Responses of Soil Cadmium Desorption under Different Saline Environments and Its Controlling Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Testing
2.2. Batch Desorption Tests
2.3. Statistical Analyses
3. Results
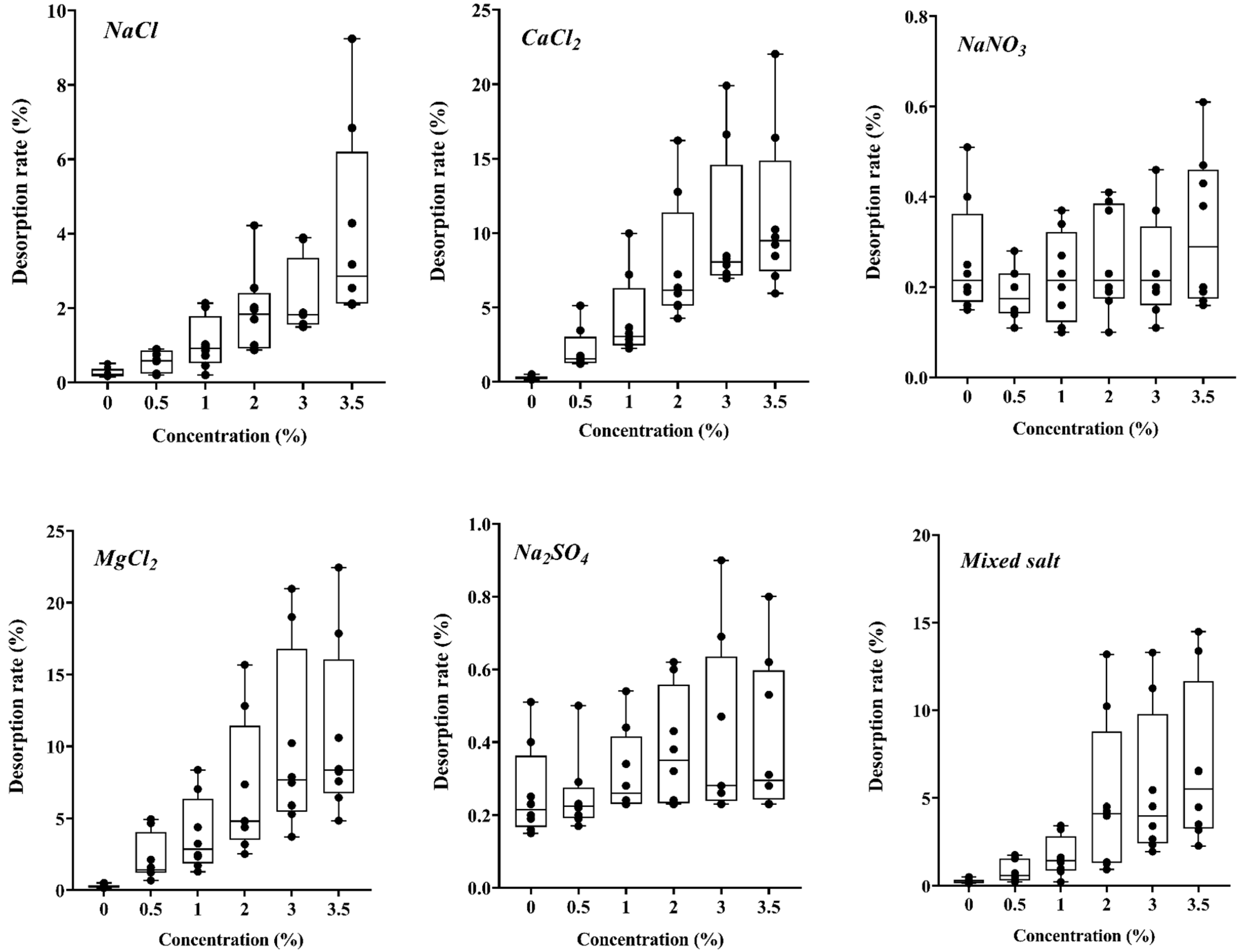
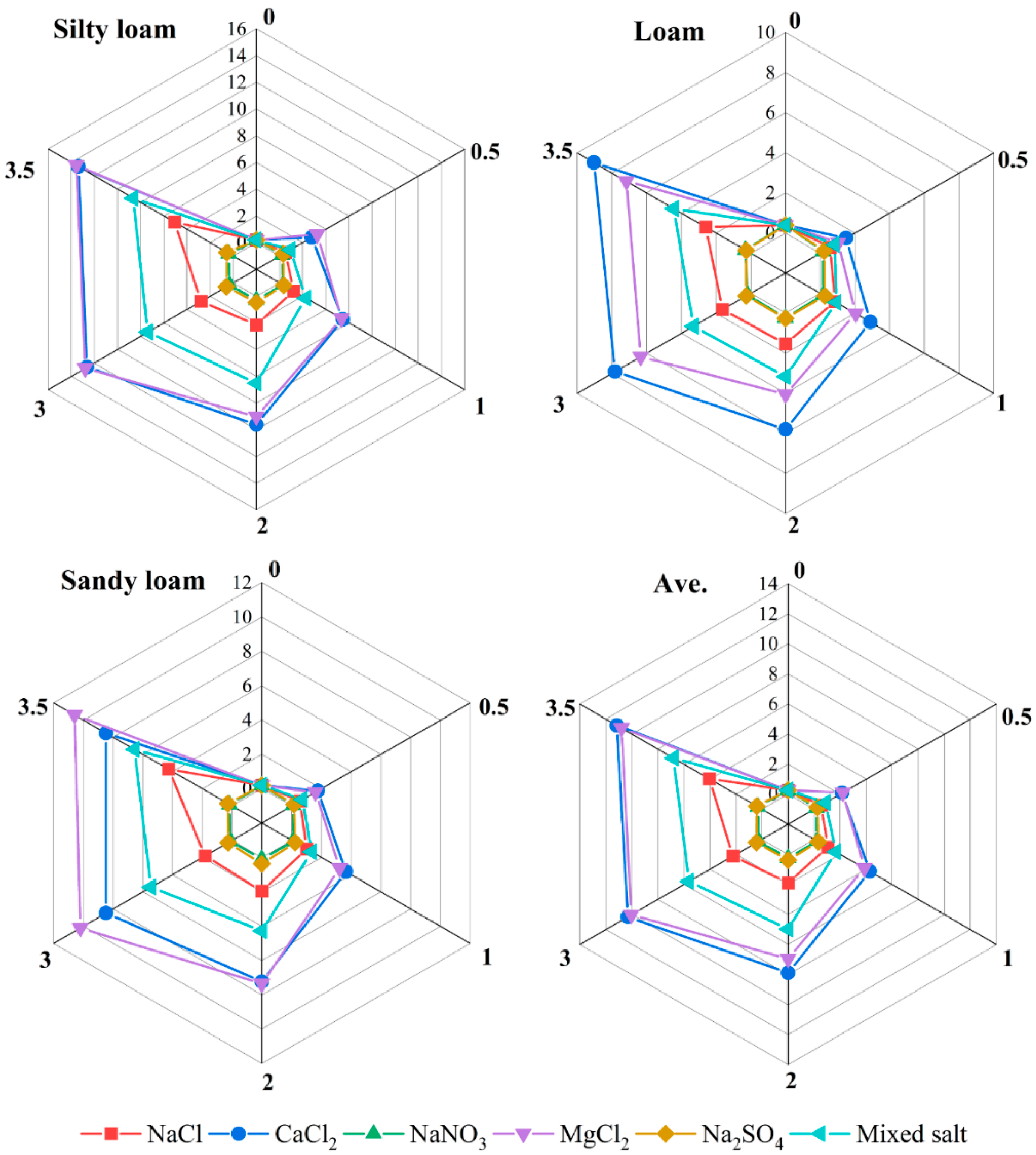
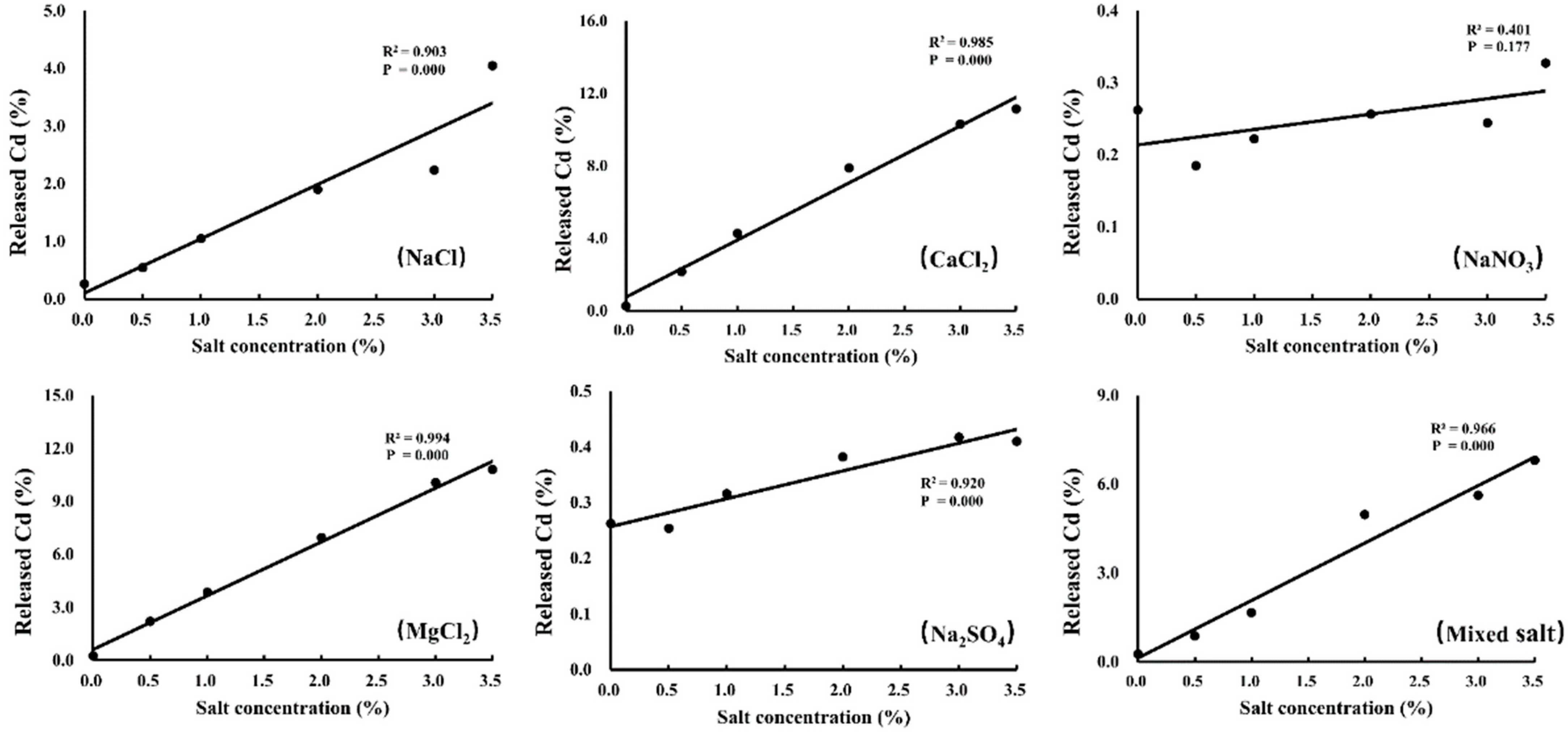
3.1. Effects of Different Salts on Cd Release
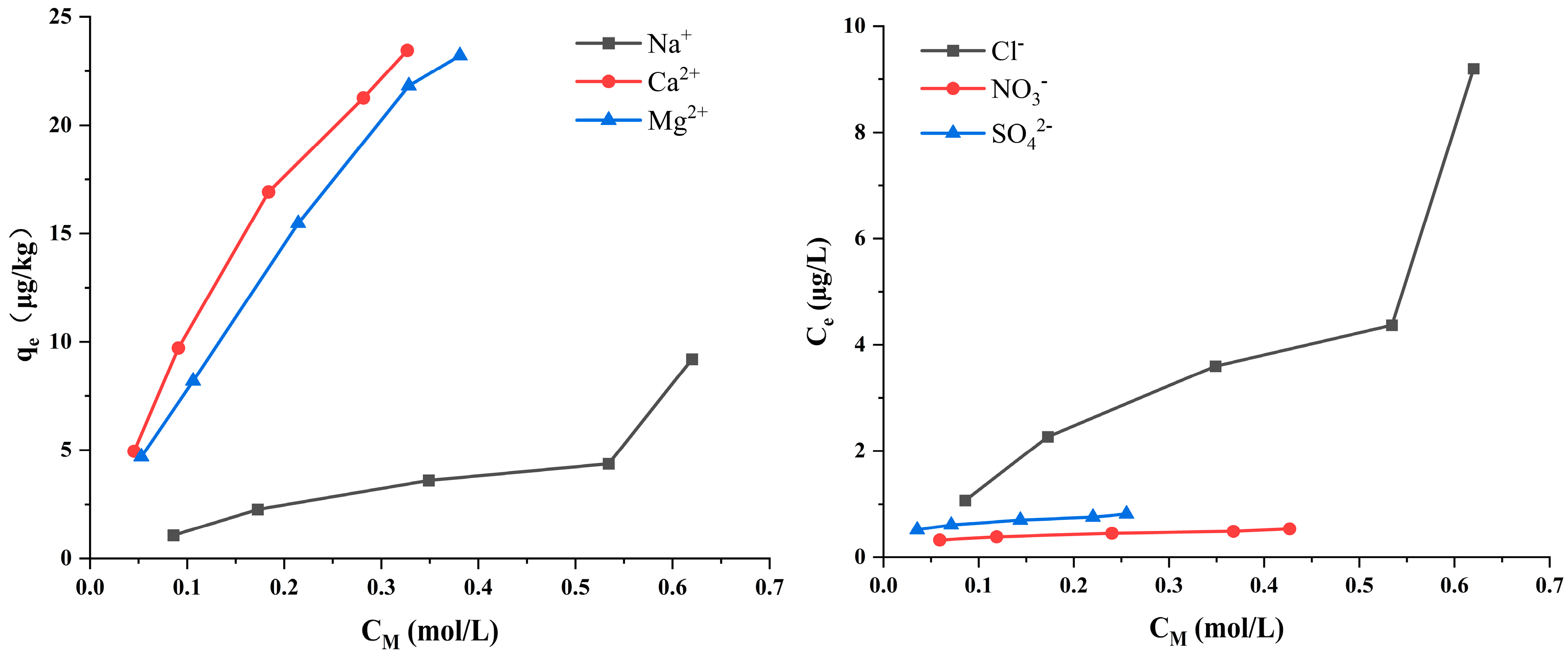
3.2. Effects of Cations and Anions on Cd Release
3.3. Simulation of the Sorption Parameters under Salt Stress
3.4. Relationships between Soil Properties and Cd Release
4. Discussion
4.1. Effects of Different Degrees of Salt Stress on Cd Desorption
4.2. Roles of Soil Properties in the Desorption Process
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chabchoubi, I.B.; Mtibaa, S.; Ksibi, M.; Hentati, O. Health risk assessment of heavy metals (Cu, Zn, and Mn) in wild oat grown in soils amended with sediment dredged from the Joumine Dam in Bizerte, Tunisia. Euro-Mediterr. J. Environ. Integr. 2020, 5, 3. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Gupt, C.B.; Kushwaha, A.; Prakash, A.; Chandra, A.; Goswami, L.; Sekharan, S. Mitigation of Groundwater Pollution: Heavy Metal Retention Characteristics of Fly Ash Based Liner Materials; Springer: Singapore, 2020; pp. 79–104. [Google Scholar]
- Dong, B.; Zhang, R.; Gan, Y.; Cai, L.; Freidenreich, A.; Wang, K.; Guo, T.; Wang, H. Multiple methods for the identification of heavy metal sources in cropland soils from a resource-based region. Sci. Total Environ. 2019, 651, 3127–3138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kalkhajeh, Y.K.; Qin, Z.; Jiao, W. Spatial distribution and assessment of the human health risks of heavy metals in a retired petrochemical industrial area, south China. Environ. Res. 2020, 188, 109661. [Google Scholar] [CrossRef]
- Yadav, A.P.S.; Dwivedi, V.; Kumar, S.; Kushwaha, A.; Goswami, L.; Reddy, B.S. Cyanobacterial extracellular polymeric substances for heavy metal removal: A mini review. J. Compos. Sci. 2021, 5, 1. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, E.; Zhang, F.; Liu, R.; Liu, X.; Zhao, Y.; Liang, X. Spatiotemporal dynamics of labile Cd in soil during rice growth. Sci. Total Environ. 2020, 738, 139832. [Google Scholar] [CrossRef]
