Effects of Different Wheat Tissues on the Population Parameters of the Fall Armyworm (Spodoptera frugiperda)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Host Plants
2.2. Insects
2.3. Life Table Trials
2.4. Life Table Analysis
2.5. Statistical Analysis
3. Results
3.1. Basic Life History Statistics of the FAW
3.2. Life Table Analysis
3.3. Effects on FAW Population Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters and Equation | Explanation |
|---|---|
| Age-stage survival rate: n01 is the number of eggs used for life table study and nxj is the number of surviving individuals at age x and stage j [28]. | |
| Nf /N, Nm/N | Nf /N: the proportion of female adults emerged from the N eggs used at the beginning of life table study. Nm/N: the proportion of male adults. |
| Age-specific survival rate: where k is the number of stages. | |
| Age-stage-specific fecundity of individuals of nxj. Because only female adults (the 10th stage) lay eggs, there is only fx10 in this study [32,33]; therefore, fx10 is the female age-stage-specific fecundity. | |
| Age-specific fecundity of cohort: where k is the number of stages [32]. It is the mean fecundity of surviving individuals. | |
| Age-specific net maternity: the mean fecundity of the cohort at age x when the survival rate is considered [34]. | |
| Net reproductive rate: the total offspring produced by an average individual during its lifetime. It is the sum of lxmx over all age groups. | |
| The Euler-Lotka equation was used to calculate the intrinsic rate of increase (r) with the age indexed from 0 [32]. | |
| Mean generation time. | |
| Finite rate of increase | |
| Age-stage life expectancy: is the probability that an individual of age x and stage j will survive to age i and stage y by assuming sxj = 1 [26]. |
| Index | Mean ± SE | Index | Mean ± SE |
|---|---|---|---|
| Larval stage duration | 16.31 ± 0.15 | Pupae weight (mg) | 180.30 ± 16.11 |
| Pupae stage duration | 10.95 ± 0.56 | Fecundity (eggs/female) | 825 ± 32 |
| Adult longevity | 13.06 ± 0.27 | R0 | 363.21 ± 31.56 |
| Nf:Nm | 92:88 | λ | 1.1978 ± 0.0037 |
| Nf/N | 0.4402 ± 0.0342 | r | 0.1805 ± 0.0031 |
| Nm/N | 0.4210 ± 0.0341 | T | 33.50 ± 0.24 |
References
- Luginbill, P. The Fall Army Worm; Technical Bulletins No.34; United States Department of Agriculture: Washington, DC, USA, 1928. [Google Scholar]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–86. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. Insect. Sci. Appl. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Meagher, R.L. Review of fall armyworm (Lepidoptera: Noctuidae) genetic complexity and migration. Fla. Entomol. 2008, 91, 546–554. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management (First Edition). 2018. Available online: https://www.usaid.gov/sites/default/files/documents/1867/Fall-Armyworm-IPM-Guide-for-Africa-Jan_30-2018.pdf (accessed on 25 June 2021).
- FAO. First Detection of Fall Army Worm on the Border of Thailand [2018-12-20]. 2018. Available online: https://www.ippc.int/en/countries/thailand/pestreports/2018/12/first-detection-of-fall-army-worm-onthe-border-of-tailan-d (accessed on 15 June 2021).
- Nakweta, G. Global Actions Needed to Combat Fall Armyworm. 2018. Available online: https://www.scidev.net/subsaharan-africa/farming/news/global-actions-combat-fallarmyworm.html (accessed on 8 October 2021).
