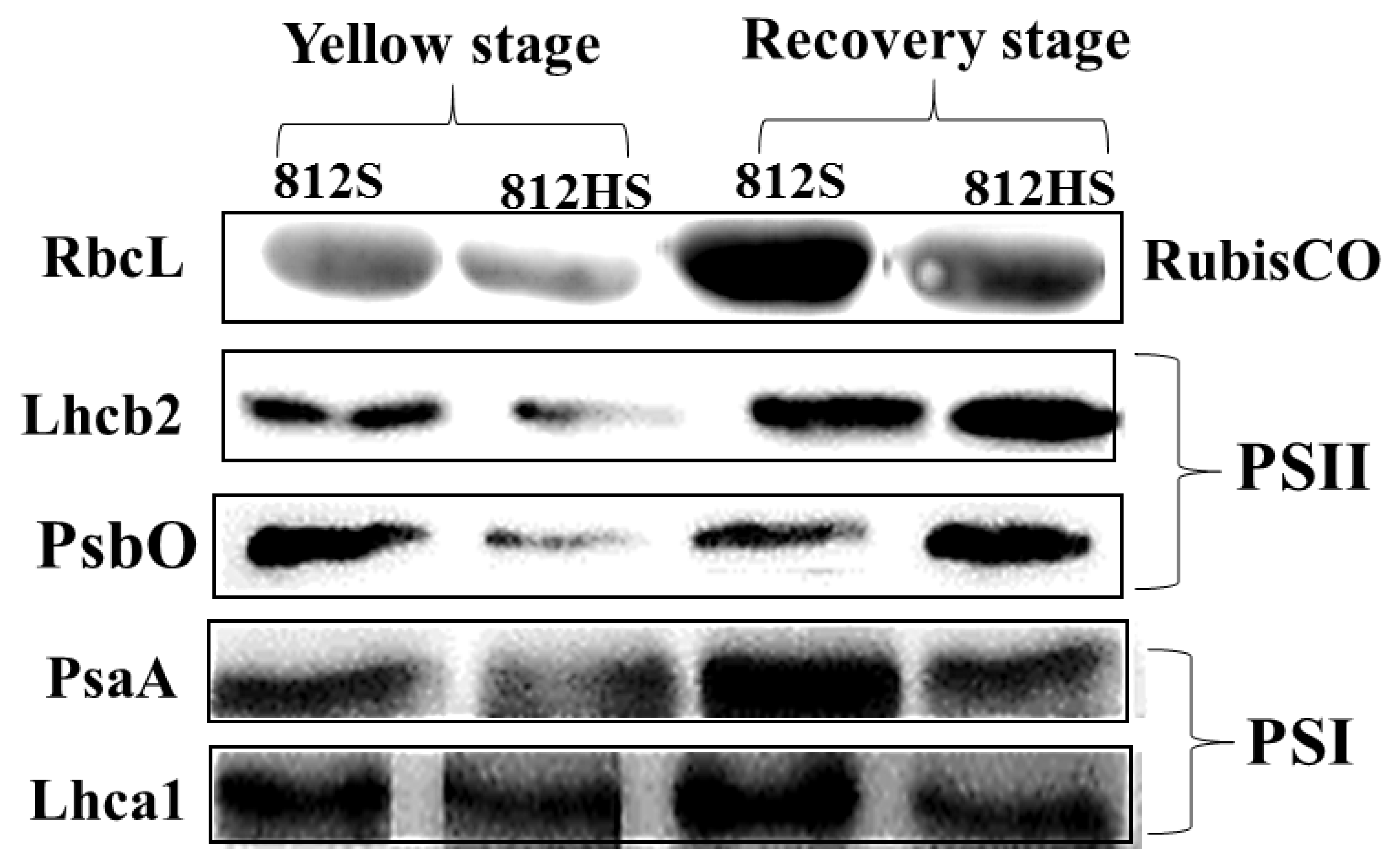
2.7. Western Blot Analysis
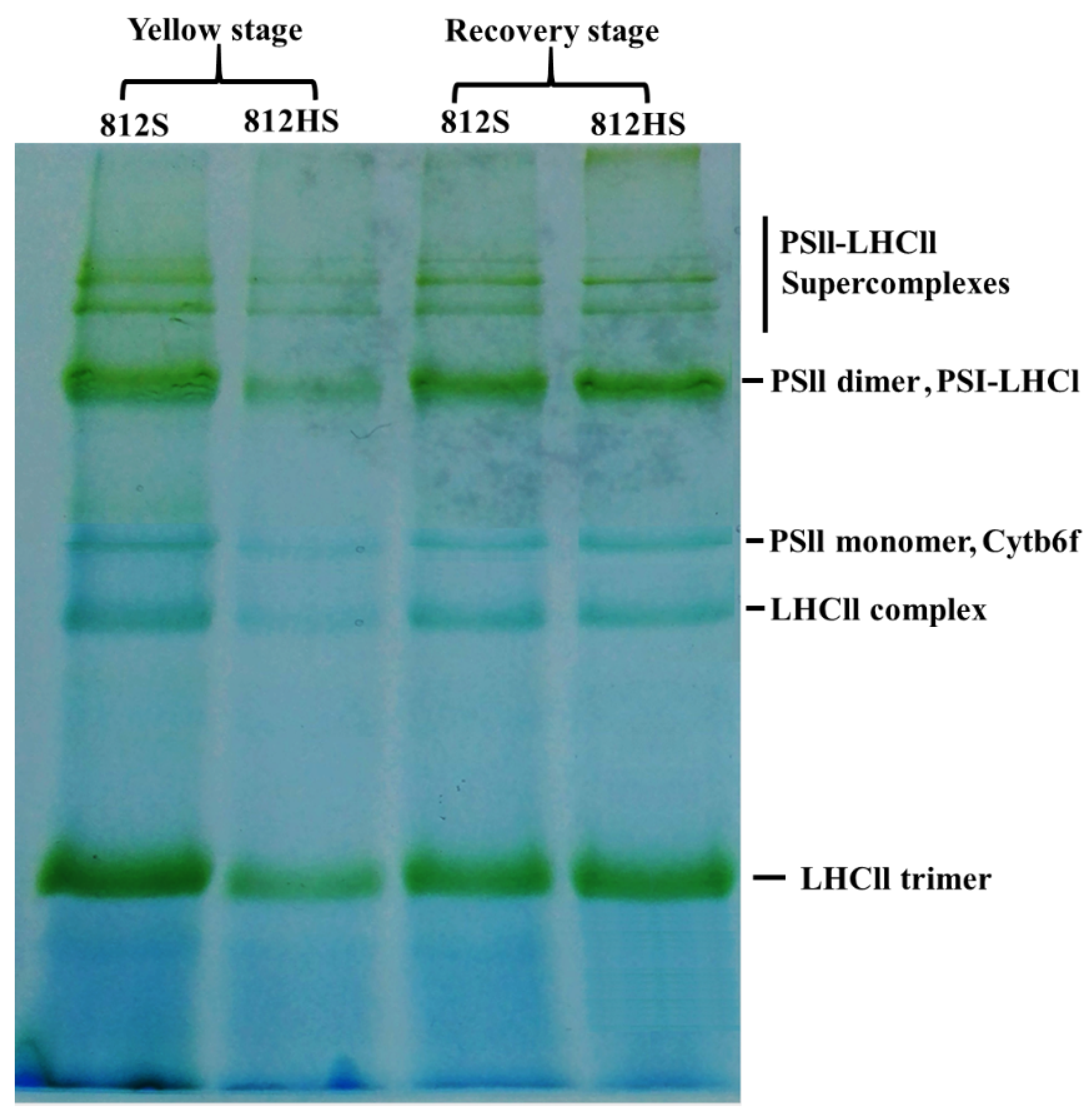
2.8. Blue Native PAGE
3. Results and Discussion
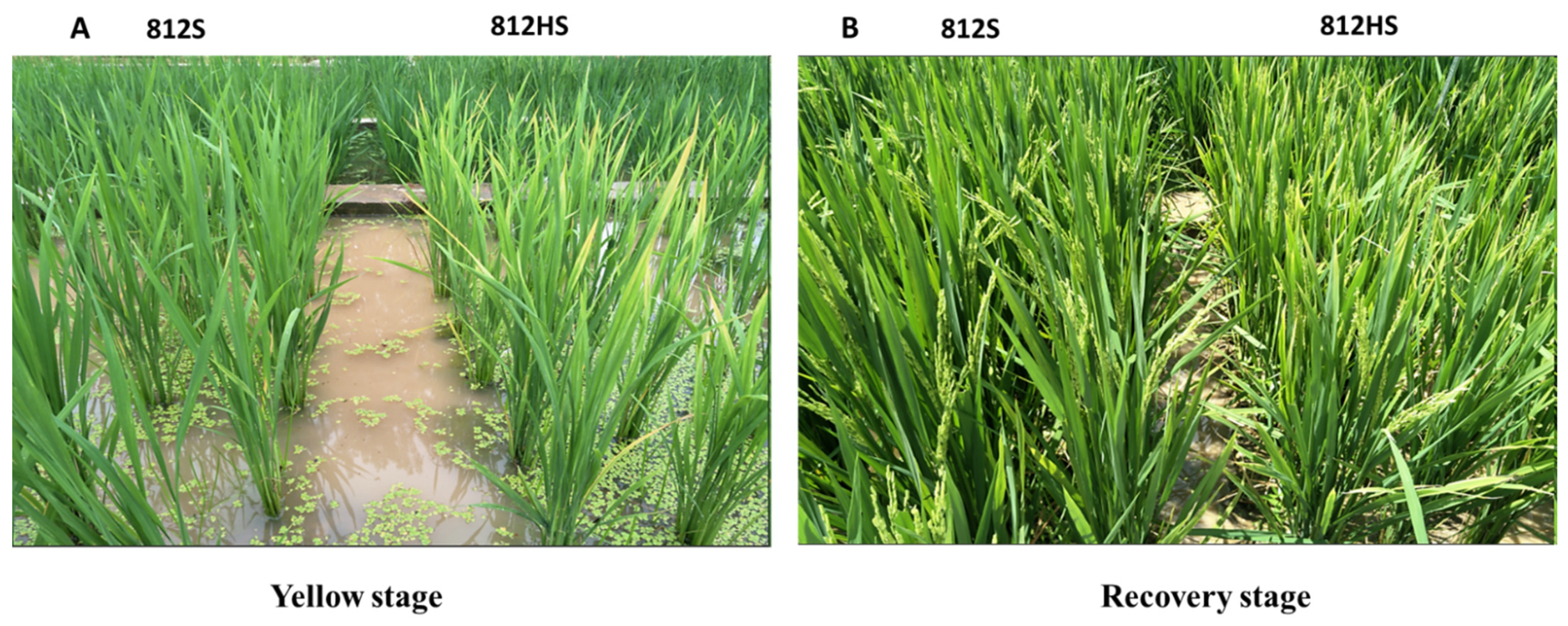
3.1. Assessment Based on Leaf Color
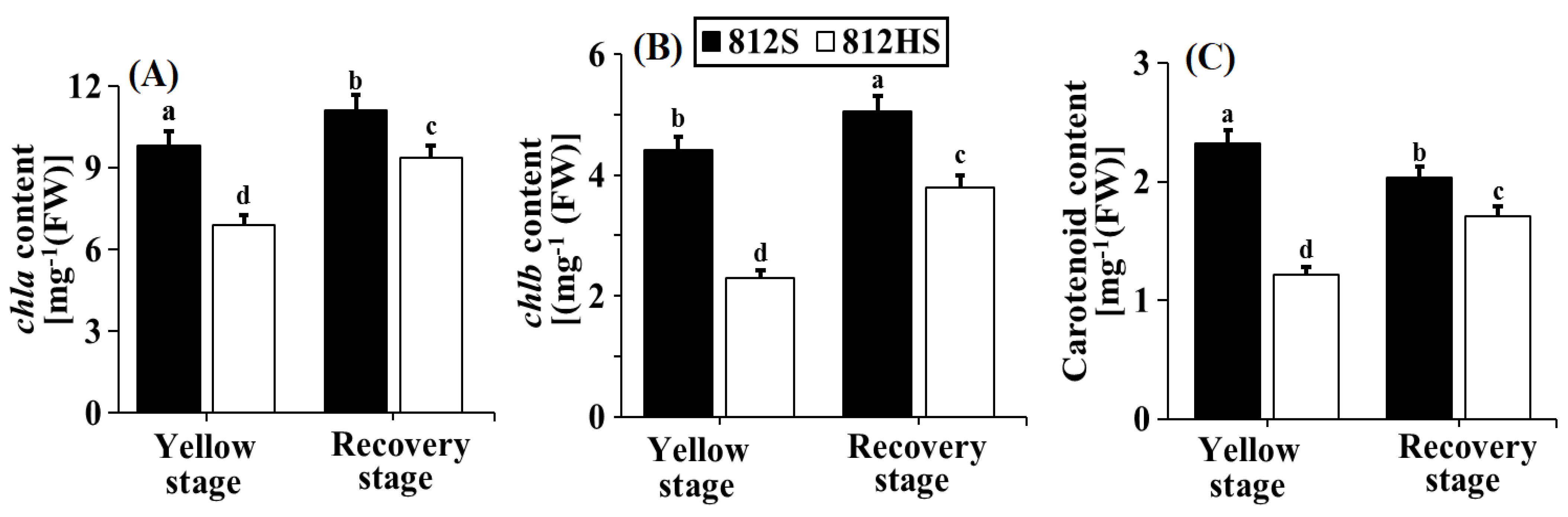
3.2. Pigment Content
3.3. Net Photosynthetic Rate
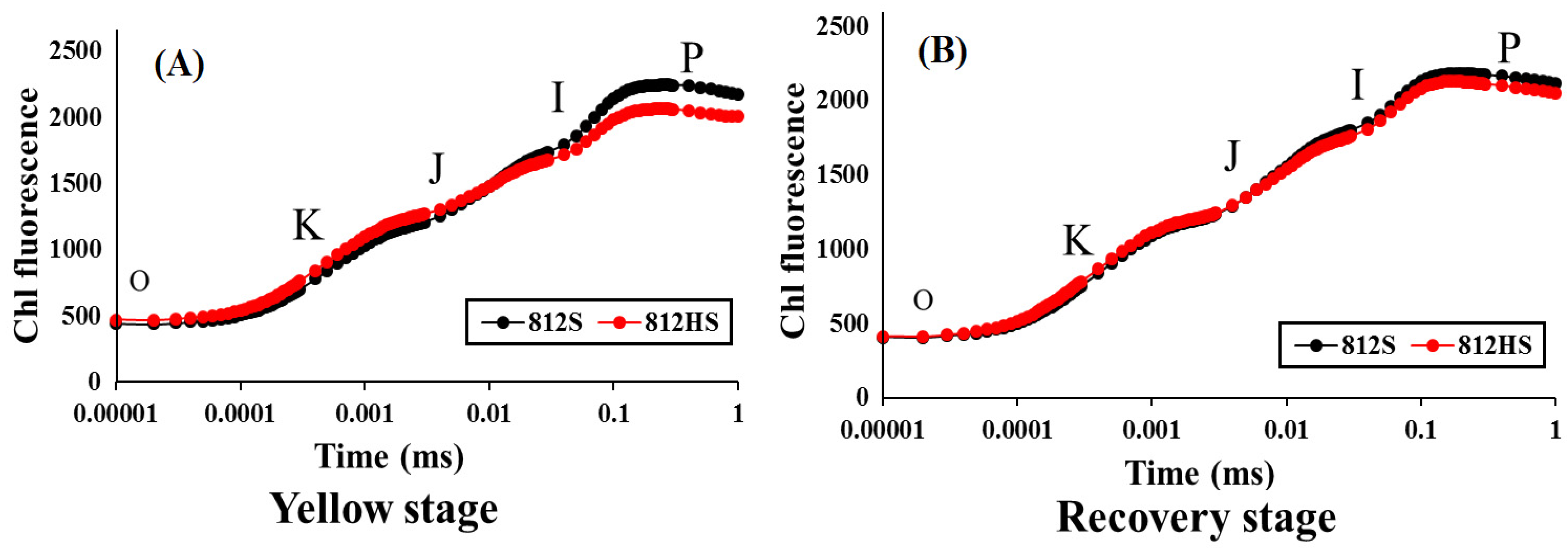
3.4. Induction of Kinetics of Chlorophyll Fluorescence
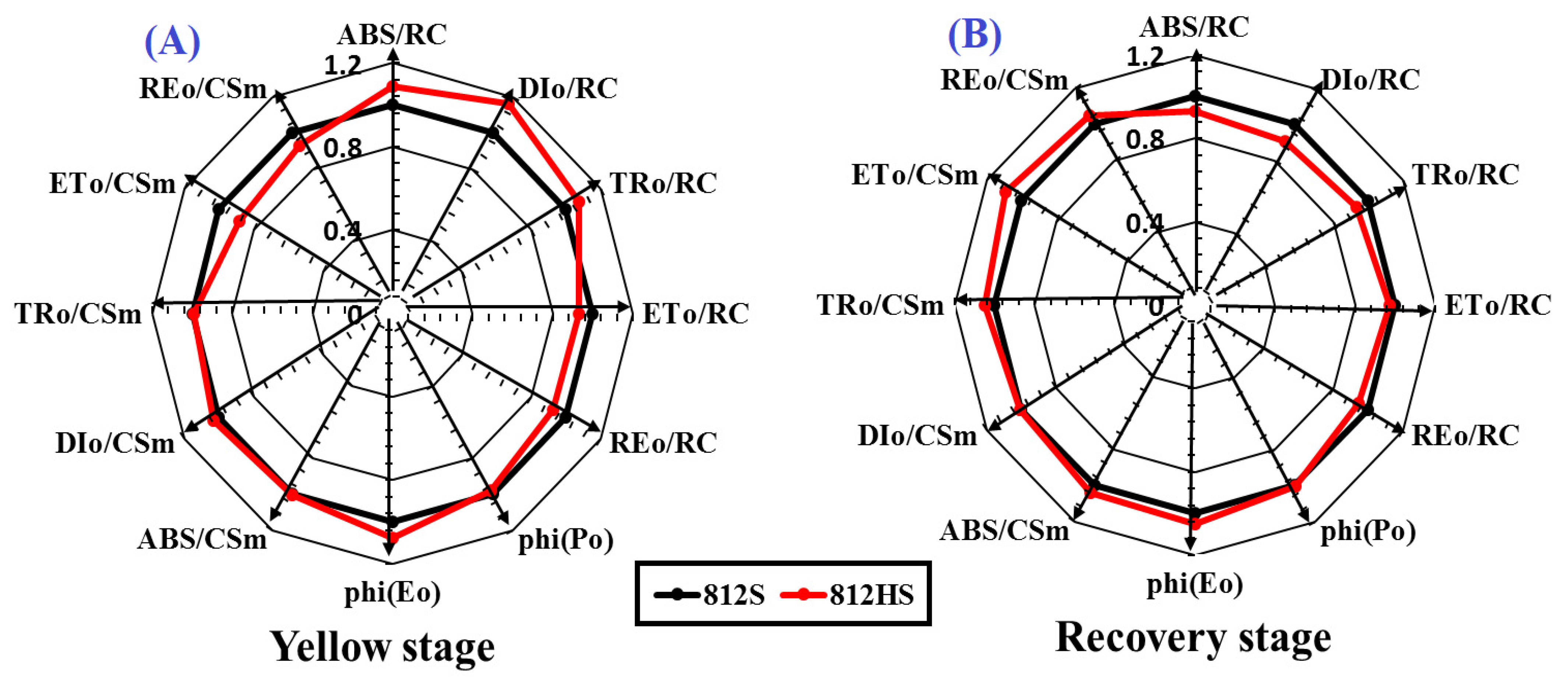
3.5. PSII Efficiency and Excitation Energy Dissipation
3.6. Ca2+-ATPase, ATP Content, and Mg2+-ATPase Activities
3.7. Contents of O2•– and H2O2 in the Leaves
3.8. SDS-PAGE
3.9. Western Blot Analysis
3.10. Blue Native PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Lin, Y.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef]
- Goff, S.A. Rice as a model for cereal genomics. Curr. Opin. Plant Biol. 1999, 2, 86–89. [Google Scholar] [CrossRef]
- Lv, C.; Ma, J.; Yuan, Z.; Wang, Y.; Chen, G.; Gao, Z.; Lv, C. Proteomics analysis of the photo-oxidation mutant 812HS rice (Oryza sativa L.) exposed to high light intensity. Plant Omics 2016, 9, 304–310. [Google Scholar] [CrossRef]
