Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design, Fertilizing Materials and Orchard Management
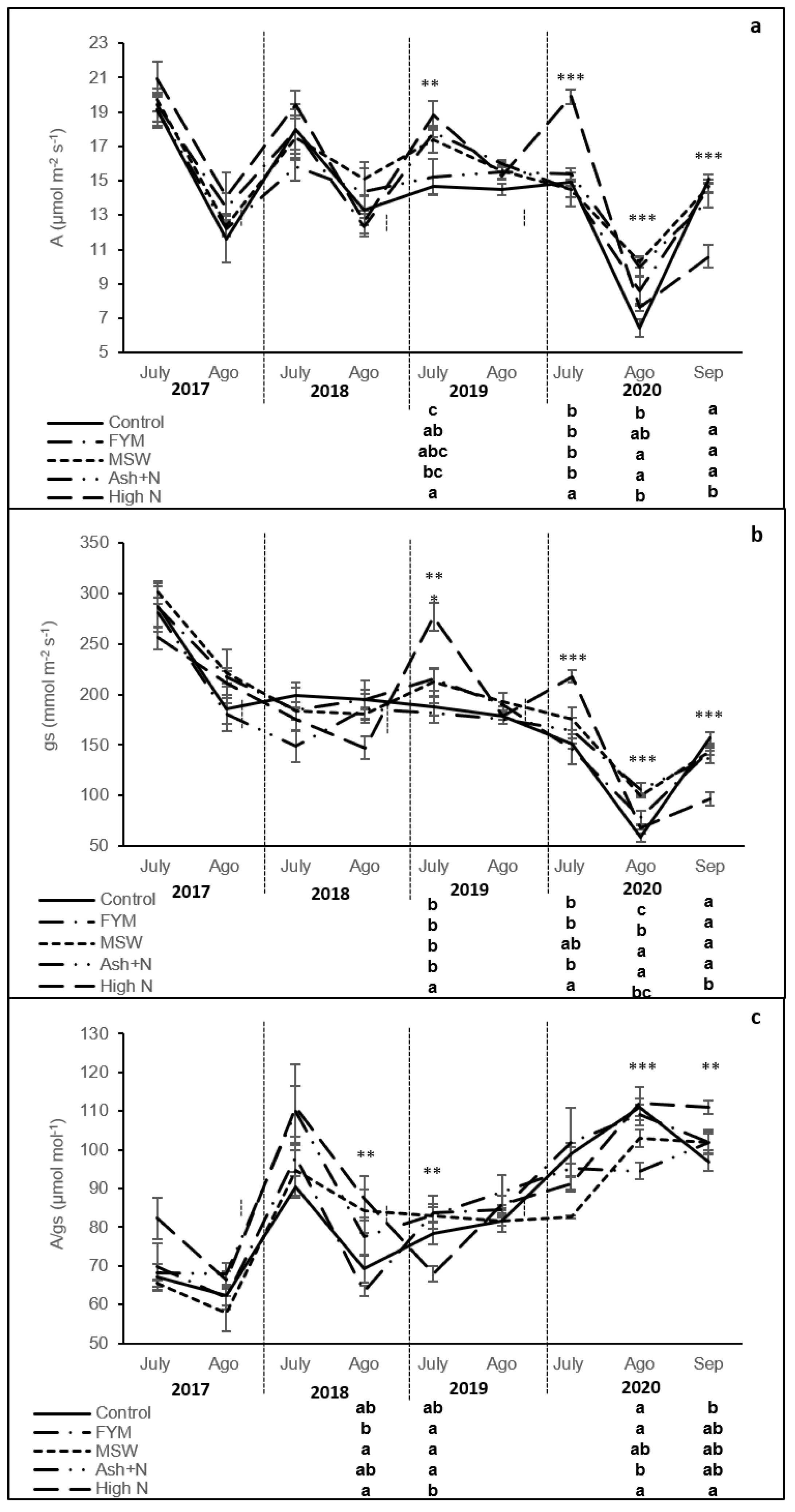
2.3. Leaf Gas Exchange Determinations
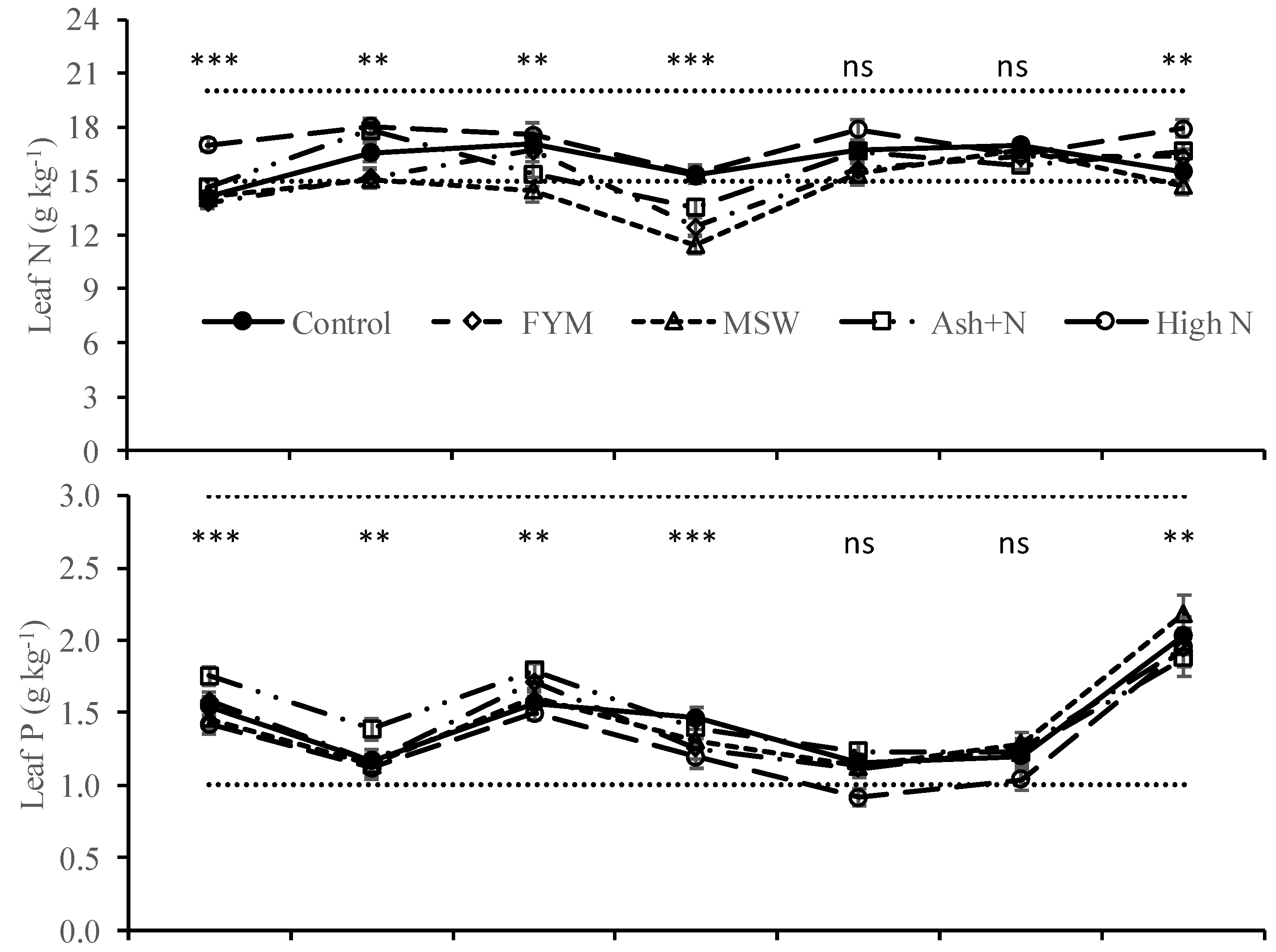
2.4. Samples Collection and Laboratory Analysis
2.5. Data Analysis
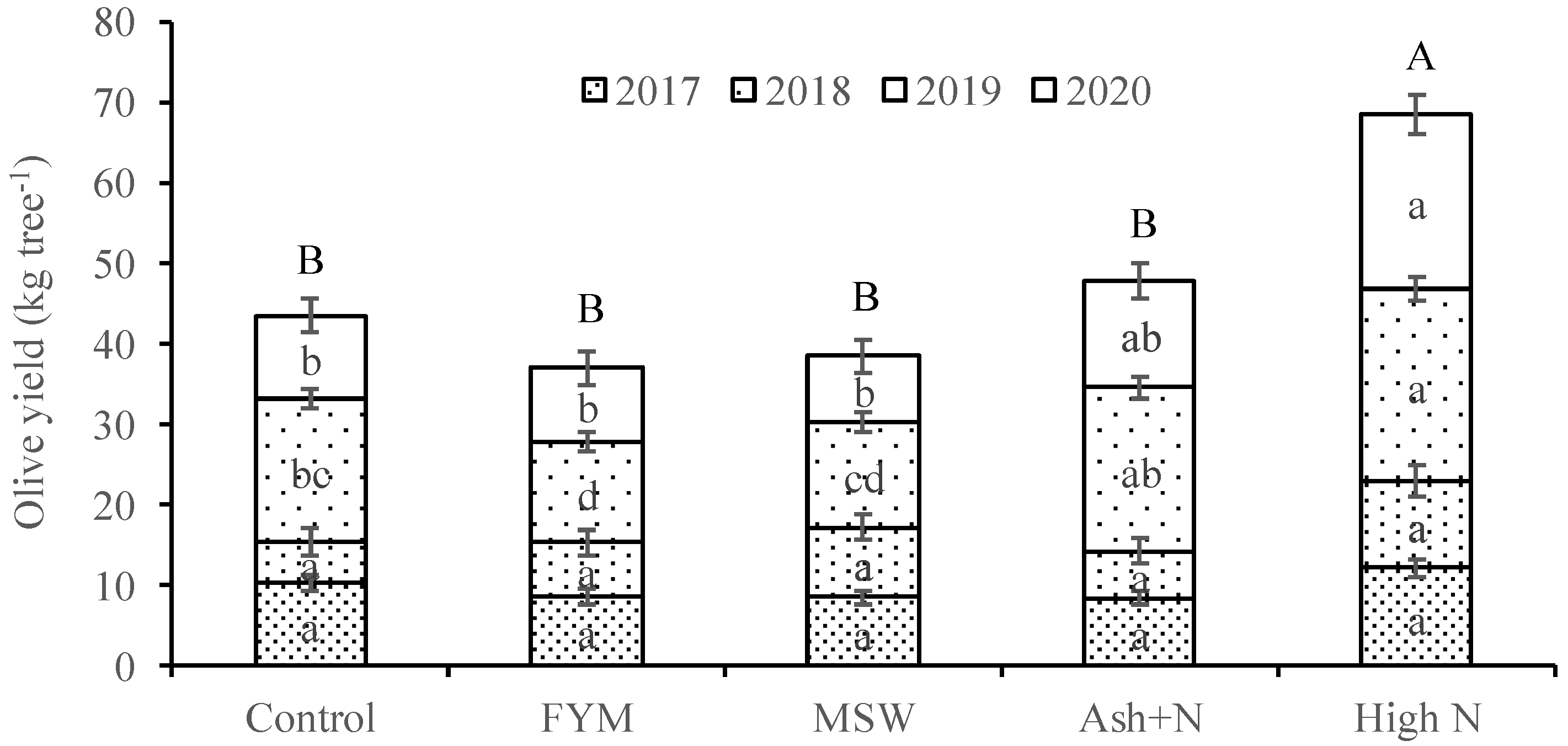
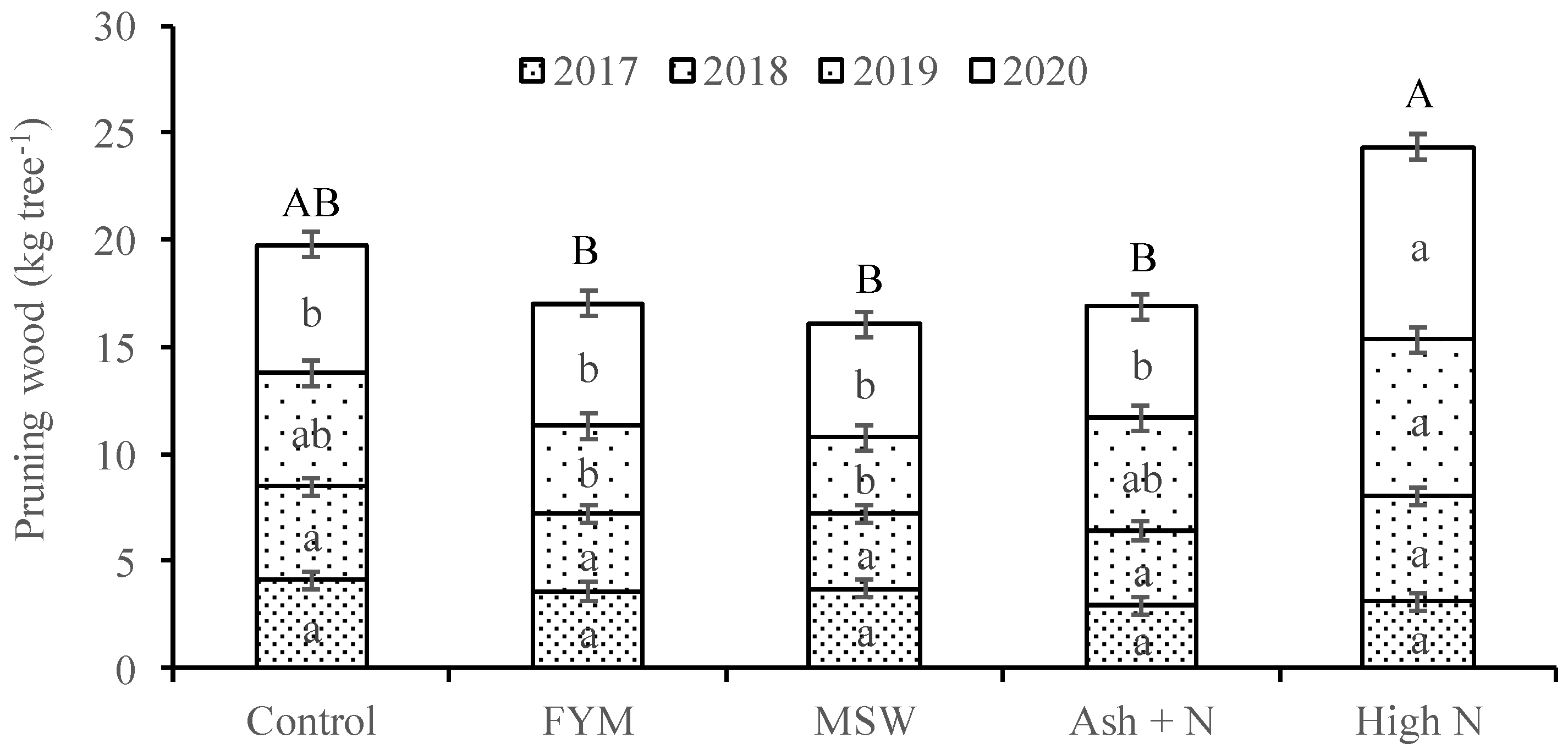
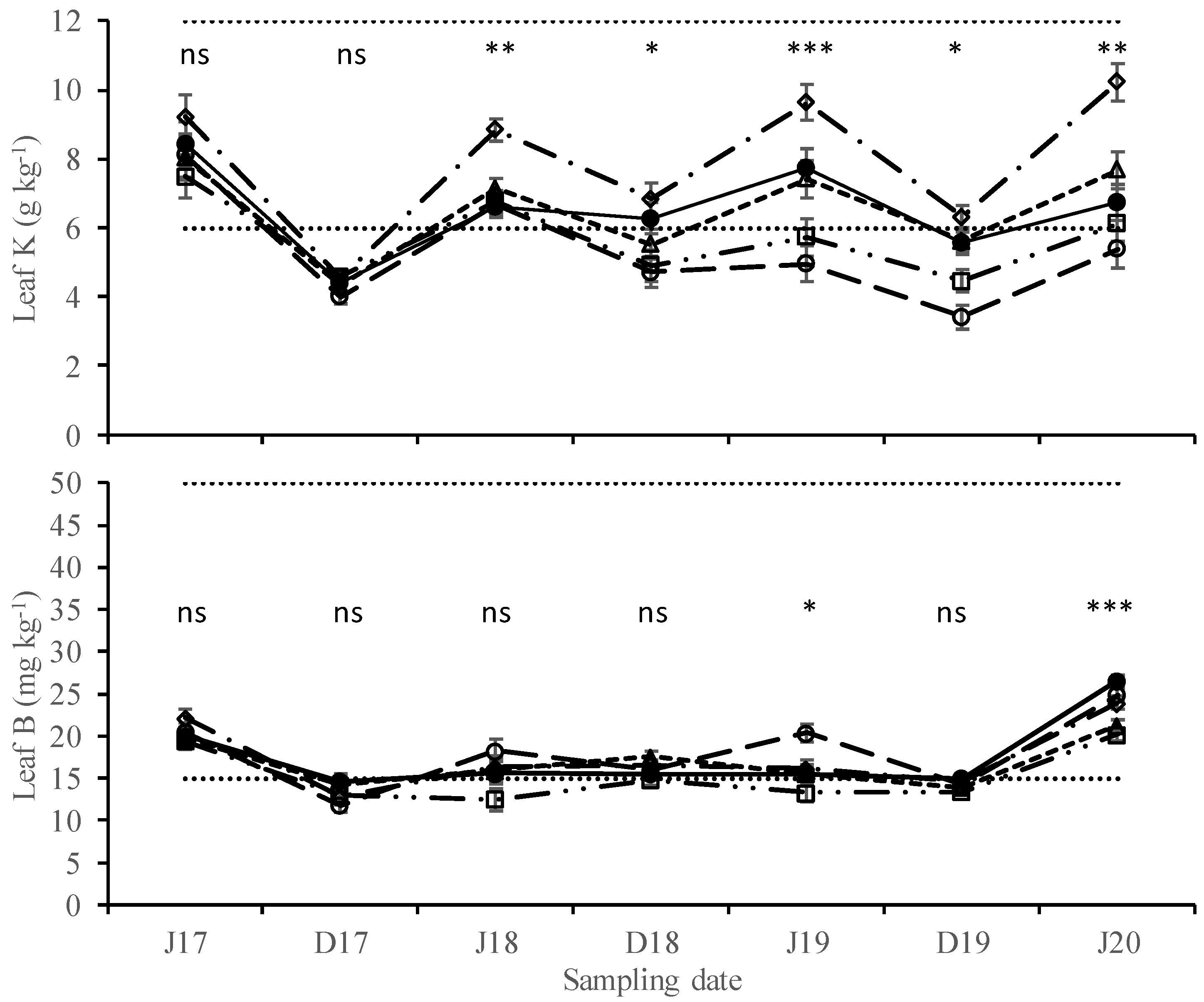
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rusinamhodzi, L.; Dahlin, S.; Corbeels, M. Living within their means: Reallocation of farm resources can help smallholder farmers improve crop yield and soil fertility. Agric. Ecosyst. Environ. 2016, 216, 125–136. [Google Scholar] [CrossRef]
- Bashagaluke, J.B.; Logah, V.; Opoku, A.; Sarkodie-Addo, J.; Quansah, C. Soil nutrient loss through erosion: Impact of different cropping systems and soil amendments in Ghana. PLoS ONE 2018, 13, e0208250. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, an Introduction to Nutrient Management, 8th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Weil, R.R.; Brady, N.C. Nature and Properties of Soils, 15th ed.; Pearson: London, UK, 2017. [Google Scholar]
- Yang, X.; Zhang, P.; Li, W.; Hu, C.; Zhang, X.; He, P. Evaluation of four seagrass species as early warning indicators for nitrogen overloading: Implications for eutrophic evaluation and ecosystem management. Sci. Total Environ. 2018, 635, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S.; Phillips, G.; Birk, S.; Free, G.; Kelly, M.G.; Willby, N.J. Deriving nutrient criteria to support ‘good’ ecological status in European lakes: An empirically based approach to linking ecology and management. Sci. Total Environ. 2019, 650, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.S. Biological denitrification. In Nitrogen in Agricultural Systems; Agronomy Monograph n.°49; Schepers, J.S., Raun, W.R., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2008; pp. 201–253. [Google Scholar]
- Pelster, D.E.; Larouche, F.; Rochette, P.; Chantigny, M.H.; Allaire, S.; Angers, D.A. Nitrogen fertilization but not soil tillage affects nitrous oxide emissions from a clay loam soil under a maize–soybean rotation. Soil Tillage Res. 2011, 115/116, 16–26. [Google Scholar] [CrossRef]
- Li, G.; Huang, G.; Li, H.; van Ittersum, M.K.; Leffelaar, P.A.; Zhang, F. Identifying potential strategies in the key sectors of China’s food chain to implement sustainable phosphorus management: A review. Nutr. Cycl. Agroecosyst. 2016, 104, 341–359. [Google Scholar] [CrossRef]
