The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. AMF Colonization
2.3. Phospholipid Fatty Acid Analysis
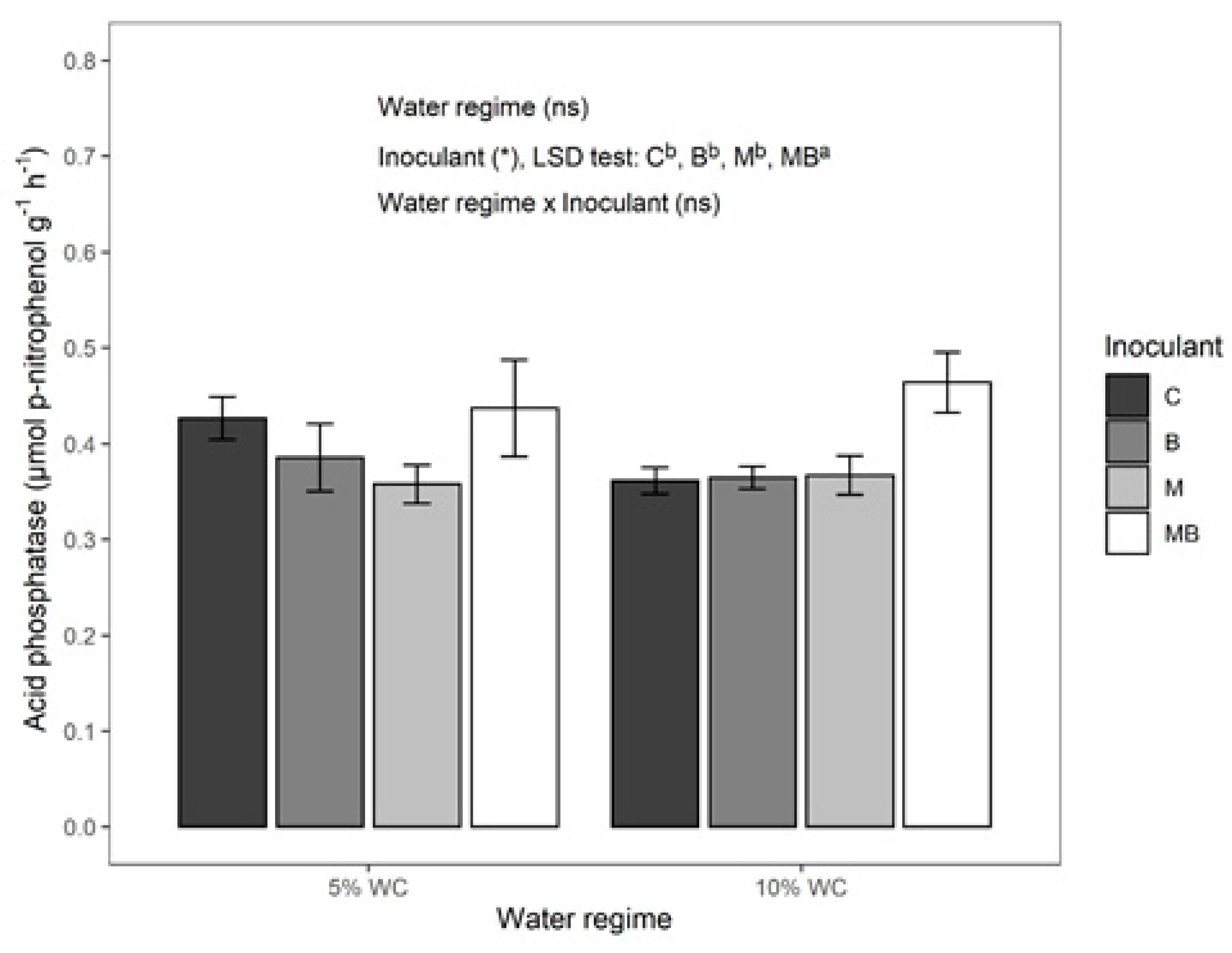
2.4. Enzyme Activity Analysis
2.5. Statistical Analysis
3. Results
3.1. AMF Colonization
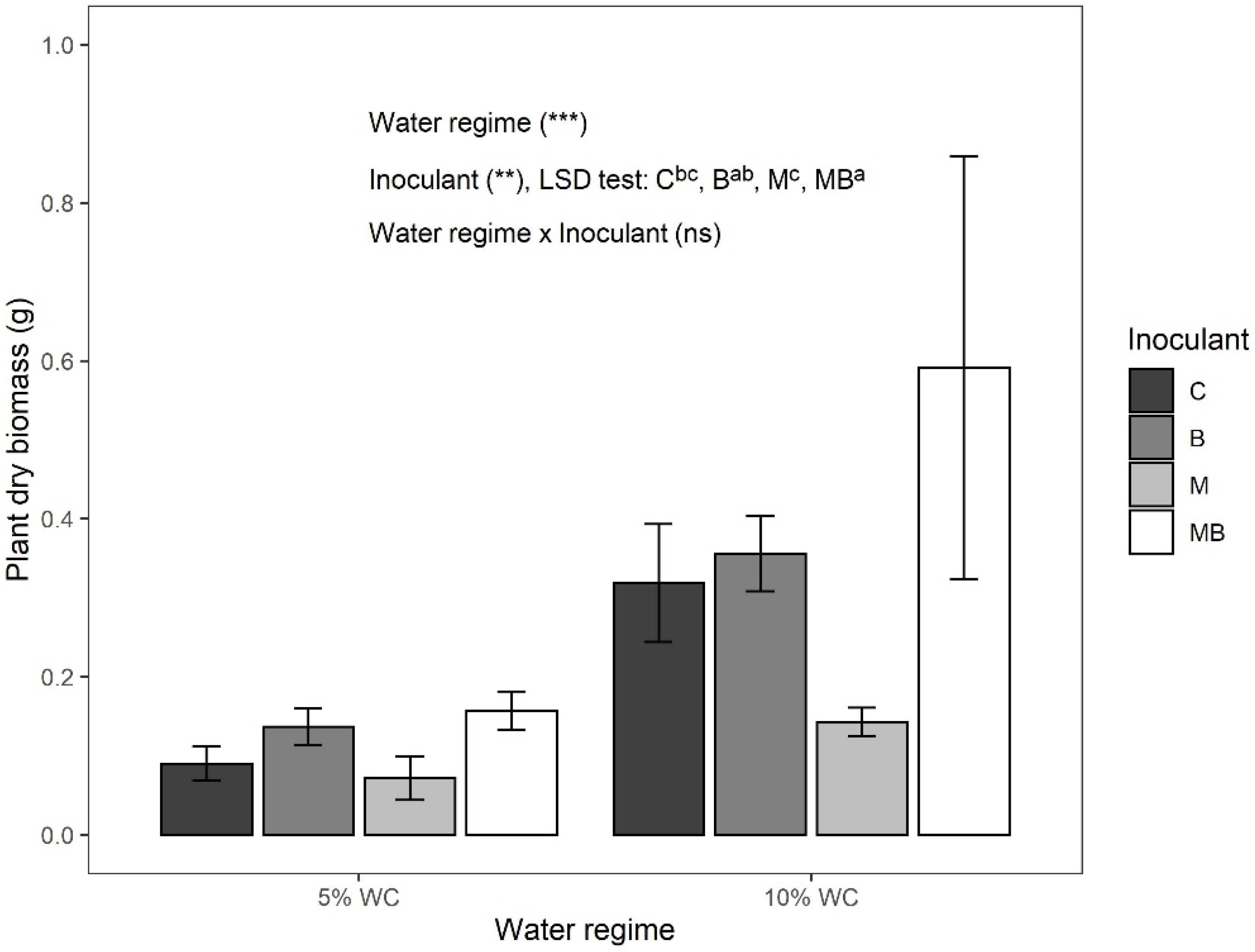
3.2. Plant Growth
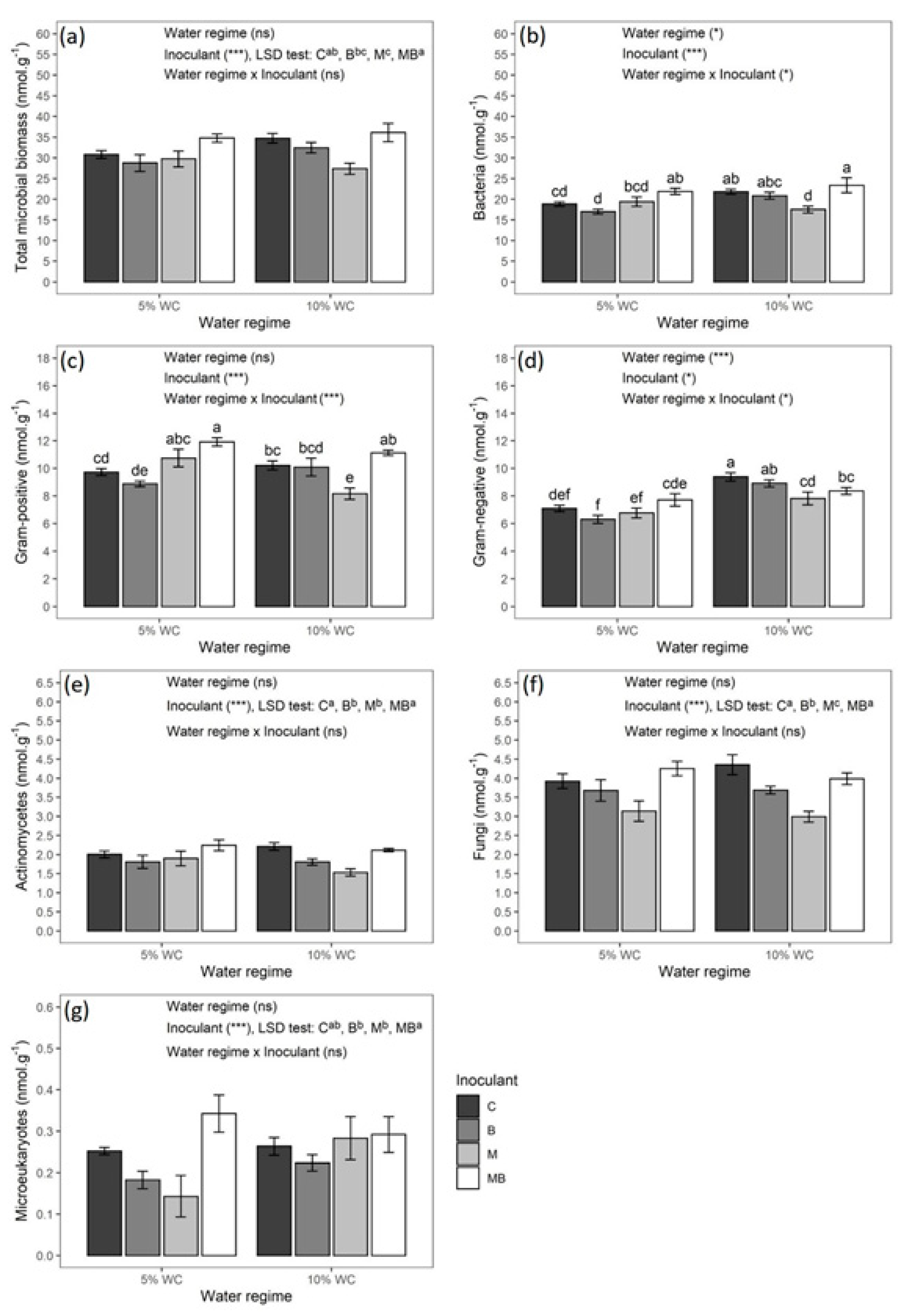
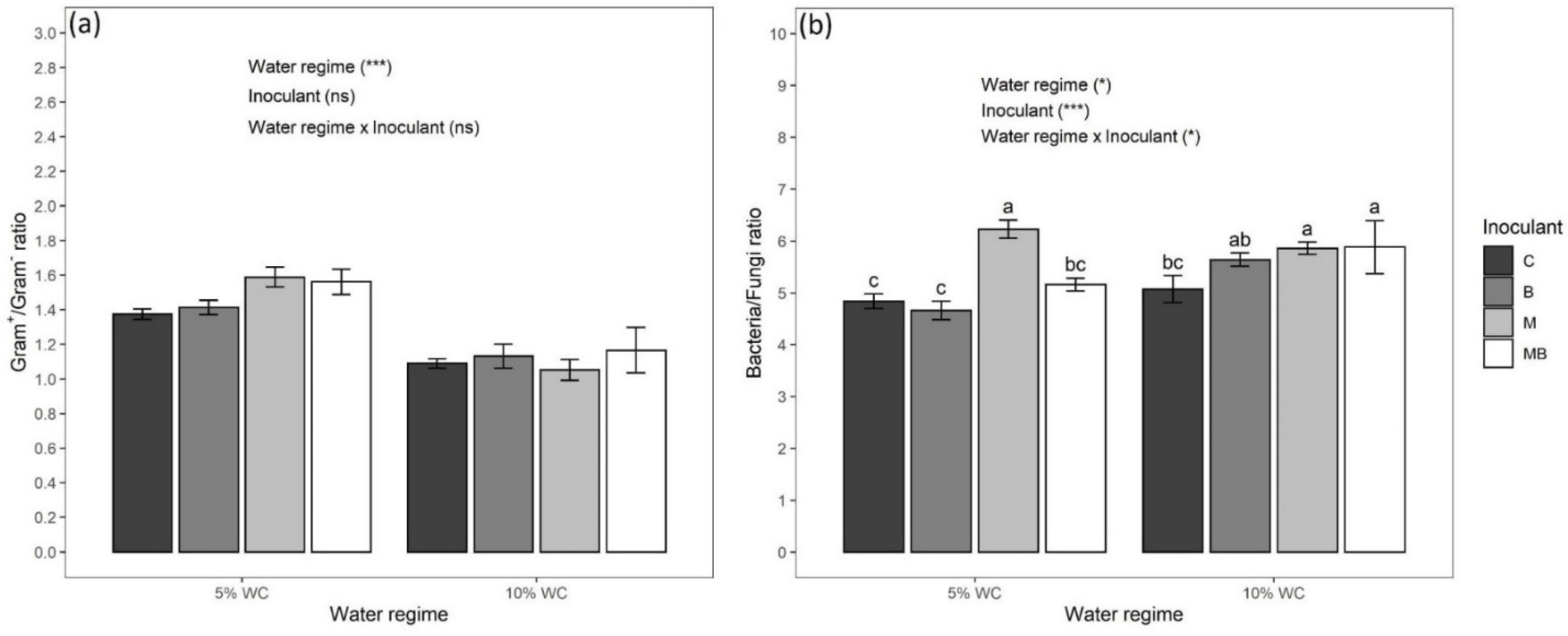
3.3. Biomass and Activity of Soil Microbial Community
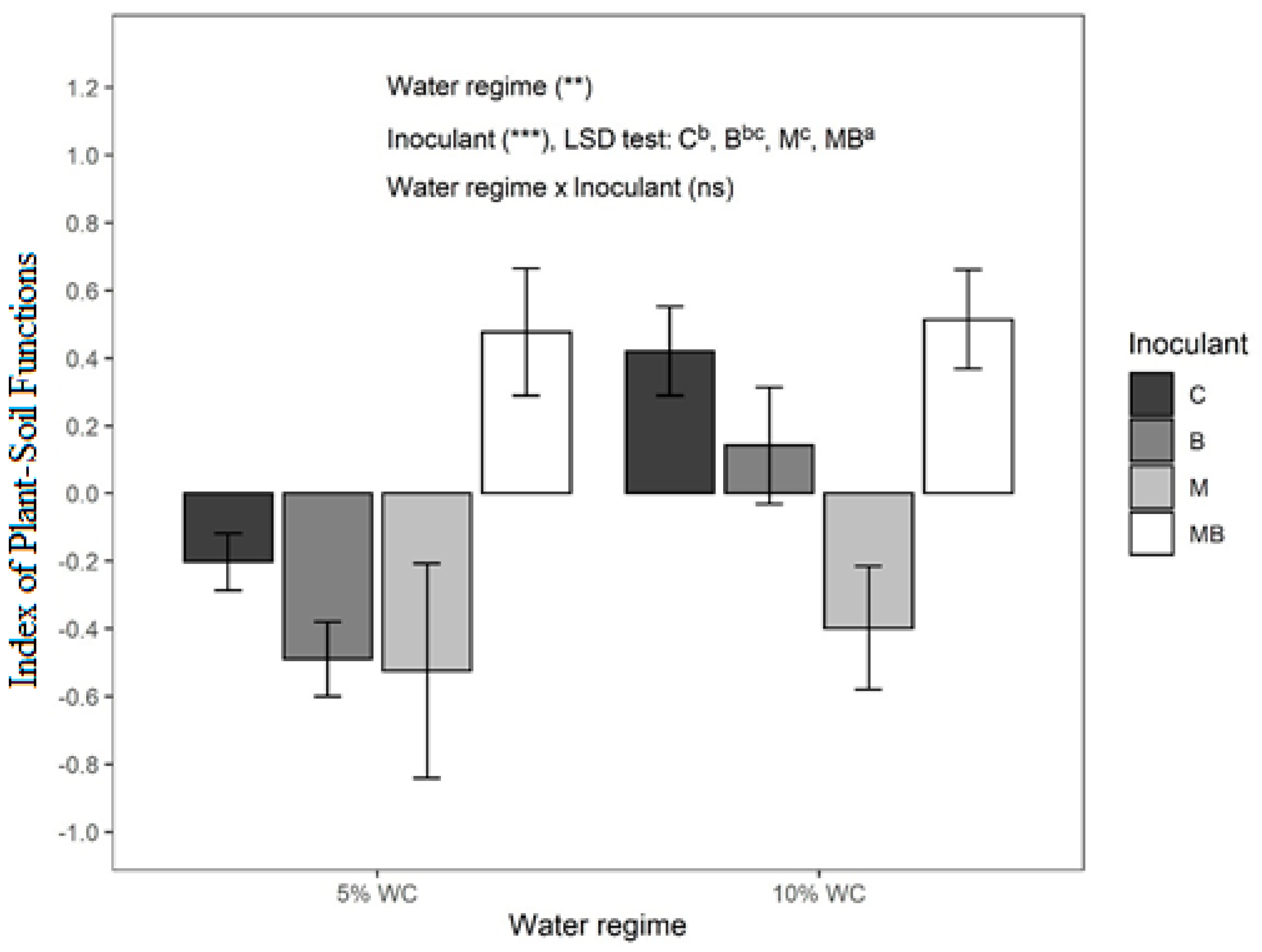
3.4. Index of Plant–Microbial Soil Functions
4. Discussion
Inocula’ Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- Alamanos, A.; Loukas, A.; Mylopoulos, N.; Xenarios, S.; Vasiliades, L.; Latinopoulos, D. Climate change effects on agriculture in southeast Mediterranean: The case of Karla Watershed in Central Greece. Geophys. Res. Abstr. 2019, 21, 1. [Google Scholar]
- Gornall, J.; Betts, R.; Burke, E.; Clark, R.; Camp, J.; Willett, K.; Wiltshire, A. Implications of climate change for agricultural productivity in the early twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2973–2989. