Blueberry Yield and Soil Mineral Nitrogen Response to Nitrogen Fertilizer and Nitrification Inhibitors under Drip-Fertigation Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Treatments
2.3. Berry Yield
2.4. Sawdust Mulch and Soil Sampling
2.5. Chemical Analysis
2.6. Statistical Analysis
3. Results
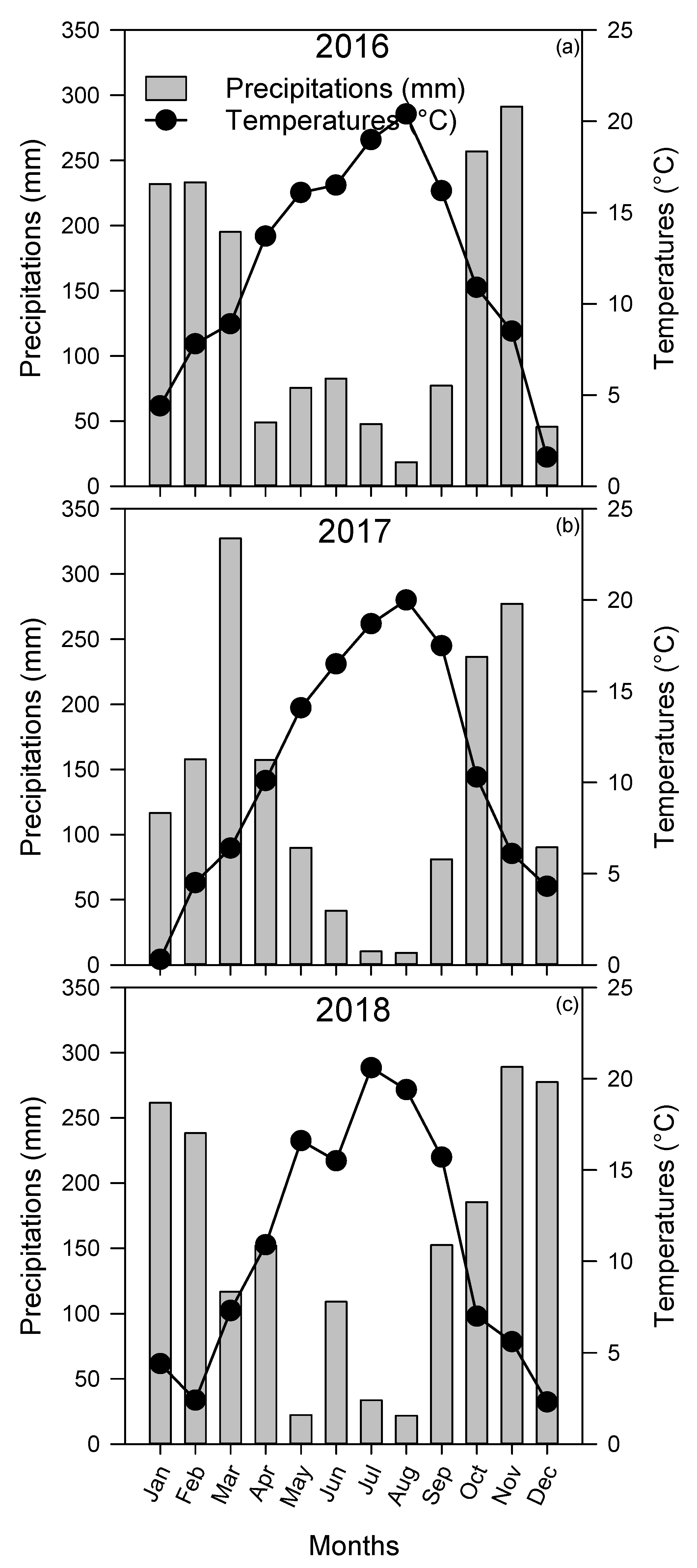
3.1. Weather Conditions
3.2. Berry Yield
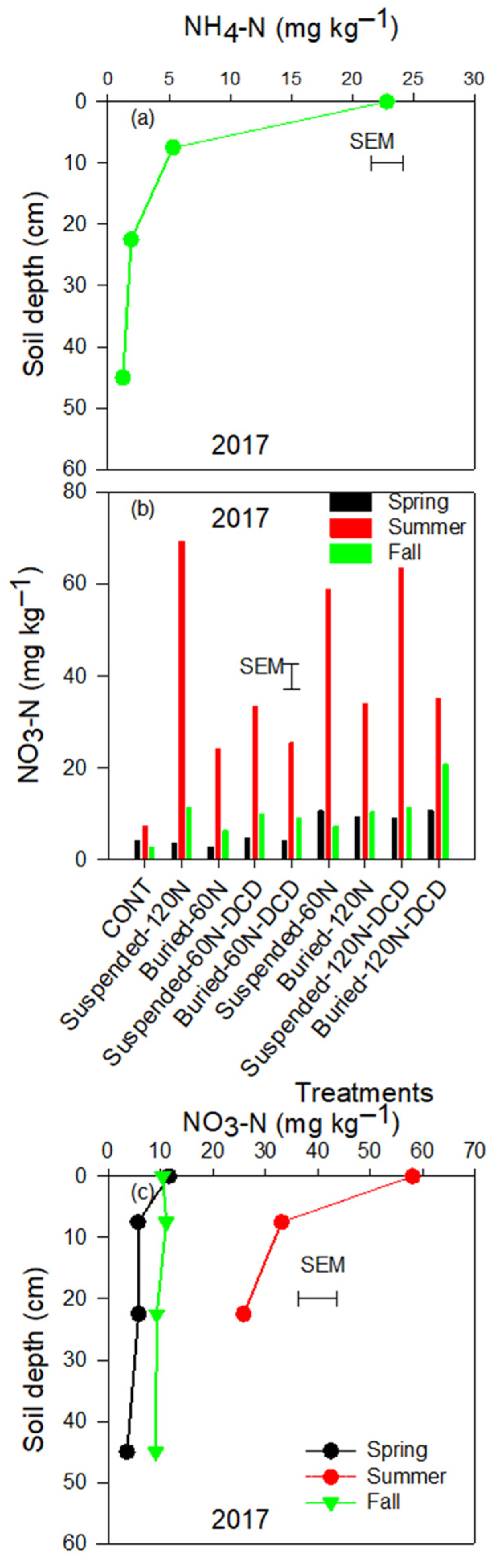
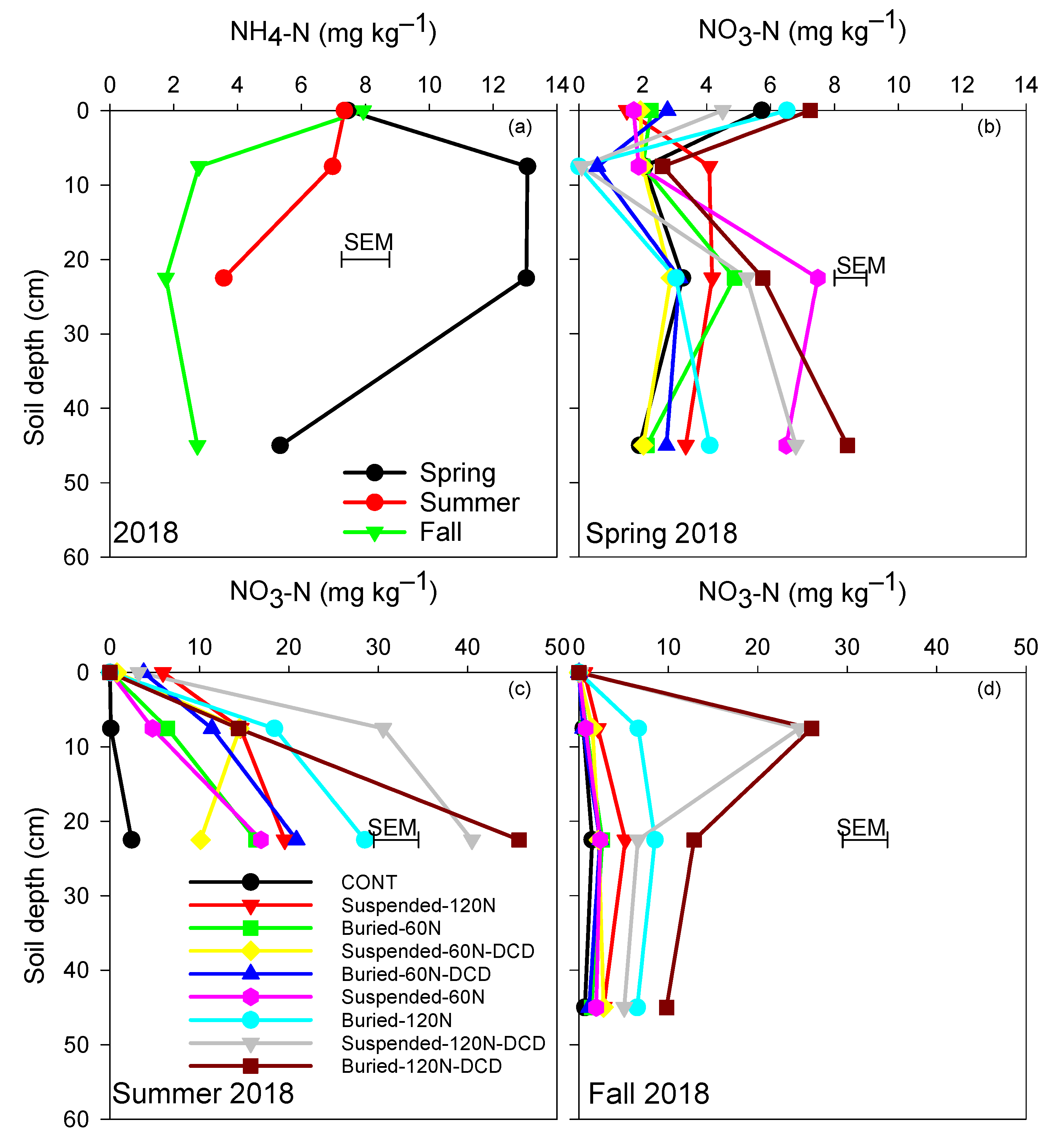
3.3. Ammonium and Nitrate Concentrations in the Soil
3.4. Soil pH and EC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arp, D.J.; Stein, L.Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 471–495. [Google Scholar] [CrossRef]
- Huber, D.; Warren, H.; Nelson, D.; Tsai, C. Nitrification inhibitors: New tools for food production. BioScience 1977, 27, 523–529. [Google Scholar] [CrossRef]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.J.; Nicol, G.W.; Smith, Z.; Fear, J.; Prosser, J.I.; Baggs, E.M. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ. Microbiol. 2006, 8, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, D.L.; Karlen, D.; Jaynes, D.B.; Kaspar, T.; Hatfield, J.; Colvin, T.S.; Cambardella, C.A. Nitrogen management strategies to reduce nitrate leaching in tile drained midwestern soils. Agron. J. 2002, 94, 153–171. [Google Scholar] [CrossRef]