- Gilbert, N. The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Function of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 135–189. [Google Scholar]
- Agegnehu, C.; Nelson, P.N.; Bird, M.I. Crop yield, nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Murtaza, B.; Zaman, G.; Imran, M.; Shah, G.M.; Amjad, M.; Ahmad, N.; Naeem, M.A.; Zakir, A.; Farooq, A.; Ahmad, S.; et al. Municipal solid waste compost improves crop productivity in saline-sodic soil: A multivariate analysis of soil chemical properties and yield response. Commun. Soil Sci. Plant Anal. 2019, 50, 1013–1029. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Dimande, P.; Pereira, E.; Ferreira, I.Q.; Freitas, S.; Correia, C.M.; Moutinho-Pereira, J.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Obriot, F.; Stauffer, M.; Goubard, Y.; Cheviron, N.; Peres, G.; Eden, M.; Revallier, A.; Vieublé-Gonod, L.; Houot, S. Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agric. Ecosyst. Environ. 2016, 232, 165–178. [Google Scholar] [CrossRef]
- Pardo, R.; Schweitzer, J.P. A Long-Term Strategy for a European Circular Economy—Setting the Course for Success; Policy Paper Produced for the Think 2030 Project; Institute for European Environmental Policy: Brussels, Belgium, 2018. [Google Scholar]
- Leogrande, R.; Lopedota, O.; Vitti, C.; Ventrella, D.; Montemurro, F. Saline water and municipal solid waste compost application on tomato crop: Effects on plant and soil. J. Plant Nutr. 2016, 39, 491–501. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Garmus, T.; Arrobas, M.; Gonçalves, A.; Silva, E.; Rocha, L.; Pinto, L.; Brito, C.; Martins, S.; Vargas, T.; et al. Combined biochar and organic waste have little effect on chemical soil properties and plant growth. Span. J. Soil Sci. 2019, 9, 199–211. [Google Scholar] [CrossRef]
- EU (European Union). Regulation (EU) 2019/1009, of the European Parliament and the Council of 5 June 2019. Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Brod, E.; Haraldsen, T.K.; Breland, T.A. Fertilization effects of organic waste resources and bottom wood ash: Results from a pot experiment. Agric. Food Sci. 2012, 21, 332–347. [Google Scholar] [CrossRef][Green Version]
- Schönegger, D.; Gómez-Brandón, M.; Mazzier, T.; Insam, H.; Hermanns, R.; Leijenhorst, E.; Bardelli, T.; Juárez, M.F.-D. Phosphorus fertilising potential of fly ash and effects on soil microbiota and crop. Resour. Conserv. Recycl. 2018, 134, 262–270. [Google Scholar] [CrossRef]
- Martinez-Santos, T.; Bonfim-Silva, E.M.; Silva, T.J.A.; Damasceno, A.P.A.B. Correction of soil compaction using wood ash in safflower crop. Aust. J. Crop. Sci. 2019, 13, 1375–1382. [Google Scholar] [CrossRef]