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef]
- Liu, X.; Tian, G.; Jiang, D.; Zhang, C.; Kong, L. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. 2016, 23, 17941–17952. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.; Lu, X.; Yang, J.; Zhang, Z.; Zhu, Q. Uptake and distribution of cadmium in different rice cultivars. J. Agro-Environ. Sci. 2003, 22, 529–532. [Google Scholar]
- Yang, Y.; Chang, A.C.; Wang, M.; Chen, W.; Peng, C. Assessing cadmium exposure risks of vegetables with plant uptake factor and soil property. Environ. Pollut. 2018, 238, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Haddioui, A. Human and animal health risk assessment of metal contamination in soil and plants from Ait Ammar abandoned iron mine, Morocco. Environ. Monit. Assess. 2015, 188, 1–12. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Wei, J.; Gong, X.; Yu, X.; Guo, H.; Zhao, Y. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zang, F.; Wang, S.; Nan, Z.; Ma, J.; Zhang, Q.; Chen, Y.; Li, Y. Accumulation, spatio-temporal distribution, and risk assessment of heavy metals in the soil-corn system around a polymetallic mining area from the Loess Plateau, northwest China. Geoderma 2017, 305, 188–196. [Google Scholar] [CrossRef]
- Xia, H.; Kong, W.; Liu, L.; Lin, K.; Li, H. Effects of harvest time and desalination of feedstock on Spartina alterniflora biochar and its efficiency for Cd2+ removal from aqueous solution. Ecotoxicol. Environ. Saf. 2021, 207, 111309. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhong, B.H.; Fang, W.M. Adsorption characterization of lead(II) and cadmium(II) on crosslinked carboxymethyl starch. J. Appl. Polym. Sci. 2012, 124, 5010–5020. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Proc. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Krčmar, D.; Tenodi, S.; Grba, N.; Kerkez, D.; Watson, M.; Rončević, S.; Dalmacija, B. Preremedial assessment of the municipal landfill pollution impact on soil and shallow groundwater in Subotica, Serbia. Sci. Total Environ. 2018, 615, 1341–1354. [Google Scholar] [CrossRef]
- Ye, W.M.; He, Y.; Chen, Y.G.; Chen, B.; Cui, Y.J. Adsorption, desorption and competitive adsorption of heavy metal ions from aqueous solution onto GMZ01 bentonite. In Engineering Geology for Society and Territory; Spinger: Cham, Switzerland, 2015; pp. 533–536. [Google Scholar]