- Zaman, M.; Blennerhassett, J.D. Effects of the different rates of urease and nitrification inhibitors on gaseous emissions of ammonia and nitrous oxide, nitrate leaching and pasture production from urine patches in an intensive grazed pasture system. Agric. Ecosyst. Environ. 2010, 136, 236–246. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.E.; Greaver, T.L.; Li, Q. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob. Chang. Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef]
- Marsden, K.A.; Marín-Martínez, A.J.; Vallejo, A.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: A comparison between DCD and DMPP. Biol. Fertil. Soils 2016, 52, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, M.; Tenuta, M.; Ma, Z.; Gui, D.; Li, X.; Zeng, F.; Gao, X. Agronomic evaluation of polymercoated urea and urease and nitrification inhibitors for cotton production under drip-fertigation in a dry climate. Sci. Rep. 2020, 10, 1472. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Sun, H.; Kronzucker, H.J.; Wang, Y.; Shi, W. Comprehensive assessment of the effects of nitrification inhibitor application on reactive nitrogen loss in intensive vegetable production systems. Agric. Ecosyst. Environ. 2021, 307, 107227. [Google Scholar] [CrossRef]
- Morales, S.E.; Jha, N.; Saggar, S. Impact of urine and the application of the nitrification inhibitor DCD on microbial communities in dairy-grazed pasture soils. Soil Biol. Biochem. 2015, 88, 344–353. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Yoshihashi, T.; Ito, O.; Ono, H.; Ohnishi-Kameyama, M.; Yoshida, M.; Kawano, N.; Berry, W.L. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 2008, 313, 89–99. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Yoshihashi, T.; Ito, O.; Ono, H.; Ohnishi-Kameyama, M.; Yoshida, M.; Kawano, N.; Berry, W.L. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardia, G.; Marsden, K.A.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messiga, A.; Haak, D.; Dorais, M. Blueberry yield and soil properties response to long-term fertigation and broadcast nitrogen. Sci. Hortic. 2018, 230, 92–101. [Google Scholar] [CrossRef]
- Messiga, A.J.; Dyck, K.; Ronda, K.; van Baar, K.; Haak, D.; Yu, S.; Dorais, M. Nutrients leaching in response to long-term fertigation and broadcast nitrogen in blueberry production. Plants 2020, 9, 1530. [Google Scholar] [CrossRef]
- Poonnachit, U.; Darnell, R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Ann. Bot. 2004, 93, 399–405. [Google Scholar] [CrossRef] [PubMed]
- BCMAL. British Columbia Ministry of Agriculture and Lands, BC Berry Production Guides. 2020. Available online: https://www2.gov.bc.ca/gov/content/industry/agriservice-bc/production-guides/berries (accessed on 3 September 2020).
- Forge, T.; Ehret, D.; Messiga, A.; Dorais, M. Influences of nitrogen inputs on nematode populations under highbush blueberry. J. Nematol. 2020, 52, e2020-56. [Google Scholar] [CrossRef]
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R.; Zebarth, B.J. Effects of nitrogen rate and application method on early production and fruit quality in highbush blueberry. Can. J. Plant Sci. 2014, 94, 1165–1179. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C.; Bañados, M.P.; Righetti, T.L. Response of highbush blueberry to nitrogen fertilizer during field establishment, II: Plant nutrient requirements in relation to nitrogen fertilizer supply. HortScience 2012, 47, 917–926. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; NRCS: Washington, DC, USA, 2010.
- Environment and Climate Change Canada (ECCC). 2020. Canadian Climate Normals 1981–2010 Station Data. Date Modified: 17 September 2020. Available online: https://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?stnID=707&autofwd=1 (accessed on 12 December 2020).
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R. Age-related changes in response of highbush blueberry plants to drip Irrigation. HortScience 2015, 50, 486–490. [Google Scholar] [CrossRef] [Green Version]
- British Columbia Ministry of Agriculture and Lands. Berry Production Guide; BC Ministry of Agriculture and Lands: Victoria, BC, Canada, 2009.