- Dahl, O.; Nurmesniemi, H.; Pöykiö, R.; Watkins, G. Heavy metal concentrations in bottom ash and fly ash fractions from a large-sized (246 MW) fluidized bed boiler with respect to their Finnish forest fertilizer limit values. Fuel Process. Technol. 2010, 91, 1634–1639. [Google Scholar] [CrossRef]
- Jayaranjan, M.L.D.; van Hullebusch, E.D.; Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev. Environ. Sci. Biotechnol. 2014, 13, 467–486. [Google Scholar] [CrossRef]
- Maschowski, C.; Zangna, M.C.; Trouvé, G.; Gieré, R. Bottom ash of trees from Cameroon as fertilizer. J. Appl. Geochem. 2016, 72, 88–96. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Dulewska, C.R.; Poluszyńska, J.; Ślęzak, E.; Łuczak, K. Ashes from sewage sludge and bottom sediments as a source of bioavailable phosphorus. Ecol. Eng. 2018, 19, 88–94. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Iftikar, W.; Sahariah, B.; Chattopadhyay, G.N. Vermicomposting converts fly ash to enrich soil fertility and sustain crop growth in red and lateritic soils. Resour. Conserv. Recycl. 2012, 65, 100–106. [Google Scholar] [CrossRef]
- Merino, A.; Omil, B.; Fonturbel, M.T.; Vega, J.A.; Balboa, M.A. Reclamation of intensively managed soils in temperate regions by addition of wood bottom ash containing charcoal: SOM composition and microbial functional diversity. Appl. Soil Ecol. 2016, 100, 195–206. [Google Scholar] [CrossRef]
- Fonseca, J.A.; Hanisch, A.L. Is biomass ash an effective product for use in cereal crops in an agroecological system? Rev. Ciênc. Agrovet. 2018, 17, 454–461. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Janas, R.; Grzesik, M. Application of Phytotoxkit in the quick assessment of ashes suitability as fertilizers in sorghum crops. Int. Agrophys. 2019, 33, 145–152. [Google Scholar] [CrossRef]
- Alcántara, C.; Soriano, M.A.; Saavedra, M.; Gómez, J.A. Sistemas de manejo del suelo. In El Cultivo del Olivo, 7th ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2017; pp. 335–417. [Google Scholar]
- Rodrigues, M.A.; Arrobas, M. Cover cropping for increasing fruit production and farming sustainability. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–295. [Google Scholar]
- Fraga, H.; Atauri, I.G.C.; Malheiro, A.; Moutinho-Pereira, J.; Santos, J.A. Viticulture in Portugal: A review of recent trends and climate change projections. OENO One 2017, 51, 61–69. [Google Scholar] [CrossRef]