- Chen, H.; Tang, L.; Wang, Z.; Su, M.; Tian, D.; Zhang, L.; Li, Z. Evaluating the protection of bacteria from extreme Cd (II) stress by P-enriched biochar. Environ. Pollut. 2020, 263, 114483A. [Google Scholar] [CrossRef]
- Yue, W.; Yang, J.; Tong, J.; Gao, H. Transfer and balance of water and salt in irrigation district of arid region. J. Hydraul. Eng. 2008, 39, 623–626. [Google Scholar]
- Kozyatnyk, I.; Lovgren, L.; Haglund, P. On the leaching of mercury by brackish seawater from permeable barriers materials and soil. J. Environ. Chem. Eng. 2015, 3, 1200–1206. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Xia, P.; Wu, S.; Lin, T.; Yi, Y. Contamination features and ecological risks of heavy metals in the farmland along shoreline of Caohai plateau wetland, China. Chemosphere 2020, 254, 126828. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Henriques, B.; Pinto, J.; Fabre, E.; Viana, T.; Ferreira, N.; Amaral, J.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. Influence of salinity and rare earth elements on simultaneous removal of Cd, Cr, Cu, Hg, Ni and Pb from contaminated waters by living macroalgae. Environ. Pollut. 2020, 266, 1153741. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Trakal, L.; Kodešová, R.; Komárek, M. Modelling of Cd, Cu, Pb and Zn transport in metal contaminated soil and their uptake by willow (Salix × smithiana) using HYDRUS-2D program. Plant Soil 2012, 366, 433–451. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, C.; Li, Y.; Li, S.; Liang, J. Characterizing the release of cadmium from 13 purple soils by batch leaching tests. Chemosphere 2013, 91, 1502–1507. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nghiem, L.D.; Hai, F.I.; Vigneswaran, S.; Nguyen, T.V. Competitive adsorption of metals on cabbage waste from multi-metal solutions. Bioresour. Technol. 2014, 160, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M. Sorption and lability of cadmium and lead in different soils from Egypt and Greece. Geoderma 2009, 153, 61–68. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Wang, D.; Liu, Y.; Shen, Z. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: Relative role of sediments’ properties and metal speciation. Chemosphere 2013, 91, 977–984. [Google Scholar] [CrossRef]