- Rehmani, M.I.A.; Ding, C.; Li, G.; Ata-Ul-Karim, S.T.; Hadifa, A.; Bashir, M.A.; Hashem, M.; Alamri, S.; Al-Zubair, F.; Ding, Y. Vulnerability of rice production to temperature extremes during rice reproductive stage in Yangtze River Valley, China. J. King Saud Univ. Sci. 2021, 33, 101599. [Google Scholar] [CrossRef]
- Rehmani, M.I.A.; Wei, G.; Hussain, N.; Ding, C.; Li, G.; Liu, Z.; Wang, S.; Ding, Y. Yield and quality responses of two indica rice hybrids to post-anthesis asymmetric day and night open-field warming in lower reaches of Yangtze River delta. Field Crops Res. 2014, 156, 231–241. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130225. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Guidi, L.; Tattini, M. Antioxidant Defenses in Plants: A Dated Topic of Current Interest. Antioxidants 2021, 10, 855. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Lv, C.; Zhang, B.; Wang, F.; Shen, W.; Chen, G.; Gao, Z.; Lv, C. Comparative analysis of ultrastructure, antioxidant enzyme activities, and photosynthetic performance in rice mutant 812HS prone to photooxidation. Photosynthetica 2017, 55, 568–578. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Hussain, S.; Nanda, S.; Zhang, J.; Rehmani, M.I.A.; Suleman, M.; Li, G.; Hou, H. Auxin and Cytokinin Interplay during Leaf Morphogenesis and Phyllotaxy. Plants 2021, 10, 1732. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.-C. Effects of different irradiation levels of light quality on Chrysanthemum. Sci. Hortic. 2018, 233, 124–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Lai, D.; Xia, S.; Wei, X.; Liu, S.; Zhang, B.; Liao, H.; Yan, W.; Zong, S.; Zhang, Q. Mapping of a leaf photo-oxidation gene LPO1 (t) in rice. Jiangsu J. Agric. Sci. 2012, 28, 1212–1217. [Google Scholar]
- Alamin, M.; Zeng, D.-D.; Sultana, M.H.; Qin, R.; Jin, X.-L.; Shi, C.-H. Photosynthesis, cellulose contents and ultrastructure changes of mutant rice leading to screw flag leaf. Plant Growth Regul. 2018, 85, 1–13. [Google Scholar] [CrossRef]
- Liao, S.; Li, J.; Yang, H.; Gan, X.; Liang, S. Diurnal changes of net photosynthetic rate and influencing environmental factors of Bauhinia championii. J. Guangxi Norm. Univ. Nat. Sci. Ed. 2010, 28, 61–65. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluo-Rescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Wang, A.-G. Quantitative relation between the reaction of hydroxylamine and superoxi-de anion radicals in plants. Plant Physiol. Commun. 1990, 26, 55–57. [Google Scholar]