- Yang, C.; Fraga, H.; van Ieperen, W.; Trindade, H.; Santos, J.A. Effects of climate change and adaptation options on winter wheat yield under rainfed Mediterranean conditions in southern Portugal. Clim. Chang. 2019, 154, 159–178. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; Technical Paper 9; ISRIC FAO: Wageningen, The Netherlands, 2002. [Google Scholar]
- Balbino, L.R. La Méthode Egner-Riehm et la Détermination du Phosfore et du Potassium «Assimilável» des Sols du Portugal. II Col. Medit Cont. Fert. Plantas Cultivadas; Facultad de Ciencias: Sevilla, Spain, 1968; pp. 55–65. [Google Scholar]
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Temminghoff, E.E.J.M.; Houba, V.G. Plant Analysis Procedures; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- FAO/WHO (Codex Alimentarius Commission). Joint FAO/WHO Food Standards Programme, Codex Committee on Contaminants in Foods, Food 2018, CF/12 INF/1, 1–169. In Proceedings of the 12th Session of the Codex Committee on Contaminants in Foods, Utrecht, The Netherlands, 12–16 March 2018. [Google Scholar]
- Arrobas, M.; Afonso, S.; Ferreira, I.Q.; Moutinho-Pereiram, J.M.; Correia, C.M.; Rodrigues, M.A. Liming and application of nitrogen, phosphorus, potassium and boron on a young plantation of Chestnut. Turk. J. Agric. For. 2017, 41, 441–451. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Marin, L.; Sánchez-Zamora, M.A.; García-Novelo, J.M.; Molina-Soria, C.; Parra, M. Long-term effects of N fertilization on cropping and growth of olive trees and on N accumulation in soil profile. Eur. J. Agron. 2009, 31, 223–232. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; García-Novelo, J.M.; Molina-Soria, C.; Parra, M.A. An approach to nitrogen balance in olive orchards. Sci. Hortic. 2012, 135, 219–226. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Pavão, F.; Lopes, J.I.; Gomes, V.; Arrobas, M.; Moutinho-Pereira, J.; Ruivo, S.; Cabanas, J.E.; Correia, C.M. Olive yields and tree nutritional status during a four year period without nitrogen and boron fertilization. Commun. Soil Sci. Plant Anal. 2011, 42, 803–814. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Shtern, N.; Zipori, I.; Erel, R.; Ben-Gal, A.; Yermiyahu, U. Significance of proper nitrogen fertilization for olive productivity in intensive cultivation. Sci. Hortic. 2019, 246, 710–717. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.A. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. For. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients, micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 191–248. [Google Scholar]
