Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Amendments Preparation
2.2. Pot Experiment Preparation
2.3. Plant Biomass
2.4. Soil Sampling and Preparation
2.5. Chemical, Biological, and Statistical Analysis
3. Results
3.1. Composition of Modified Digestates
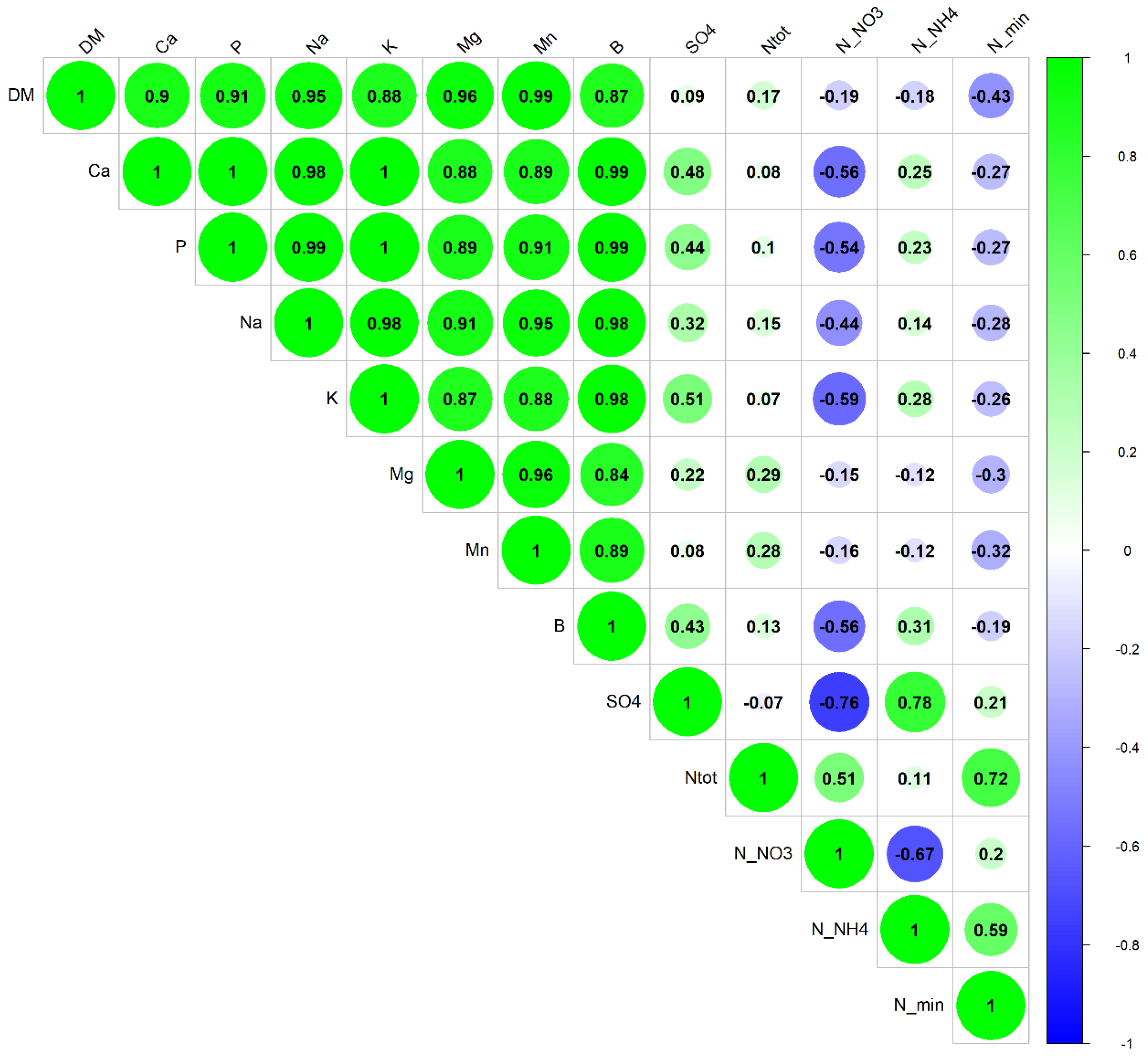
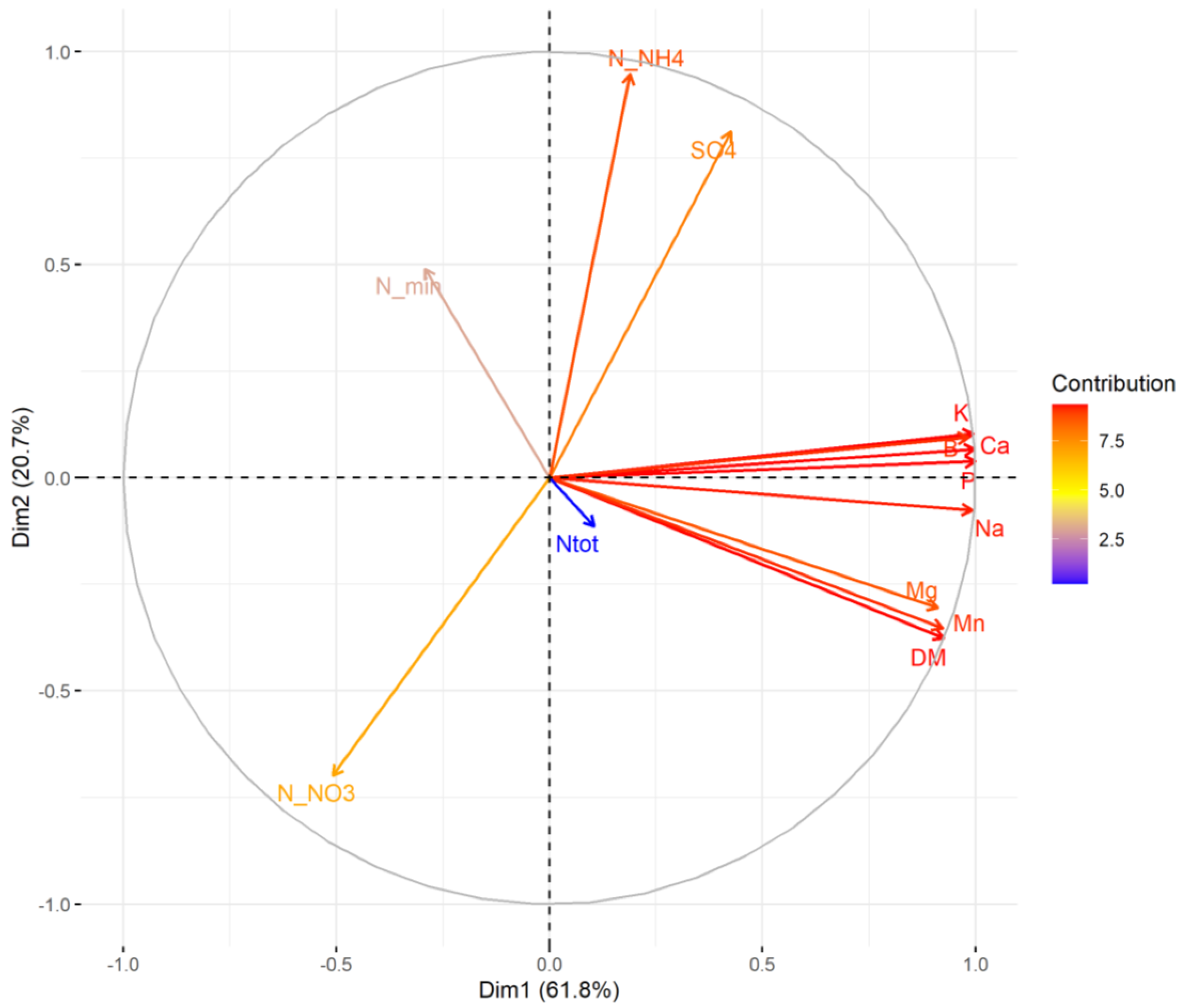
3.2. Soil Nutrients, Microbial Abundance, and Plant Biomass
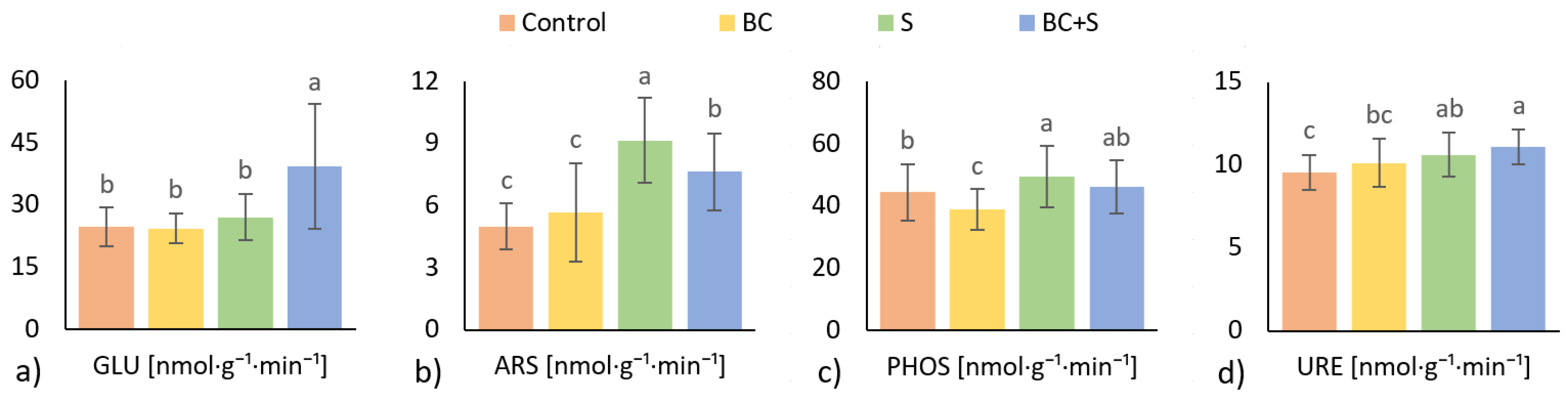
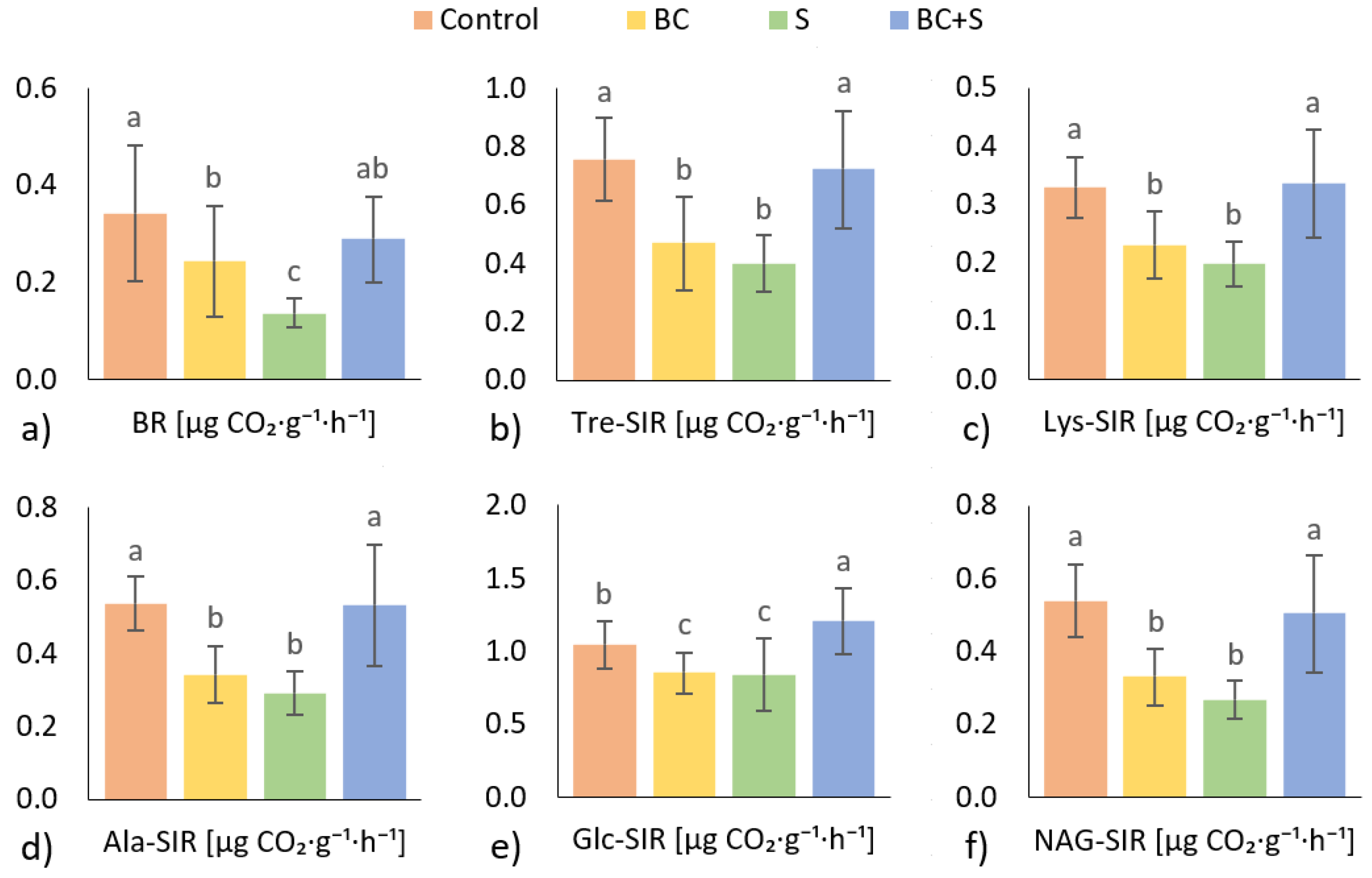
3.3. Soil Microbial Activity
4. Discussion
4.1. Modified Digestates
4.2. Soil Nutrients, Microbial Abundance, and Plant Biomass
4.3. Soil Microbial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Pawlett, M.; Owen, A.; Tibbett, M. Amenity grassland quality following anaerobic digestate application. Grassl. Sci. 2018, 64, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source-Review, in Biogas, Dr. Sunil Kumar; IntechOpen: London, UK, 2012; pp. 295–310. ISBN 978-953-51-0204-5. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Acea, M.J.; Carballas, T. Microbial biomass and its contribution to nutrient concentrations in forest soils. Soil Biol. Biochem. 1993, 25, 25–31. [Google Scholar] [CrossRef]
- Khan, S.; Hesham, A.E.L.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on soil microbial community structure and activities, Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, S.; Tinello, A.; Cavinato, C.; Zabeo, A.; Semenzin, E. Sustainability assessment of two digestate treatments: A comparative life cycle assessment. Environ. Eng. Manag. J. 2019, 18, 2193–2202. [Google Scholar]
- Möller, K.; Stinner, W. Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur. J. Agron. 2009, 30, 1–16. [Google Scholar] [CrossRef]