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant. Prot. 2020, 46, 1–5. [Google Scholar]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant. Prot. 2019, 45, 10–19. [Google Scholar]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Westbrook, J.; Fleischer, S.; Jairam, S.; Meagher, R.; Nagoshi, R. Multigenerational migration of fall armyworm, a pest insect. Ecosphere 2019, 10, e02919. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.M.d.; Bueno, A.d.F.; Andrade, K.; Stecca, C.d.S.; Neves, P.M.O.J.; Oliveira, M.C.d. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci. Agric. 2017, 74, 18–31. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Y.F.; Sun, X.X.; Shen, X.J.; Wu, Q.L.; Zhang, H.W.; Zhang, D.D.; Zhao, S.Y.; Liang, G.M.; Wu, K.M. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, P.J.; Chen, Z.Y.; Ba, T.X.; Jiang, Y.Y.; Mu, C.Q.; Guo, S.C.; Wang, S.L.; Lu, R.G.; Qi, J.F.; et al. Observation on the damage char-acteristics of Spodoptera frugiperda to wheat in middle and late stages. Plant. Prot. 2021, 47, 297–301. [Google Scholar] [CrossRef]
- Scriber, J.M.; Slansky, F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Ba, T.X.; Zhang, Y.H.; Zhang, Z.; Guan, D.D.; Li, C.C.; Ji, S.Y.; Yin, X.T.; Zhang, A.H.; Tang, Q.B.; Liu, Y.H.; et al. The host preference and population life tables of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on maize and wheat. Plant. Prot. 2020, 46, 17–23. [Google Scholar] [CrossRef]
- He, L.M.; Wang, T.L.; Chen, Y.C.; Ge, S.S.; Wyckhuys, K.A.G.; Wu, K.M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 736–744. [Google Scholar] [CrossRef]
- Capinera, J.L. Featured creatures: Fall armyworm. 2017. Available online: https://entnemdept.ufl.edu/creatures/field/fall_armyworm.htm (accessed on 8 October 2021).