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Dodd, I.C.; Belimov, A.A.; Sobeih, W.Y.; Safronova, V.I.; Grierson, D.; Davies, W.J. Will modifying plant ethylene status improve plant productivity in water-limited environments. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; p. 134. [Google Scholar]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of Agrochemicals on Soil Health. In Agrichemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 161–187. ISBN 9780081030172. [Google Scholar]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, I.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Abitha, B.; Henry, A.J.; Sa, T.; Joe, M.M. Interaction of rhizobacteria with arbuscularmycorrhizal fungi (AMF) and their role in stress abetment in agriculture. In Recent Advances on Mycorrhizal Fungi; Springer: Cham, Switzerland, 2016; pp. 117–142. ISBN 978-3-319-24353-5. [Google Scholar]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Gagné-Bourque, F.; Mayer, B.F.; Charron, J.B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated growth rate and increased drought stress resilience of the model grass brachypodium distachyon colonized by bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular Mycorrhizal Fungi and Tolerance of Drought Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 25–41. ISBN 9789811041150. [Google Scholar]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Cano, C.; Bago, A.; Ruiz-Lozano, J.M. Identification of a gene from the arbuscular mycorrhizal fungus Glomus intraradices encoding for a 14-3-3 protein that is up-regulated by drought stress during the AM symbiosis. Microb. Ecol. 2006, 52, 575–582. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, H.; Chen, H.; Wang, J.; Ren, C.; Wu, L. Impact of Bacillus subtilis JA, a biocontrol strain of fungal plant pathogens, on arbuscular mycorrhiza formation in Zea mays. World J. Microbiol. Biotechnol. 2008, 24, 1133–1137. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A.; Palni, L.M.S. Bacterial inoculants for field applications under mountain ecosystem: Present initiatives and future prospects. In Bacteria in Agrobiology: Plant Probiotics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 15–44. ISBN 9783642275159. [Google Scholar]