- Knudsen, M.; Kristensen, I.; Berntsen, J.; Petersen, B.; Kristensen, E. Estimated N leaching losses for organic and conventional farming in Denmark. J. Agric. Sci. 2006, 144, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Rubæk, G.; Henriksen, K.; Petersen, J.; Rasmussen, B.; Sommer, S. Effects of application technique and anaerobic digestion on gaseous nitrogen loss from animal slurry applied to ryegrass (Lolium perenne). J. Agric. Sci. 1996, 126, 481–492. [Google Scholar] [CrossRef]
- Wulf, S.; Maeting, M.; Clemens, J. Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading: I. Ammonia volatilization. J. Environ. Qual. 2002, 31, 1789–1794. [Google Scholar] [CrossRef]
- Maucieri, C.; Barbera, A.; Borin, M. Effect of injection depth of digestate liquid fraction on soil carbon dioxide emission and maize biomass production. Ital. J. Agron. 2016, 11, 6. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.L.; Clarke, M.L.; Othman, M.; Ramsden, S.J.; West, H.M. Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenerg 2015, 79, 39–49. [Google Scholar] [CrossRef]
- Dicke, C.; Andert, J.; Ammon, C.; Kern, J.; Meyer-Aurich, A.; Kaupenjohann, M. Effects of different biochars and digestate on N2O fluxes under field conditions. Sci. Total Environ. 2015, 524–525, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Plaimart, J.; Acharya, K.; Mrozik, W.; Davenport, R.J.; Vinitnantharat, S.; Werner, D. Coconut husk biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ. Pollut. 2021, 268, 115684. [Google Scholar] [CrossRef] [PubMed]
- Elbashier, M.M.A.; Xiaohou, S.; Ali, A.A.S.; Mohmmed, A. Effect of digestate and biochar amendments on photosynthesis rate, growth parameters, wateruse efficiency and yield of Chinese melon (Cucumis melo L.) under saline irrigation. Agronomy 2018, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Izaurralde, R.C.; McGill, W.B.; Williams, J.R. Development and application of the EPIC model for carbon