- Kolodynska, D.; Wnetrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Coelho, G.F.; GonCalves, A.C., Jr.; Novoa-Munoz, J.C.; Fernandez-Calvino, D.; Arias-Estevez, M.; Fernandez-Sanjurjo, M.J.; Alvarez-Rodriguez, E.; Nunez-Delgado, A. Competitive and non-competitive cadmium, copper and lead sorption/desorption on wheat straw affecting sustainability in vineyards. J. Clean. Prod. 2016, 139, 1496–1503. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Chen, L.; Wang, D. Saline stress modifies the effect of cadmium toxicity on soil archaeal communities. Ecotoxicol. Environ. Saf. 2019, 182, 109431. [Google Scholar] [CrossRef]
- Raiesi, F.; Razmkhah, M.; Kiani, S. Salinity stress accelerates the effect of cadmium toxicity on soil N dynamics and cycling: Does joint effect of these stresses matter? Ecotoxicol. Environ. Saf. 2018, 153, 160–167. [Google Scholar] [CrossRef]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J. Hazard. Mater. 2007, 143, 419–430. [Google Scholar] [CrossRef]
- Behbahani, A.; Ryan, R.J.; McKenzie, E.R. Impacts of salinity on the dynamics of fine particles and their associated metals during stormwater management. Sci. Total Environ. 2021, 777, 146135. [Google Scholar] [CrossRef]
- Fernandez-Calvino, D.; Cutillas-Barreiro, L.; Paradelo-Nunez, R.; Novoa-Munoz, J.C.; Fernandez-Sanjurjo, M.J.; Alvarez-Rodriguez, E.; Nunez-Delgado, A.; Arias-Estevez, M. Heavy metals fractionation and desorption in pine bark amended mine soils. J. Environ. Manag. 2017, 192, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, L.; Gao, W.; Zeng, X.; Wang, Z. Impact of inorganic anions on the cadmium effective fraction in soil and its phytoavailability during salinization in alkaline soils. Acta Ecol. Sinica 2012, 32, 7512–7518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sale, P.W.G.; Tang, C. Cadmium uptake by Carpobrotus rossii (Haw.) Schwantes under different saline conditions. Environ. Sci. Pollut. Res. 2016, 23, 13480–13488. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Romic, D.; Savic, R. Salinity decreases dissolved organic carbon in the rhizosphere and increases trace element phyto-accumulation. Eur. J. Soil Sci. 2012, 63, 685–693. [Google Scholar] [CrossRef]