- Ferreira, I.Q.; Rodrigues, M.A.; Arrobas, M. Soil and foliar applied boron in olive: Tree crop growth and yield, and boron remobilization within plant tissue. Span. J. Agric. Res. 2019, 17, e0901. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Moutinho-Pereira, J.M.; Correia, C.; Arrobas, M. Olive tree response to applied phosphorus in field and pot experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Piroli, L.B.; Forcelini, D.; Raimundo, S.; Domingues, L.C.; Cassol, L.C.; Correia, C.M.; Arrobas, M. Use of commercial mycorrhizal fungi in stress-free growing conditions of potted olive cuttings. Sci. Hortic. 2021, 275, 109712. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.A. Leaf nutrient concentration standards for lemon verbena (Aloysia citrodora Paláu) obtained from field and pot fertilization experiments. J. Appl. Res. Med. Aromat. Plants 2018, 8, 33–40. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Ferreira, I.Q.; Claro, A.M.; Arrobas, M. Fertiliser recommendations for olive based upon nutrients removed in crop and pruning. Sci. Hortic. 2012, 142, 205–211. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Fertilization. In El Cultivo del Olivo, 7th ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2017; pp. 419–460. (In Spanish) [Google Scholar]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.; Rodrigues, M.A. Olive response to potassium applications under different water regimes and cultivars. Nutr. Cycl. Agroecosyst. 2018, 112, 387–401. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Anderson, A.M.; Stewart, D.W.; Ma, B.L.; Tollenaar, M. Changes in maize hybrid photosynthetic response to leaf nitrogen, from pre-anthesis to grain fill. Agron. J. 1995, 87, 1221–1225. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.R.; Padjung, R.; Hodges, H.F. Nitrogen nutrition and photosynthesis in leaves of pima cotton. J. Plant Nutr. 1996, 19, 755–770. [Google Scholar] [CrossRef]
- Correia, C.M.; Moutinho-Pereira, J.M.; Coutinho, J.F.; Björn, L.O.; Torres-Pereira, J.M.G. Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: A Mediterranean field study. Eur. J. Agron. 2005, 22, 337–347. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.A.; El-Tohamy, W.A.; Zaghloul, A.M. Yield and water use efficiency of potato grown under different irrigation andnitrogen levels in an arid region. Agric. Water Manag. 2012, 110, 9–15. [Google Scholar] [CrossRef]
- Gheysari, M.; Loescher, H.W.; Sadeghi, S.H.; Mirlatifi, S.M.; Zareian, M.J.; Hoogenboom, G. Water-yield relations and water use efficiency of maize under nitrogen fertigation for semiarid environments: Experiment and synthesis. Adv. Agron. 2015, 130, 175–229. [Google Scholar]