cycle, greenhouse-gas mitigation, and biofuel studies. In Managing Agricultural Greenhouse Gases: Coordinated Agricultural Research through GRACEnet to Address Our Changing Climate, 1st ed.; Liebig, M.M., Franzluebbers, A.J., Follett, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123868978. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Kimetu, J.; Lehmann, J. Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Soil Res. 2010, 48, 577–585. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Brewer, C.; Unger, R.; Schmidt-Rohr, K.; Brown, R.C. Criteria to select biochars for field studies based on biochar chemical properties. Bioenerg. Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. Glob Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- José, M.; Knicker, H. Bioavailability of N released from N-rich pyrogenic organic matter: An incubation study. Soil Biol. Biochem. 2011, 43, 2368–2373. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J.; Ok, Y.S. Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J Env. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef]
- Scherer, W.H. Sulfur in soils. J. Plant. Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhao, F.J.; McGrath, S.P. Sulphur fractionation in calcareous soils and bioavailability to plants. Plant Soil 2005, 268, 103–109. [Google Scholar] [CrossRef]
- Wainwright, M.; Nevell, W.; Grayston, S.J. Effects of organic matter on sulphur oxidation in soil and influence of sulphur oxidation on soil nitrification. Plant Soil 1986, 96, 369–376. [Google Scholar] [CrossRef]
- ISO 11465: 1993. Soil Quality—Determination of Dry Matter and Water Content on A Mass Basis—Gravimetric Method; International Organization for Standardization: Geneva, Switzerland, 1993. [Google Scholar]