- Liu, G.; Xue, W.; Wang, J.; Liu, X. Transport behavior of variable charge soil particle size fractions and their influence on cadmium transport in saturated porous media. Geoderma 2019, 337, 945–955. [Google Scholar] [CrossRef]
- Zong, Y.; Xiao, Q.; Lu, S. Distribution, bioavailability, and leachability of heavy metals in soil particle size fractions of urban soils (northeastern China). Environ. Sci. Pollut. Res. 2016, 23, 14600–14607. [Google Scholar]
- Elbana, T.A.; Selim, H.M. Modeling of cadmium and nickel release from different soils. Geoderma 2019, 338, 78–87. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, E.F.; Andrade, L.; Vega, F.A. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma 2011, 162, 20–26. [Google Scholar] [CrossRef]
- Nguemezi, C.; Tematio, P.; Yemefack, M.; Tsozue, D.; Silatsa, T.B.F. Soil quality and soil fertility status in major soil groups at the Tombel area, South-West Cameroon. Heliyon 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, R.; Kanbar, H.J.; Kazpard, V.; Wazne, M.; El Samrani, A.G.; Amacha, N.; Saad, Z.; Chou, L. Role of phosphogypsum and NPK amendments on the retention or leaching of metals in different soils. J. Environ. Manag. 2016, 178, 20–29. [Google Scholar] [CrossRef]
- Imoto, Y.; Yasutaka, T. Comparison of the impacts of the experimental parameters and soil properties on the prediction of the soil sorption of Cd and Pb. Geoderma 2020, 376, 114538. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Sun, X.; Sun, Y.; Cen, Y. Predicting nickel concentration in soil using reflectance spectroscopy associated with organic matter and clay minerals. Geoderma 2018, 327, 25–35. [Google Scholar] [CrossRef]

| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
|---|---|---|---|---|---|---|---|---|
| pH | 7.65 | 7.77 | 7.50 | 7.73 | 7.79 | 7.89 | 7.37 | 7.80 |
| Eh | 136.0 | 156.6 | 144.1 | 170.6 | 176.1 | 159.7 | 186.2 | 186.0 |
| EC | 249.47 | 166.57 | 99.60 | 655.00 | 731.00 | 165.63 | 80.17 | 905.67 |
| Total Cd (mg/kg) | 0.218 | 0.215 | 2.470 | 2.000 | 0.462 | 0.336 | 0.244 | 0.639 |
| SOM (mg/g) | 28.30 | 38.10 | 15.45 | 20.30 | 19.70 | 17.10 | 10.20 | 25.30 |
| CEC (cmol/kg) | 19.50 | 21.10 | 11.20 | 33.60 | 34.90 | 19.90 | 9.80 | 40.60 |
| SIO (mg/g) | 13.66 | 12.83 | 14.91 | 12.00 | 13.97 | 14.63 | 13.78 | 14.10 |