- Chi, H. TWOSEX-MS Chart Version 2021.06.21. 2021. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 8 October 2021).
- Chi, H.; Liu, H. Two new methods for study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 2, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect. Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Chen, Z.Z.; Zheng, F.Q.; Shi, A.J.; Guo, T.T.; Yeh, B.H.; Chi, H.; Xu, Y.Y. Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: A case study of Chrysopa pallens (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.S.; Rodrigues, D.; Stireman III, J.O.; Carrière, Y. Roles of food quality and enemy-free space in hos use by a generalist insect herbivore. Ecology 2004, 85, 2747–2753. [Google Scholar] [CrossRef]
- Sokame, B.M.; Musyoka, B.; Obonyo, J.; Rebaudo, F.; Abdel-Rahman, E.M.; Subramanian, S.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Impact of an exotic invasive pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on resident communities of pest and natural enemies in maize fields in Kenya. Agronomy 2021, 11, 1074. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chen, G.-M.; Chi, H.; Wang, R.-C.; Wang, Y.-P.; Xu, Y.-Y.; Li, X.-D.; Yin, P.; Zheng, F.-Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef]
- Carey, J.M. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
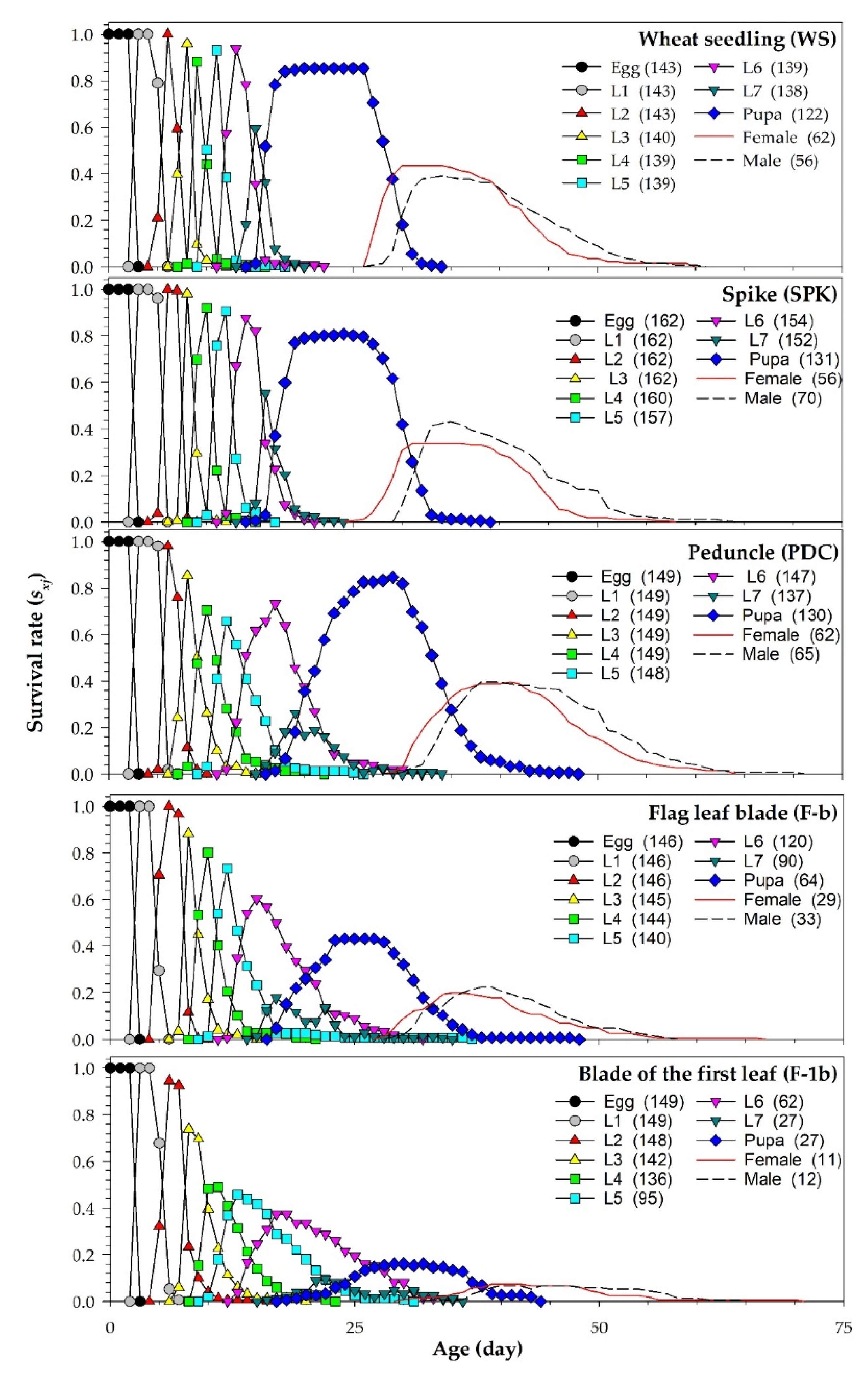
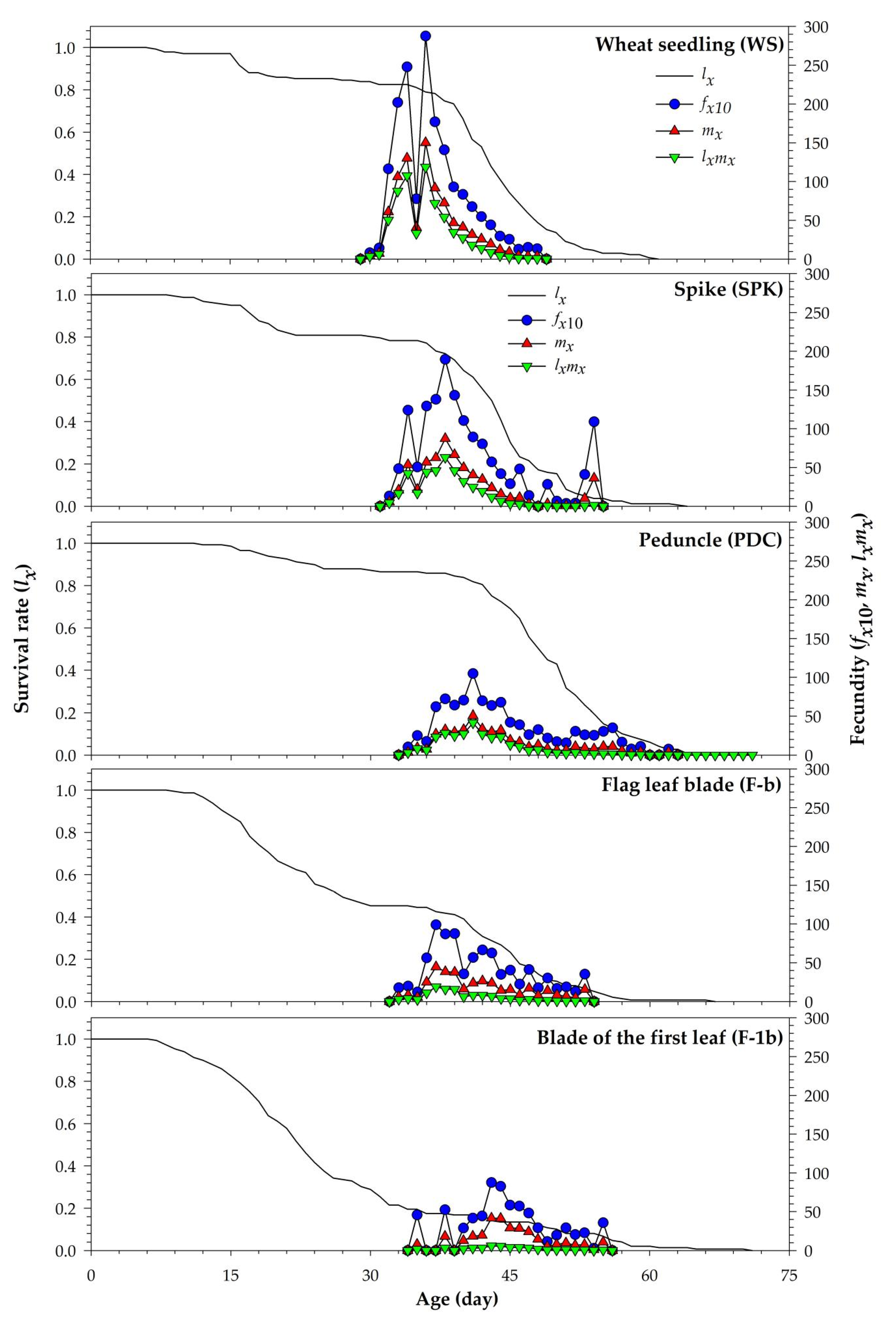

| Stages | Developmental Duration (Mean ± SE) (Day) | ||||
|---|---|---|---|---|---|
| WS | SPK | PDC | F-b | F-1b | |
| 1st instar | 2.79 ± 0.03 b | 2.96 ± 0.01 a | 3.00 ± 0.02 a | 2.29 ± 0.04 c | 2.72 ± 0.04 b |
| 2nd instar | 1.81 ± 0.03 d | 2.05 ± 0.02 c | 1.89 ± 0.05 d | 2.79 ± 0.05 a | 2.60 ± 0.09 b |