- Hossain, M.A.; Fujita, M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol. Mol. Biol. Plants 2010, 16, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketcham, S.R.; Davenport, J.W.; Warncke, K.; McCarty, R.E. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J. Biol. Chem. 1984, 259, 7286–7293. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Lv, C.; Wu, M.; Cai, X.; Liu, Z.; Song, X.; Chen, G.; Lv, C. Chlorophyll a fluorescence analysis of high-yield rice (Oryza sativa L.) LYPJ during leaf senescence. Photosynthetica 2016, 54, 422–429. [Google Scholar] [CrossRef]
- Wang, L.; Gao, M.; Chen, J.; Yang, Z.; Sun, J.; Wang, Z.; Huang, X.; Yuan, T.; Shen, X.; Xian, S. Resveratrol Ameliorates Pressure Overload–induced Cardiac Dysfunction and Attenuates Autophagy in Rats. J. Cardiovasc. Pharmacol. 2015, 66, 376–382. [Google Scholar] [CrossRef]
- Vallejos, R.; Arana, J.; Ravizzini, R. Changes in activity and structure of the chloroplast proton ATPase induced by illumination of spinach leaves. J. Biol. Chem. 1983, 258, 7317–7321. [Google Scholar] [CrossRef]
- Kügler, M.; Jansch, L.; Kruft, V.; Schmitz, U.K.; Braun, H.-P. Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 1997, 53, 35–44. [Google Scholar] [CrossRef]