- Maynard, D.G.; Kalra, Y.P.; Crambaugh, J.A. Nitrate and exchangeable ammonium nitrogen. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 607–616. [Google Scholar]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Ion exchange and exchangeable cations. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 183–205. [Google Scholar]
- SAS Institute. SAS User’s Guide: Statistics, 3rd ed.; Version 9; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York, NY, USA, 2013; pp. 205–397. [Google Scholar]
- Liu, T.; Liang, Y.; Chu, G. Nitrapyrin addition mitigates nitrous oxide emissions and raises nitrogen use efficiency in plastic-film mulched drip-fertigated cotton field. PLoS ONE 2017, 12, e0176305. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef] [PubMed]
- Serna, M.D.; Legaz, F.; PrimoMillo, E. Improvement of the n fertilizer efficiency with dicyandiamide (dcd) in citrus trees. Fert. Res. 1996, 43, 137–142. [Google Scholar] [CrossRef]
- Holzapfel, E.A.; Hepp, R.F.; Mariño, M.A. Effect of irrigation on fruit production in blueberry. Agric. Water Manag. 2004, 67, 173–184. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries; Crop Production Science in Horticulture Series 21; CAB International: Wallingford, UK, 2012. [Google Scholar]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Shepherd, M.; Wyatt, J.; Welten, B. Effect of soil type and rainfall on dicyandiamide concentrations in drainage from lysimeters. Soil Res. 2012, 50, 67–75. [Google Scholar] [CrossRef]
- Kim, D.-G.; Giltrap, D.; Saggar, S.; Palmada, T.; Berben, P.; Drysdale, D. Fate of the nitrification inhibitor dicyandiamide (DCD) sprayed on a grazed pasture: Effect of rate and time of application. Soil Res. 2012, 50, 337–347. [Google Scholar] [CrossRef]
- Kelliher, F.M.; Clough, T.J.; Clark, H.; Rys, G.; Sedcole, J.R. The temperature dependence of dicyandiamide (DCD) degradation in soils: A data synthesis. Soil Biol. Biochem. 2008, 40, 1878–1882. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, R.J.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 940, 222–232. [Google Scholar] [CrossRef]


| Modifications of Treatments during the Three Successive Trials | Treatment Description between 2016 and 2018 | ||||
|---|---|---|---|---|---|
| Treatment Plots | 2006–2012 | 2013–2015 | 2016–2018 | Rate of Nitrogen Application and Method of Application | Placement of Fertigation Lines |
| 1 | CONT | CONT | CONT | CONT, 0 kg N ha−1 yr−1 | |
| 2 | Heavy water | - | Suspended-120N | 120 N ha−1 yr−1 applied by fertigation (high N rate) | Suspended line |
| 3 | Heavy water | - | Buried-60N | 60 N ha−1 yr−1 applied by fertigation (low N rate) | Buried line |
| 4 | Heavy water | No Humic Acid | Suspended-60N-DCD | 60 N ha−1 yr−1 applied by fertigation (low rate) | Suspended line |
| 5 | Heavy water | With Humic Acid | Buried-60N-DCD | 60 N ha−1 yr−1 combined with inhibitor (DCD) applied by fertigation (low rate) | Buried line |
| 6 | Moderate water | - | Suspended-60N | 60 N ha−1 yr−1 applied by fertigation (low rate) | Suspended line |
| 7 | Moderate water | - | Buried-120N | 120 N ha−1 yr−1 applied by fertigation (high N rate) | Buried line |