- ISO 13878: 1998. Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis); International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- ISO 15476: 2009. Fertilizers—Determination of Nitric and Ammoniacal Nitrogen According to Devarda; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 10084: 1992. Solid Fertilizers—Determination of Mineral-Acid-Soluble Sulfate Content—Gravimetric Method; International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- ISO 6878: 2004. Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Spencer, R.R.; Erdmann, D.E. Azomethine H colorimetric method for determining dissolved boron in water. Environ. Sci. Technol. 1979, 13, 954–956. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Richards, J.R.; Payton, M.E. Interlaboratory validation of the Mehlich 3 method as a universal extractant for plant nutrients. J. AOAC Int. 2019, 92, 995–1008. [Google Scholar] [CrossRef] [Green Version]
- Holatko, J.; Hammerschmiedt, T.; Datta, R.; Baltazar, T.; Kintl, A.; Latal, O.; Pecina, V.; Sarec, P.; Novak, P.; Balakova, L.; et al. Humic acid mitigates the negative effects of high rates of biochar application on microbial activity. Sustainability 2020, 12, 9524. [Google Scholar] [CrossRef]
- ISO 10694: 1995. Soil Quality–Determination of Organic and Total Carbon after Dry Combustion (Elemental Analysis); International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- ISO 20130: 2018. Soil quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colorimetric Substrates in Micro-Well Plates; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.G.; Prasad, D. Biosolids from two-stage bioleaching could produce compost for unrestricted use. Environ. Technol. 2006, 27, 665–672. [Google Scholar] [CrossRef]