- Jäderlund, L.; Arthurson, V.; Granhall, U.; Jansson, J.K. Specific interactions between arbuscular mycorrhizal fungi and plant growth-promoting bacteria: As revealed by different combinations. FEMS Microbiol. Lett. 2008, 287, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Cornejo, P.; Barea, J.M. Interactions between the arbuscular mycorrhizal fungus Glomus intraradices and the plant growth promoting rhizobacteria Paenibacillus polymyxa and P. macerans in the mycorrhizosphere of Cucumis sativus. Soil Biol. Biochem. 2009, 41, 286–292. [Google Scholar] [CrossRef]
- Azaizeh, H.A.; Marschner, H.; Römheld, V.; Wittenmayer, L. Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 1995, 5, 321–327. [Google Scholar] [CrossRef]
- Gupta Sood, S. Chemotactic response of plant-growth-promoting bacteria towards roots of vesicular-arbuscular mycorrhizal tomato plants. FEMS Microbiol. Ecol. 2003, 45, 219–227. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldán, A. Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl. Soil Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Ramos, B.; García, J.A.L.; Probanza, A.; Barrientos, M.L.; Gutierrez Mañero, F.J. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis. Environ. Exp. Bot. 2003, 49, 61–68. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant growth promoting Rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008, 54, 876–886. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Malviya, M.K.; Sharma, A.; Pandey, A.; Rinu, K.; Sati, P.; Palni, L.M.S. Bacillus subtilis NRRL B-30408: A potential inoculant for crops grown under rainfed conditions in the mountains. J. Soil Sci. Plant Nutr. 2012, 12, 811–824. [Google Scholar] [CrossRef][Green Version]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Sakineh, A.; Ayme, S.; Akram, S.; Safaie, N. Streptomyces strains modulate dynamics of soil bacterial communities and their efficacy in disease suppression caused by Phytophthora capsici. Sci. Rep. 2021, 11, 9317. [Google Scholar]
- Wang, J.; Xu, S.; Yang, R.; Zhao, W.; Zhu, D.; Zhang, X.; Huang, Z. Bacillus amyloliquefaciens FH-1 significantly affects cucumber seedlings and the rhizosphere bacterial community but not soil. Sci. Rep. 2021, 11, 12055. [Google Scholar] [CrossRef] [PubMed]
- Troelstra, S.R.; Wagenaar, R.; Smant, W.; Peters, B.A.M. Interpretation of bioassays in the study of interactions between soil organisms and plants: Involvement of nutrient factors. New Phytol. 2001, 150, 697–706. [Google Scholar] [CrossRef]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, K.M. Hydrology and Surface Supplies. In Water Supply; Butterworth-Heinemann: Oxford, UK, 2009; pp. 63–107. ISBN 978-0-7506-6843-9. [Google Scholar]
- Carter, M.R.; Gerard, G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Orfanoudakis, M.; Wheeler, C.T.; Hooker, J.E. Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 2010, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae: Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, 1–5 July 1985; Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Institut National de la Recherche Agronomique, INRA: Paris, French, 1986; pp. 217–221. [Google Scholar]
- Available online: http://www2.dijon.inra.fr/mychintec/Mycocalc-prg/download.html (accessed on 20 March 2019).