| 3rd instar | 1.52 ± 0.05 c | 1.32 ± 0.04 d | 2.03 ± 0.08 b | 1.65 ± 0.08 c | 2.53 ± 0.13 a |
| 4th instar | 1.45 ± 0.05 d | 1.90 ± 0.03 c | 2.41 ± 0.08 b | 2.17 ± 0.07 b | 2.68 ± 0.10 a |
| 5th instar | 1.91 ± 0.03 c | 2.16 ± 0.04 c | 2.90 ± 0.08 b | 2.62 ± 0.09 b | 3.99 ± 0.19 a |
| 6th instar | 2.79 ± 0.04 d | 3.28 ± 0.06 c | 5.03 ± 0.11 b | 5.46 ± 0.28 b | 7.19 ± 0.37 a |
| Pre-pupa | 1.30 ± 0.04 b | 1.35 ± 0.04 b | 1.48 ± 0.05 b | 1.52 ± 0.09 a | 1.48 ± 0.14 b |
| Larva | 13.48 ± 0.07 e | 14.85 ± 0.10 d | 18.81 ± 0.26 b | 17.16 ± 0.34 c | 21.44 ± 0.59 a |
| Pupa | 12.73 ± 0.11 a | 12.75 ± 0.11 a | 12.75 ± 0.09 a | 12.37 ± 0.13 a | 12.43 ± 0.27 a |
| Pre-adult | 29.19 ± 0.14 e | 30.59 ± 0.17 d | 34.50 ± 0.28 b | 32.26 ± 0.32 c | 37.04 ± 0.74 a |
| Adult | 15.58 ± 0.47 a | 14.72 ± 0.45 a | 15.99 ± 0.45 a | 14.32 ± 0.66 a | 16.52 ± 1.30 a |
| APOP | 6.08 ± 0.33 b | 7.14 ± 0.41 a | 7.76 ± 0.57 a | 7.34 ± 0.62 a | 8.18 ± 1.28 a |
| TPOP | 34.19 ± 0.26 d | 36.32 ± 0.34 c | 41.39 ± 0.61 a | 38.55 ± 0.71 b | 43.55 ± 1.63 a |
| Oviposition days | 6.63 ± 0.35 a | 5.75 ± 0.28 b | 5.55 ± 0.34 b | 5.21 ± 0.54 b | 5.55 ± 0.99 b |
| Female longevity | 15.45 ± 0.63 a | 15.00 ± 0.52 a | 15.77 ± 0.60 a | 14.47 ± 0.99 a | 17.27 ± 1.78 a |
| Male longevity | 15.71 ± 0.69 a | 14.49 ± 0.70 a | 16.23 ± 0.66 a | 14.17 ± 0.84 a | 15.83 ± 1.89 a |
| Index | Treatments | ||||
|---|---|---|---|---|---|
| WS | SPK | PDC | F-b | F-1b | |
| Pupae weight (mg) | 230.38 ± 2.06 a | 186.33 ± 2.70 b | 154.52 ± 2.66 c | 122.31 ± 3.90 d | 127.84 ± 6.06 d |
| Fecundity (eggs/female) | 1488 ± 92 a | 1108 ± 64 b | 698 ± 58 c | 590 ± 79 c | 561 ± 110 c |
| Index | Treatments | ||||
|---|---|---|---|---|---|
| WS | SPK | PDC | F-b | F-1b | |
| Nf:Nm | 62:56 | 56:70 | 62:65 | 29:33 | 11:12 |