- Kiani, M.; Gheysari M- Mostafazadeh-Fard, B.; Majidi, M.M.; Karchani, K.; Hoogenboom, G. Effect of the interaction of water and nitrogen on sunflower under drip irrigation in an arid region. Agric. Water Manag. 2015, 171, 162–172. [Google Scholar] [CrossRef]
- Brueck, H. Effects of nitrogen supply on water-use efficiency of higher plants. J. Plant Nutr. Soil Sci. 2008, 171, 210–219. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Pereira, A.; Cabanas, J.E.; Dias, L.; Pires, J.; Arrobas, M. Crops use-efficiency of nitrogen from manures permitted in organic farming. Eur. J. Agron. 2006, 25, 328–335. [Google Scholar] [CrossRef]
- Almagro, M.; de Vente, J.; Boix-Fayos, C.; García-Franco, N.; Aguilar, J.M.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strateg. Glob. Chang. 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Fox, R.H.; Rayner, J.H. Interactions between fertilizer nitrogen and soil nitrogen—The so-called “priming effect”. J. Soil Sci. 1985, 36, 425–444. [Google Scholar] [CrossRef]
- Schnier, H.F. Nitrogen-15 recovery fraction in flooded tropical rice as affected by added nitrogen interaction. Eur. J. Agron. 1994, 3, 161–167. [Google Scholar] [CrossRef]
- Micó, C.; Peris, M.; Recatalá, L.; Sánchez, J. Baseline values for heavy metals in agricultural soils in an European Mediterranean region. Sci. Total Environ. 2007, 378, 13–17. [Google Scholar] [CrossRef] [PubMed]

| Soil Properties | Soil Properties | ||
|---|---|---|---|
| 1 Organic carbon (g kg−1) | 8.54 ± 0.59 | 5 Extract. Zn (mg kg−1) | 1.9 ± 0.25 |
| 2 pH (H2O) | 5.90 ± 0.11 | 5 Extract. Cu (mg kg−1) | 3.2 ± 0.20 |
| 2 pH (KCl) | 4.69 ± 0.09 | 6 Exchang. Ca (cmolc kg−1) | 3.88 ± 0.62 |
| 3 Extract. P (mg P2O5 kg−1) | 93.1 ± 9.5 | 6 Exchang. Mg (cmolc kg−1) | 0.71 ± 0.13 |
| 3 Extract. K (mg K2O kg−1) | 157.6 ± 17.5 | 6 Exchang. K (cmolc kg−1) | 0.31 ± 0.05 |
| 4 Extract. B (mg kg−1) | 1.0 ± 0.32 | 6 Exchang. Na (cmolc kg−1) | 0.81 ± 0.11 |
| 5 Extract. Fe (mg kg−1) | 43.6 ± 3.25 | 7 Exchang. acidity (cmolc kg−1) | 0.08 ± 0.02 |
| 5 Extract. Mn (mg kg−1) | 62.4 ± 9.24 | 8 CEC (cmolc kg−1) | 5.79 ± 0.79 |
| Municipal Solid Waste | Farmyard Manure | Bottom Ash * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Properties | 2017 | 2018 | 2019 | 2020 | 2017 | 2018 | 2019 | 2020 | Properties | 2017/2018 |
| Dry matter (%) | 68.3 ± 3.0 | 78.5 ± 6.5 | 87.4 ± 7.1 | 78.3 ± 6.3 | 34.5 ± 3.8 | 51.5 ± 7.5 | 51.1 ± 6.5 | 63.0 ± 3.4 | Dry matter (%) | 59 |