- Papadopoulou, E.S.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U. Extraction parameters significantly influence and the quantity and the profile of PLFAs extracted from soil. Microb. Ecol. 2011, 62, 704–714. [Google Scholar] [CrossRef]
- Ntalli, N.; Monokrousos, N.; Rumbos, C.; Kontea, D.; Zioga, D.; Argyropoulou, M.D.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N.G. Greenhouse biofumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J. Pest Sci. 2018, 91, 29–40. [Google Scholar] [CrossRef]
- Stamou, G.; Konstadinou, S.; Monokrousos, N.; Mastrogianni, A.; Orfanoudakis, M.; Hassiotis, C.; Menkissoglu-Spiroudi, U.; Vokou, D.; Papatheodorou, E.M. The effects of arbuscular mycorrhizal fungi and essential oil on soil microbial community and N-related enzymes during the fungal early colonization phase. AIMS Microbiol. 2017, 3, 938–959. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microb. Ecol. 2006, 29, 303–310. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Reynolds, H.; Long, T.M. Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biol. Biochem. 2000, 32, 2095–2097. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Hueso, S.; García, C.; Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 2012, 50, 167–173. [Google Scholar] [CrossRef]
- Hartmann, M.; Brunner, I.; Hagedorn, F.; Bardgett, R.D.; Stierli, B.; Herzog, C.; Chen, X.; Zingg, A.; Graf-Pannatier, E.; Rigling, A.; et al. A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol. Ecol. 2017, 26, 1190–1206. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Breulmann, M.; Masyutenko, N.P.; Kogut, B.M.; Schroll, R.; Dorfler, U.; Buscot, F.; Schulz, E. Short-term bioavailability of carbon in soil organic matter fractions of different particle sizes and densities in grassland ecosystems. Sci. Total Environ. 2014, 497–498. [Google Scholar] [CrossRef]
- Citernesi, A.S.; Filippi, C.; Bagnoli, G.; Giovannetti, M. Effects of the antimycotic molecule Iturin A2, secreted by Bacillus subtilis strain M51, on arbuscular mycorrhizal fungi. Microbiol. Res. 1994, 149, 241–246. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Janos, D.P. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza. 2007, 442, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.; Azcón, R.; Barea, J.M. Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability (32P) and nutrient cycling. Appl. Environ. Microbiol. 1997, 63, 4408–4412. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, S.; Ma, L.; Zhou, X.; Luo, S.; Zhang, J.; Lu, B.; Tian, C. The effect of Glomus intraradices on the physiological properties of Panax ginseng and on rhizospheric microbial diversity. J. Ginseng Res. 2019, 43, 77–85. [Google Scholar] [CrossRef]
- Konstantinou, S.; Monokrousos, N.; Kapagianni, P.; Menkissoglu-Spiroudi, U.; Gwynn-Jones, D.; Stamou, G.P.; Papatheodorou, E.M. Instantaneous responses of microbial communities to stress in soils pretreated with Mentha spicata essential oil and/or inoculated with arbuscular mycorrhizal fungus. Ecol. Res. 2019, 34, 701–710. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Mechri, B.; Manga, A.G.B.; Tekaya, M.; Attia, F.; Cheheb, H.; Meriem, F.B.; Braham, M.; Boujnah, D.; Hammami, M. Changes in microbial communities and carbohydrate profiles induced by the mycorrhizal fungus (Glomus intraradices) in rhizosphere of olive trees (Olea europaea L.). Appl. Soil Ecol. 2014, 75, 124–133. [Google Scholar] [CrossRef]