- Sears, K.; Alleman, J.E.; Barnard, J.L.; Oleszkiewicz, J.A. Impacts of reduced sulfur components on active and resting ammonia oxidizers. J. Ind. Microbiol. Biotechnol. 2004, 31, 369–378. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manage. 2017, 61, 138–149. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Yun, S.N.; Wang, Z.Q.; Zhang, Y.W.; Zhang, X.M.; Wang, K.J. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Renew. Sust. Energ. Rev. 2021, 135, 16. [Google Scholar] [CrossRef]
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004, 55, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Gharmakher, N.H.; Machet, J.M.; Beaudoin, N.; Recous, S. Estimation of sulfur mineralization and relationships with nitrogen and carbon in soils. Biol. Fertil. Soils 2009, 45, 297–304. [Google Scholar] [CrossRef]
- Saren, S.; Burman, S.; Mishra, A.; Saha, D. Effect of added organic matter and sulphur on transformation of different fractions of sulphur in soil. Bioscan 2016, 11, 2399–2403. [Google Scholar]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef] [Green Version]
- Soaud, A.A.; Saleh, M.E.; El-Tarabily, K.A.; Azirun, M.S.; Rahman, M.M. Effect of elemental sulfur application on ammonia volatilization from surface applied urea fertilizer to calcareous sandy soils. Aust. J. Crop Sci. 2011, 5, 611–619. [Google Scholar]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 10. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Castellarin, J.; Miralles, D.; Pedrol, H. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Res. 2009, 113, 170–177. [Google Scholar] [CrossRef]
- Lee, M.S.; Urgun-Demirtas, M.; Shen, Y.; Zumpf, C.; Anderson, E.K.; Rayburn, A.L.; Lee, D.K. Effect of digestate and digestate supplemented with biochar on switchgrass growth and chemical composition. Biomass Bioenerg. 2021, 144, 9. [Google Scholar] [CrossRef]
- LeBauer, D.S. Litter degradation rate and beta-glucosidase activity increase with fungal diversity. Can. J. For. Res. 2010, 40, 1076–1085. [Google Scholar] [CrossRef]