| Clay (%) | 17.81 | 10.30 | 26.91 | 11.84 | 15.16 | 19.43 | 23.23 | 12.21 |
| Silt (%) | 68.10 | 47.71 | 73.09 | 40.78 | 46.45 | 69.15 | 76.77 | 33.59 |
| Sand (%) | 14.10 | 41.99 | 0.00 | 47.37 | 38.39 | 11.42 | 0.00 | 54.21 |
| BET | 15.92 | 12.68 | 10.94 | 11.89 | 16.58 | 17.33 | 12.92 | 15.21 |
| C | Salt | Kf | 1/n | R2 | n |
|---|---|---|---|---|---|
| 0.5% | NaCl | 370.79 | 0.893 | 0.960 | 1.128 |
| CaCl2 | 18.324 | 0.539 | 0.827 | 1.856 | |
| NaNO3 | 264.272 | 0.697 | 0.900 | 1.435 | |
| MgCl2 | 17.517 | 0.527 | 0.733 | 1.899 | |
| Na2SO4 | 103.131 | 0.608 | 0.865 | 1.644 | |
| mixed salt | 50.588 | 0.618 | 0.729 | 1.618 | |
| 1% | NaCl | 40.986 | 0.607 | 0.871 | 1.647 |
| CaCl2 | 11.960 | 0.532 | 0.807 | 1.878 | |
| NaNO3 | 202.600 | 0.678 | 0.875 | 1.476 | |
| MgCl2 | 13.705 | 0.543 | 0.762 | 1.841 | |
| Na2SO4 | 136.071 | 0.664 | 0.873 | 1.505 | |
| mixed salt | 25.334 | 0.566 | 0.759 | 1.767 | |
| 2% | NaCl | 114.556 | 0.873 | 0.948 | 1.145 |
| CaCl2 | 9.461 | 0.566 | 0.814 | 1.766 | |
| NaNO3 | 157.432 | 0.658 | 0.851 | 1.519 | |
| MgCl2 | 8.586 | 0.523 | 0.751 | 1.911 | |
| Na2SO4 | 122.800 | 0.664 | 0.831 | 1.505 | |
| mixed salt | 8.656 | 0.485 | 0.777 | 2.061 | |
| 3% | NaCl | 34.678 | 0.658 | 0.890 | 1.520 |
| CaCl2 | 8.316 | 0.577 | 0.809 | 1.733 | |
| NaNO3 | 111.024 | 0.614 | 0.881 | 1.629 | |
| MgCl2 | 7.370 | 0.547 | 0.771 | 1.829 | |
| Na2SO4 | 102.805 | 0.646 | 0.824 | 1.549 | |
| mixed salt | 8.991 | 0.503 | 0.717 | 1.989 | |
| 3.5% | NaCl | 13.009 | 0.545 | 0.816 | 1.835 |
| CaCl2 | 7.485 | 0.566 | 0.827 | 1.768 | |
| NaNO3 | 154.931 | 0.673 | 0.826 | 1.487 | |
| MgCl2 | 6.978 | 0.544 | 0.766 | 1.840 | |
| Na2SO4 | 72.580 | 0.599 | 0.824 | 1.670 | |
| mixed salt | 9.460 | 0.542 | 0.741 | 1.847 |
| NaCl | CaCl2 | NaNO3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | PCA1 | PCA2 | PCA3 | PCA4 | PCA1 | PCA2 | PCA3 | PCA4 | PCA1 | PCA2 | PCA3 | PCA4 |
| pH | −0.45 | 0.74 | −0.44 | 0.23 | −0.46 | 0.70 | −0.22 | 0.48 | −0.58 | 0.49 | 0.64 | 0.08 |
| Eh | −0.41 | 0.12 | 0.78 | 0.20 | −0.48 | −0.05 | 0.76 | −0.10 | −0.63 | −0.59 | −0.15 | −0.18 |
| EC | −0.68 | 0.54 | 0.37 | −0.11 | −0.68 | 0.53 | 0.41 | −0.12 | −0.91 | −0.16 | 0.06 | 0.27 |
| BET | −0.26 | 0.59 | −0.07 | 0.64 | −0.35 | 0.35 | 0.21 | 0.72 | −0.47 | −0.03 | 0.77 | −0.19 |