- Kim, Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F.; Casper, B.B. Plant and nutrient controls on microbial functional characteristics in a tropical Oxisol. Plant Soil 2013, 373, 893–905. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
| Soil Physicochemical Properties | |
|---|---|
| pH | 5.72 |
| Electrical conductivity (mS/cm) | 0.35 |
| Organic C (%) | 1.26 |
| NO3-N (mg/kg) | 15.68 |
| P (ppm) | 20 |
| K (ppm) | 125 |
| Ca (ppm) | 577 |
| Mg (ppm) | 100 |
| PLFA Biomarkers | |
|---|---|
| i15:0, a15:0, 15:0, i16:0, i17:0, 17:0 | Gram-positive bacteria |
| 16:1ω9cis, 16:1ω9trans, cy17:0 | Gram-negative bacteria |
| 10Me16:0, 10Me17:0, 10Me18:0 | Actinomycetes |
| 20:0, 22:0, 24:0 | Microeukaryotes |
| 18:2ω9,12 | Fungi |
| 18:1ω9t, 18:1ω9c | Gram-negative bacteria and fungi |
| 16:0 | Bacteria and fungi |
| 11:0, 13:0, 14:0, 18:0 | Microbial origin |
| 16:1ω5 | AM fungal mycelium |
| % AMF Colonization Rate | ||
|---|---|---|
| Inoculant | 5% WC | 10% WC |
| R. irregularis | 31.67 ± 1.67 b | 58.80 ± 1.39 a |
| R. irregularis + B. subtilis | 16.40 ± 0.93 d | 22.00 ± 1.38 c |
| Microbial Groups | Water Regime (1 df) | Inoculant Type (3 df) | Water Regime × Inoculant Type (3 df) | |||
|---|---|---|---|---|---|---|
| F Value | p-Value | F Value | p Value | F Value | p Value | |
| Bacteria | 6.22 | 0.018 | 8.18 | 0.000 | 3.79 | 0.020 |
| Gram+ | 2.09 | 0.158 | 10.64 | 0.000 | 7.90 | 0.000 |
| Gram− | 46.33 | 0.000 | 3.14 | 0.039 | 3.69 | 0.022 |
| Gram+/Gram− | 66.56 | 0.000 | 1.63 | 0.202 | 1.72 | 0.183 |
| Bacteria/Fungi | 6.35 | 0.017 | 9.65 | 0.000 | 3.48 | 0.027 |
| Total microbial biomass | 2.60 | 0.116 | 8.03 | 0.000 | 2.03 | 0.129 |
| Fungi | 0.00 | 0.958 | 12.03 | 0.000 | 1.21 | 0.322 |
| Actinomycetes | 0.74 | 0.395 | 7.19 | 0.000 | 2.14 | 0.115 |
| Microeukaryotes | 2.05 | 0.162 | 4.21 | 0.013 | 2.46 | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidou, C.; Monokrousos, N.; Kapagianni, P.D.; Orfanoudakis, M.; Dermitzoglou, T.; Papatheodorou, E.M. The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa. Agronomy 2021, 11, 2183. https://doi.org/10.3390/agronomy11112183
Nikolaidou C, Monokrousos N, Kapagianni PD, Orfanoudakis M, Dermitzoglou T, Papatheodorou EM. The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa. Agronomy. 2021; 11(11):2183. https://doi.org/10.3390/agronomy11112183
Chicago/Turabian StyleNikolaidou, Charitini, Nikolaos Monokrousos, Pantelitsa D. Kapagianni, Michael Orfanoudakis, Triantafyllia Dermitzoglou, and Efimia M. Papatheodorou. 2021. "The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa" Agronomy 11, no. 11: 2183. https://doi.org/10.3390/agronomy11112183
APA StyleNikolaidou, C., Monokrousos, N., Kapagianni, P. D., Orfanoudakis, M., Dermitzoglou, T., & Papatheodorou, E. M. (2021). The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa. Agronomy, 11(11), 2183. https://doi.org/10.3390/agronomy11112183