- Castellano, S.D.; Dick, R.P. Cropping and sulfur fertilization influence on sulfur transformations in soil. Soil Sci. Soc. Am. J. 1991, 55, 114–121. [Google Scholar] [CrossRef]
- Godlewska, A. Assessment of the effect of NPK fertilisation and elemental sulphur on soil enzyme activity. Fresenius Environ. Bull. 2018, 27, 180–186. [Google Scholar]
- Ye, R.Z.; McCray, J.M.; Wright, A.L. Microbial response of a calcareous histosol to sulfur amendment. Soil Sci. 2011, 176, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Grego, S.; Ceccanti, B. Ecological significance of the biological activity in soil. In Soil Biochemistry; Bollag, J.M., Stotzky, G., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1990; Volume 6, pp. 293–355. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Blennerhassett, J.D.; Quin, B.F. Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Page, A.L.; Miller, R.H.; Keeney, D.R. Soil respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar] [CrossRef]
- Mukherjee, S.; Weihermueller, L.; Tappe, W.; Vereecken, H.; Burauel, P. Microbial respiration of biochar- and digestate-based mixtures. Biol. Fertil. Soils 2016, 52, 151–164. [Google Scholar] [CrossRef]
- Visser, S.; Parkinson, D. Microbial respiration and biomass in soil of a lodgepole pine stand acidified with elemental sulfur. Can. J. For. Res.-Rev. Can. Rech. For. 1989, 19, 955–961. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Monreal, M.A.; Clayton, G.W.; Grant, C.A.; Johnston, A.M.; Rice, W.A. Soil microbial biomass and diversity respond to tillage and sulphur fertilizers. Can. J. Soil Sci. 2001, 81, 577–589. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Digestate [L·Barrel−1] | Biochar [kg·Barrel−1] | Sulphur [g·Barrel−1] |
|---|---|---|---|
| Control | 10 | 0 | 0 |
| Biochar (BC) | 10 | 4 | 0 |
| Sulphur (S) | 10 | 0 | 8 |