| Clay | 0.93 | −0.22 | 0.23 | 0.05 | 0.90 | −0.30 | 0.26 | 0.03 | 0.84 | −0.51 | 0.07 | 0.04 |
| Silt | 0.84 | −0.44 | −0.07 | 0.29 | 0.79 | −0.55 | −0.03 | 0.25 | 0.91 | −0.25 | 0.10 | −0.29 |
| Sand | −0.88 | 0.39 | −0.01 | −0.24 | −0.84 | 0.50 | −0.05 | −0.20 | −0.91 | 0.32 | −0.09 | 0.21 |
| TOC | −0.60 | −0.09 | −0.74 | −0.20 | −0.52 | 0.14 | −0.80 | −0.02 | −0.30 | 0.90 | −0.16 | −0.08 |
| CEC | −0.76 | 0.60 | 0.17 | −0.06 | −0.75 | 0.60 | 0.23 | −0.01 | −0.94 | 0.05 | 0.16 | 0.23 |
| SIO | 0.71 | 0.24 | 0.06 | 0.27 | 0.67 | 0.13 | 0.25 | 0.41 | 0.49 | −0.33 | 0.48 | −0.03 |
| Total Cd | 0.37 | 0.29 | 0.21 | −0.80 | 0.45 | 0.46 | 0.10 | −0.68 | 0.21 | −0.17 | −0.17 | 0.93 |
| Salt–0.0% | −0.63 | −0.06 | −0.12 | 0.05 | −0.60 | −0.04 | −0.24 | −0.03 | −0.45 | 0.38 | −0.28 | −0.28 |
| Salt–0.5% | 0.26 | 0.89 | 0.15 | 0.05 | 0.88 | 0.47 | −0.01 | −0.07 | 0.85 | 0.29 | −0.40 | 0.00 |
| Salt–1.0% | 0.64 | 0.71 | −0.19 | −0.17 | 0.86 | 0.50 | 0.01 | −0.02 | 0.87 | 0.30 | 0.11 | 0.00 |
| Salt–2.0% | 0.52 | 0.79 | −0.03 | 0.19 | 0.83 | 0.55 | 0.01 | 0.03 | 0.76 | 0.44 | 0.25 | 0.10 |
| Salt–3.0% | 0.85 | 0.48 | −0.21 | −0.04 | 0.87 | 0.46 | −0.04 | 0.06 | 0.86 | 0.09 | 0.30 | 0.36 |
| Salt–3.5% | 0.79 | 0.52 | −0.04 | −0.29 | 0.84 | 0.47 | −0.24 | −0.05 | 0.62 | 0.75 | 0.05 | 0.01 |
| Eigenvalue | 7.27 | 4.51 | 1.74 | 1.57 | 8.68 | 3.34 | 1.78 | 1.53 | 8.77 | 3.06 | 1.76 | 1.41 |
| Cumulative variance | 42.77 | 69.31 | 79.54 | 88.79 | 51.05 | 70.70 | 81.19 | 90.16 | 51.56 | 69.53 | 79.90 | 88.21 |
| MgCl2 | Na2SO4 | Mixed salt | ||||||||||
| Variables | PCA1 | PCA2 | PCA3 | PCA4 | PCA1 | PCA2 | PCA3 | PCA4 | PCA1 | PCA2 | PCA3 | PCA4 |
| pH | −0.48 | 0.66 | 0.55 | 0.13 | −0.7 | 0.01 | 0.52 | 0.41 | −0.44 | 0.71 | 0.5 | 0.11 |
| Eh | −0.35 | 0.23 | −0.71 | 0.44 | −0.27 | 0.8 | −0.47 | 0 | −0.35 | 0.2 | −0.75 | 0.4 |
| EC | −0.64 | 0.63 | −0.32 | 0.03 | −0.73 | 0.59 | 0.19 | −0.1 | −0.61 | 0.64 | −0.36 | −0.01 |
| BET | −0.28 | 0.5 | 0.32 | 0.60 | −0.45 | 0.22 | 0.22 | 0.73 | −0.23 | 0.56 | 0.23 | 0.56 |
| Clay | 0.94 | −0.21 | −0.16 | 0.11 | 0.97 | 0.06 | 0.03 | 0.09 | 0.94 | −0.24 | −0.16 | 0.09 |
| Silt | 0.81 | −0.51 | 0.13 | 0.23 | 0.88 | −0.3 | −0.17 | 0.29 | 0.80 | −0.51 | 0.14 | 0.25 |
| Sand | −0.86 | 0.44 | −0.06 | −0.2 | −0.93 | 0.21 | 0.12 | −0.24 | −0.86 | 0.45 | −0.06 | −0.21 |