- Ma, J.; Lv, C.; Xu, M.; Chen, G.; Lv, C.; Gao, Z. Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ. Sci. Pollut. Res. 2016, 23, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarfordoei, A.; Samson, R.; Van Damme, P.J.P. Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 2011, 49, 107. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Eapen, S.; D’Souza, S. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Faiza, S.; Hafiz, N.A.; Zain, M.; Amina, H.; Naqshe, Z.; Rizwan, A.; Muhammad, A.A. Role of endomycorrhizae, rhizobacteria and compost to improve phosphorus availability in onion. Asian J. Agric. Biol. 2020, 8, 194–200. [Google Scholar]
- Song, Y.G.; Liu, B.; Wang, L.F.; Li, M.H.; Liu, Y. Damage to the oxygen-evolving complex by superoxide anion, hydrogen peroxide, and hydroxyl radical in photoinhibition of photosystem II. Photosynth. Res. 2006, 90, 67–78. [Google Scholar] [CrossRef]
- Fang, J.; Chai, C.; Qian, Q.; Li, C.; Tang, J.; Sun, L.; Huang, Z.; Guo, X.; Sun, C.; Liu, M. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J. 2008, 54, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Lv, C.; Xu, M.; Hao, P.; Wang, Y.; Shen, W.; Gao, Z.; Chen, G.; Lv, C. Analysis of chlorophyll a fluorescence and proteomic differences of rice leaves in response to photooxidation. Acta Physiol. Plant. 