| Nf/N | 0.43 ± 0.04 a | 0.35 ± 0.04 a | 0.42 ± 0.04 a | 0.19 ± 0.03 b | 0.07 ± 0.02 c |
| Nm/N | 0.39 ± 0.04 a | 0.43 ± 0.04 a | 0.44 ± 0.04 a | 0.23 ± 0.03 b | 0.08 ± 0.02 c |
| Index | Treatments | ||||
|---|---|---|---|---|---|
| WS | SPK | PDC | F-b | F-1b | |
| R0 | 645.1748 ± 73.1169 a | 383.1481 ± 47.0621 b | 290.7987 ± 37.1213 b | 117.2877 ± 24.9445 c | 41.4430 ± 14.3762 d |
| λ | 1.1954 ± 0.0045 a | 1.1681 ± 0.0043 b | 1.1459 ± 0.0042 c | 1.1270 ± 0.0065 d | 1.0878 ± 0.0099 e |
| r | 0.1785 ± 0.0038 a | 0.1554 ± 0.0037 b | 0.1362 ± 0.0036 c | 0.1196 ± 0.0058 d | 0.0841 ± 0.0091 e |
| T | 36.2480 ± 0.2719 e | 38.2740 ± 0.3487 c | 41.6423 ± 0.4825 b | 39.8491 ± 0.6113 d | 44.2694 ± 1.1160 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Batuxi; Jiang, Y.; Li, X.; Zhang, A.; Zhu, X.; Zhang, Y. Effects of Different Wheat Tissues on the Population Parameters of the Fall Armyworm (Spodoptera frugiperda). Agronomy 2021, 11, 2044. https://doi.org/10.3390/agronomy11102044
Zhang Z, Batuxi, Jiang Y, Li X, Zhang A, Zhu X, Zhang Y. Effects of Different Wheat Tissues on the Population Parameters of the Fall Armyworm (Spodoptera frugiperda). Agronomy. 2021; 11(10):2044. https://doi.org/10.3390/agronomy11102044
Chicago/Turabian StyleZhang, Zhi, Batuxi, Yanan Jiang, Xiangrui Li, Aihuan Zhang, Xun Zhu, and Yunhui Zhang. 2021. "Effects of Different Wheat Tissues on the Population Parameters of the Fall Armyworm (Spodoptera frugiperda)" Agronomy 11, no. 10: 2044. https://doi.org/10.3390/agronomy11102044
APA StyleZhang, Z., Batuxi, Jiang, Y., Li, X., Zhang, A., Zhu, X., & Zhang, Y. (2021). Effects of Different Wheat Tissues on the Population Parameters of the Fall Armyworm (Spodoptera frugiperda). Agronomy, 11(10), 2044. https://doi.org/10.3390/agronomy11102044