| TOC | −0.64 | −0.12 | 0.59 | −0.39 | −0.66 | −0.64 | 0.11 | −0.14 | −0.65 | −0.09 | 0.63 | −0.32 |
| CEC | −0.73 | 0.65 | −0.12 | 0.02 | −0.85 | 0.45 | 0.25 | −0.02 | −0.70 | 0.67 | −0.16 | −0.02 |
| SIO | 0.72 | 0.24 | 0.14 | 0.31 | 0.63 | 0.2 | 0.34 | 0.42 | 0.76 | 0.26 | 0.1 | 0.29 |
| Total Cd | 0.39 | 0.35 | −0.35 | −0.71 | 0.32 | 0.24 | 0.52 | −0.67 | 0.38 | 0.26 | −0.34 | −0.77 |
| Salt–0.0% | −0.64 | −0.14 | 0.08 | 0.01 | −0.53 | −0.14 | −0.06 | −0.09 | −0.58 | 0.06 | 0.16 | 0.09 |
| Salt–0.5% | 0.93 | 0.33 | 0.11 | 0.04 | 0.76 | −0.16 | 0.57 | −0.22 | 0.53 | 0.73 | 0.08 | 0.16 |
| Salt–1.0% | 0.94 | 0.31 | −0.06 | 0.01 | 0.74 | 0.15 | 0.6 | 0.09 | 0.91 | 0.4 | −0.06 | 0.08 |
| Salt–2.0% | 0.83 | 0.53 | 0.07 | −0.12 | 0.88 | 0.41 | −0.07 | −0.04 | 0.79 | 0.55 | 0.06 | −0.24 |
| Salt–3.0% | 0.81 | 0.57 | 0.12 | −0.08 | 0.88 | 0.27 | −0.36 | −0.05 | 0.82 | 0.51 | 0.12 | −0.17 |
| Salt–3.5% | 0.85 | 0.5 | 0.06 | −0.14 | 0.95 | 0.25 | 0.1 | −0.06 | 0.81 | 0.56 | 0.09 | −0.09 |
| Eigenvalue | 8.94 | 3.31 | 1.61 | 1.47 | 9.4 | 2.25 | 1.91 | 1.58 | 8.03 | 3.95 | 1.64 | 1.51 |
| Cumulative variance | 52.57 | 72.03 | 81.5 | 90.13 | 55.3 | 68.52 | 79.78 | 89.07 | 47.24 | 70.5 | 80.13 | 88.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, N.; Yue, W.; Wu, J.; Hou, K.; Wu, L.; Guo, M.; Teng, Y. Responses of Soil Cadmium Desorption under Different Saline Environments and Its Controlling Factors. Agronomy 2021, 11, 2175. https://doi.org/10.3390/agronomy11112175
Zheng N, Yue W, Wu J, Hou K, Wu L, Guo M, Teng Y. Responses of Soil Cadmium Desorption under Different Saline Environments and Its Controlling Factors. Agronomy. 2021; 11(11):2175. https://doi.org/10.3390/agronomy11112175
Chicago/Turabian StyleZheng, Nengzhan, Weifeng Yue, Jin Wu, Kaixuan Hou, Lijun Wu, Mengshen Guo, and Yanguo Teng. 2021. "Responses of Soil Cadmium Desorption under Different Saline Environments and Its Controlling Factors" Agronomy 11, no. 11: 2175. https://doi.org/10.3390/agronomy11112175
APA StyleZheng, N., Yue, W., Wu, J., Hou, K., Wu, L., Guo, M., & Teng, Y. (2021). Responses of Soil Cadmium Desorption under Different Saline Environments and Its Controlling Factors. Agronomy, 11(11), 2175. https://doi.org/10.3390/agronomy11112175