2017, 39, 46. [Google Scholar] [CrossRef]
- Zeng, S.; Li, M. Changes of Ca2+-ATPase activities in cell of rice seedlings during the enhancement of chilling resistance induced by cold and salt pretreatment. Acta Bot. Sin. 1999, 41, 156–160. [Google Scholar]
- Krishnamurthy, A.; Rathinasabapathi, B.J.P.S. Oxidative stress tolerance in plants: Novel interplay between auxin and reactive oxygen species signaling. Plant Signal. Behav. 2013, 8, e25761. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Hu, J.; Xie, K.; Yang, H.; Du, K.; Shi, G. Accumulation and acute toxicity of silver in Potamogeton crispus L. J. Hazard. Mater. 2010, 173, 186–193. [Google Scholar] [CrossRef]
- Li, X.-G.; Meng, Q.-W.; Jiang, G.-Q.; Zou, Q. The Susceptibility of Cucumber and Sweet Pepper to Chilling Under Low Irradiance is Related to Energy Dissipation and Water-Water Cycle. Photosynthetica 2003, 41, 259–265. [Google Scholar] [CrossRef]
- Peltier, J.-B.; Ytterberg, A.J.; Sun, Q.; van Wijk, K.J. New Functions of the Thylakoid Membrane Proteome of Arabidopsis thaliana Revealed by a Simple, Fast, and Versatile Fractionation Strategy. J. Biol. Chem. 2004, 279, 49367–49383. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almakas, A.; Chen, G.; Wattoo, F.M.; Rana, R.M.; Saleem, M.A.; Gao, Z.; Amjid, M.W.; Rehmani, M.I.A.; Hashem, A.; Abd_Allah, E.F. Comparative Physiological, Biochemical, and Proteomic Responses of Photooxidation-Prone Rice Mutant 812HS under High Light Conditions. Agronomy 2021, 11, 2225. https://doi.org/10.3390/agronomy11112225
Almakas A, Chen G, Wattoo FM, Rana RM, Saleem MA, Gao Z, Amjid MW, Rehmani MIA, Hashem A, Abd_Allah EF. Comparative Physiological, Biochemical, and Proteomic Responses of Photooxidation-Prone Rice Mutant 812HS under High Light Conditions. Agronomy. 2021; 11(11):2225. https://doi.org/10.3390/agronomy11112225
Chicago/Turabian StyleAlmakas, Aisha, Guoxiang Chen, Fahad Masoud Wattoo, Rashid Mehmood Rana, Muhammad Asif Saleem, Zhiping Gao, Muhammad Waqas Amjid, Muhammad Ishaq Asif Rehmani, Abeer Hashem, and Elsayed Fathi Abd_Allah. 2021. "Comparative Physiological, Biochemical, and Proteomic Responses of Photooxidation-Prone Rice Mutant 812HS under High Light Conditions" Agronomy 11, no. 11: 2225. https://doi.org/10.3390/agronomy11112225