| 1 Cond (mS cm−1) | 5.6 ± 0.2 | 6.2 ± 0.3 | 4.6 ± 0.4 | 4.8 ± 0.3 | 5.6 ± 0.3 | 8.0 ± 0.7 | 7.0 ± 0.8 | 4.3 ± 0.4 | Organic matter (%) | 11 |
| 2 pH (H2O) | 8.2 ± 0.1 | 7.8 ± 0.1 | 7.9 ± 0.1 | 8.1 ± 0.1 | 8.6 ± 0.1 | 9.0 ± 0.1 | 8.2 ± 0.2 | 8.6 ± 0.1 | pH (23.4 °C) | 12 |
| 3 C (g kg−1) | 236.1 ± 17.0 | 247.0 ± 23.1 | 218.1 ± 22.3 | 222.4 ± 15.9 | 306.4 ± 19.8 | 258.6 ± 19.3 | 223.6 ± 12.8 | 249.3 ± 55.2 | Total N (g kg−1) | <5.6 |
| 4 N (g kg−1) | 17.5 ± 2.0 | 15.7 ± 1.3 | 16.8 ± 1.2 | 17.5 ± 1.6 | 22.6 ± 1.9 | 14.9 ± 1.2 | 13.7 ± 1.2 | 15.2 ± 1.4 | NO3−-N (mg kg−1) | <4.5 |
| 5 P (g kg−1) | 4.5 ± 0.1 | 4.7 ± 0.2 | 3.2 ± 0.1 | 3.4 ± 0.2 | 6.5 ± 1.2 | 6.1 ± 0.4 | 7.4 ± 0.6 | 4.4 ± 0.4 | NH4+-N (mg kg−1) | <4.2 |
| 6 K (g kg−1) | 14.1 ± 2.4 | 15.9 ± 1.6 | 13.6 ± 1.4 | 13.1 ± 2.1 | 55.1 ± 4.4 | 28.5 ± 3.8 | 23.5 ± 3.1 | 23.1 ± 2.7 | P (g kg−1) | 1.2 |
| 6 Ca (g kg−1) | 74.3 ± 3.0 | 63.0 ± 5.1 | 64.8 ± 4.4 | 27.8 ± 2.5 | 33.5 ± 3.3 | 22.3 ± 2.1 | 12.3 ± 1.5 | 18.8 ± 1.4 | K (g kg−1) | 9.7 |
| 6 Mg (g kg−1) | 8.3 ± 0.3 | 8.3 ± 0.7 | 7.9 ± 0.7 | 8.8 ± 0.8 | 8.5 ± 0.9 | 8.4 ± 1.3 | 9.4 ± 1.6 | 8.5 ± 0.7 | Ca (g kg−1) | 20 |
| 5 B (mg kg−1) | 49.0 ± 4.4 | 74.4 ± 3.7 | 65.3 ± 6.2 | 58.1 ± 4.3 | 46.1 ± 6.4 | 39.7 ± 7.1 | 30.7 ± 8.1 | 56.1 ± 7.6 | Mg (g kg−1) | 5.2 |
| 6 Cu (mg kg−1) | 265.7 ± 68.1 | 184.0 ± 33.2 | 169.5 ± 28.6 | 249.4 ± 31.8 | 32.8 ± 2.6 | 56.1 ± 4.9 | 36.1 ± 2.9 | 49.4 ± 5.1 | Na (g kg−1) | 2.1 |
| 6 Fe (g kg−1) | 12.3 ± 2.6 | 11.9 ± 2.1 | 12.8 ± 1.9 | 13.8 ± 1.2 | 6.1 ± 0.9 | 17.3 ± 1.5 | 17.7 ± 1.3 | 13.4 ± 1.1 | Zn (mg kg−1) | 47 |
| 6 Zn (mg kg−1) | 487.9 ± 17.0 | 419.0 ± 38.0 | 528.0 ± 41.2 | 428.4 ± 41.9 | 200.2 ± 9.5 | 144.1 ± 12.6 | 124.9 ± 11.2 | 228.4 ± 25.8 | Cu (mg kg−1) | <17 |
| 6 Mn (mg kg−1) | 474.8 ± 31.1 | 569.7 ± 43.2 | 414.2 ± 38.5 | 429.1 ± 31.3 | 366.3 ± 37.3 | 479.5 ± 52.2 | 579.8 ± 61.1 | 319.3 ± 41.6 | Ni (mg kg−1) | <10 |
| 6 Cd (mg kg−1) | 6.4 ± 0.8 | 7.2 ± 0.7 | 5.5 ± 0.6 | 5.8 ± 0.4 | 0.7 ± 0.0 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.2 | Pb (mg kg−1) | 18 |
| 6 Cr (mg kg−1) | 57.5 ± 4.2 | 40.2 ± 4.1 | 93.8 ± 8.6 | 62.4 ± 6.5 | 35.8 ± 0.5 | 41.8 ± 6.9 | 31.8 ± 4.9 | 29.6 ± 3.4 | Cr (mg kg−1) | 24 |
| 6 Ni (mg kg−1) | 44.8 ± 2.8 | 77.9 ± 3.7 | 60.0 ± 4.1 | 53.2 ± 5.2 | 18.4 ± 1.6 | 18.2 ± 3.8 | 14.2 ± 4.2 | 21.8 ± 3.6 | Cd (mg kg−1) | <0.33 |
| 6 Pb (mg kg−1) | 198.6 ± 60.8 | 149.2 ± 25.5 | 101.8 ± 12.5 | 132.6 ± 14.1 | 36.0 ± 3.2 | 33.8 ± 4.9 | 23.8 ± 4.7 | 28.6 ± 5.1 | Hg (mg kg−1) | <0.33 |
| Cadmium | Chromium | Lead | Nickel | |
|---|---|---|---|---|
| Control | 0.54 ab | 2.88 b | 5.15 a | 9.03 ab |