| 8 | Moderate water | With Humic Acid | Suspended-120N-DCD | 120 N ha−1 yr−1 combined with inhibitor (DCD) applied by fertigation (high rate) | Suspended line |
| 9 | Moderate water | No Humic Acid | Buried-120N-DCD | 120 N ha−1 yr−1 combined with inhibitor (DCD) applied by fertigation (high rate) | Buried line |
| Treatments | 2016 | 2017 | 2018 |
|---|---|---|---|
| CONT a | 49.51 | 8.60 | 26.18 |
| Suspended-60N | 48.23 | 11.65 | 27.04 |
| Suspended-60N-DCD | 44.52 | 9.24 | 26.29 |
| Suspended-120N | 51.45 | 11.66 | 25.75 |
| Suspended-120N-DCD | 46.83 | 10.35 | 27.16 |
| Buried-60N | 50.36 | 10.48 | 28.96 |
| Buried-60N-DCD | 46.44 | 9.37 | 26.81 |
| Buried-120N | 42.58 | 11.21 | 25.90 |
| Buried-120N-DCD | 45.93 | 9.70 | 27.61 |
| SEM b | 3.80 | 0.466 | 3.321 |
| p values c | 0.376 | <0.001 | 0.991 |
| Contrasts | p values | ||
| CONT vs. All | 0.072 | 0.266 | 0.669 |
| Suspended-60N-DCD vs. Suspended-60N | 0.198 | <0.001 | 0.752 |
| Suspended-120N-DCD vs. Suspended-120N | 0.549 | <0.001 | 0.864 |
| Buried-60N-DCD vs. Buried-60N | 0.825 | 0.005 | 0.409 |
| Buried-120N-DCD vs. Buried-120N | 0.548 | <0.001 | 0.794 |
| Buried-60N-DCD vs. Buried-120N | 0.487 | 0.001 | 0.768 |
| Buried-60N-DCD vs. Suspended-120N | 0.354 | 0.010 | 0.669 |
| Suspended-60N-DCD vs. Suspended-120N | 0.894 | 0.046 | 0.811 |
| 2016 | 2017 | 2018 | ||||
|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | NH4+-N | NO3−-N | NH4+-N | NO3−-N | |
| Treatment | 0.081 a | <0.001 | 0.589 | 0.104 | 0.762 | <0.001 |
| Depth | <0.001 | <0.001 | <0.001 | 0.004 | 0.009 | <0.001 |
| Treatment × Depth | 0.001 | <0.001 | 0.863 | 0.175 | 0.885 | <0.001 |
| Season | 0.070 | <0.001 | 0.174 | <0.001 | <0.001 | <0.001 |
| Treatment × Season | 0.004 | <0.001 | 0.678 | 0.009 | 0.156 | <0.001 |
| Depth × Season | 0.002 | <0.001 | 0.127 | 0.011 | 0.001 | <0.001 |
| Treatment × Depth × Season | <0.001 | 0.001 | 0.7867 | 0.125 | 0.722 | <0.001 |
| 2016 | 2017 | 2018 | ||||
|---|---|---|---|---|---|---|
| pH | EC | pH | EC | pH | EC | |
| Treatment | 0.002 a | 0.016 | 0.045 | 0.854 | 0.008 | 0.899 |
| Depth | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Treatment × Depth | 0.833 | 0.313 | 0.729 | 0.974 | 0.339 | 0.657 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by Her Magesty the Queen in Right of Canada as represented by the Ministry of Agriculture and Agri-Food Canada. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messiga, A.J.; Nyamaizi, S.; Yu, S.; Dorais, M. Blueberry Yield and Soil Mineral Nitrogen Response to Nitrogen Fertilizer and Nitrification Inhibitors under Drip-Fertigation Systems. Agronomy 2021, 11, 2144. https://doi.org/10.3390/agronomy11112144
Messiga AJ, Nyamaizi S, Yu S, Dorais M. Blueberry Yield and Soil Mineral Nitrogen Response to Nitrogen Fertilizer and Nitrification Inhibitors under Drip-Fertigation Systems. Agronomy. 2021; 11(11):2144. https://doi.org/10.3390/agronomy11112144
Chicago/Turabian StyleMessiga, Aimé J., Sylvia Nyamaizi, Shaobing Yu, and Martine Dorais. 2021. "Blueberry Yield and Soil Mineral Nitrogen Response to Nitrogen Fertilizer and Nitrification Inhibitors under Drip-Fertigation Systems" Agronomy 11, no. 11: 2144. https://doi.org/10.3390/agronomy11112144