| FYM | 0.60 ab | 3.83 ab | 4.38 a | 11.87 a |
| MSW | 0.62 ab | 4.29 a | 4.92 a | 11.51 a |
| Ash + N | 0.81 a | 4.07 a | 6.09 a | 12.62 a |
| High N | 0.46 b | 2.73 b | 4.01 a | 7.29 b |
| Organic C | Extrac. P | Extrac. K | Exch Ca | Exch. Mg | Exch. K | CEC | Boron | Zinc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | pH(H2O) | (mg P2O5 kg−1) | (mg K2O kg−1) | (cmolc kg−1) | (mg kg−1) | |||||
| Soil depth (Z) | ||||||||||
| 0.0–0.1 m | 11.6 a | 6.2 a | 185.6 a | 282.6 a | 4.9 a | 0.9 a | 0.6 a | 7.9 a | 1.3 a | 4.1 a |
| 0.1–0.2 m | 8.3 b | 6.0 b | 88.7 b | 152.1 b | 3.9 b | 0.9 a | 0.3 b | 6.8 ab | 0.9 ab | 2.0 ab |
| 0.2–0.3 m | 5.2 c | 5.9 b | 43.7 b | 96.2 b | 3.3 b | 0.8 a | 0.2 b | 6.2 b | 0.6 b | 1.6 b |
| Treatment (T) | ||||||||||
| Control | 8.5 ab | 5.8 bc | 71.5 bc | 142.7 bc | 3.5 bc | 0.7 c | 0.3 b | 5.6 b | 1.7 a | 1.4 b |
| FYM | 9.2 ab | 6.3 a | 150.8 ab | 350.7 a | 3.9 bc | 1.1 a | 0.8 a | 7.2 ab | 0.4 b | 2.0 b |
| MSW | 10.1 a | 6.4 a | 216.1 a | 170.6 bc | 5.6 a | 0.9 ab | 0.3 b | 8.8 a | 0.3 b | 6.4 a |
| Ash + N | 7.8 bc | 5.9 b | 57.1 bc | 73.8 c | 4.2 b | 0.9 ab | 0.2 b | 7.9 a | 0.3 b | 1.6 b |
| High N | 6.2 c | 5.6 c | 34.3 c | 147.1 bc | 2.9 c | 0.8 bc | 0.3 b | 5.5 b | 1.9 a | 1.4 b |
| Prob > F (Z) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0703 | <0.0001 | 0.0055 | 0.0016 | 0.0182 |
| Prob > F (T) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.I.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Raimundo, S.; Arrobas, M.; Rodrigues, M.Â.; Correia, C.M. Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments. Agronomy 2021, 11, 2172. https://doi.org/10.3390/agronomy11112172
Lopes JI, Gonçalves A, Brito C, Martins S, Pinto L, Moutinho-Pereira J, Raimundo S, Arrobas M, Rodrigues MÂ, Correia CM. Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments. Agronomy. 2021; 11(11):2172. https://doi.org/10.3390/agronomy11112172
Chicago/Turabian StyleLopes, João I., Alexandre Gonçalves, Cátia Brito, Sandra Martins, Luís Pinto, José Moutinho-Pereira, Soraia Raimundo, Margarida Arrobas, Manuel Ângelo Rodrigues, and Carlos M. Correia. 2021. "Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments" Agronomy 11, no. 11: 2172. https://doi.org/10.3390/agronomy11112172
APA StyleLopes, J. I., Gonçalves, A., Brito, C., Martins, S., Pinto, L., Moutinho-Pereira, J., Raimundo, S., Arrobas, M., Rodrigues, M. Â., & Correia, C. M. (2021). Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments. Agronomy, 11(11), 2172. https://doi.org/10.3390/agronomy11112172