| Biochar + sulphur (BC + S) | 10 | 4 | 8 |
| Property | Abbrev. | Method | Unit | Reference |
|---|---|---|---|---|
| Dry matter | DM | Gravimetric, on a mass basis | % | [31] |
| Total nitrogen | Ntotal | Dry combustion using TruSpec analyzer (LECO, USA) | g·kg−1 | [32] |
| Nitrate nitrogen | N-NO3− | Reduction using Devarda’s alloy | g·kg−1 | [33] |
| Ammonium nitrogen | N-NH4+ | Reduction using Devarda’s alloy | g·kg−1 | [34] |
| Mineral nitrogen | Nmin | Sum of N-NO3- and N-NH4+ | g·kg−1 | - |
| Sulphur in sulphates | S-SO42− | Acid-soluble sulphates | mg·kg−1 | [35] |
| Boron | B | Azomethine H colorimetric method | mg·kg−1 | [36] |
| Nutrients | P, Ca, Na, K, Mg | Atomic absorption spectrometry (AAS) in Mehlich 3 extract | g·kg−1 | [37] |
| Mn | mg·kg−1 |
| pH [-] | TC [%] | TN [%] | Nmin [mg·kg−1] | N-NO3 [mg·kg−1] | N-NH4 [mg·kg−1] |
|---|---|---|---|---|---|
| 7.29 | 1.40 | 0.16 | 62.84 | 56.80 | 6.04 |
| C:N [-] | S [%] | K [mg·kg−1] | Ca [mg·kg−1] | Mg [mg·kg−1] | P [mg·kg−1] |
| 8.77 | 0.01 | 231 | 3259 | 236 | 97 |
| Property | Method | Unit | Reference |
|---|---|---|---|
| Total soil carbon | Dry combustion using, TruSpec analyzer (LECO, USA) | mg·g−1 | [39] |
| Total soil nitrogen | [32] | ||
| Microbial biomass carbon | Fumigation extraction method | mg·g−1 | [40] |
| Soil enzyme activities | Microplate incubation, UV-Vis spectrophotometry | µmol PNP·g−1·min−1, µmol NH3·g−1·min−1 | [41] |
| Basal soil respiration | MicroResp® device (The James Hutton Institute, UK) | μg CO2·g−1·h−1 | [42] |
| Substrate induced soil respiration | MicroResp® device + inducers (sugars, amino acids) | ||
| Processing | Tool | Method | Reference |
| Statistical analysis | Program R version 3.6.1. | Multivariate analysis of variance (MANOVA), principal component analysis (PCA), one-way analysis of variance (ANOVA), Tukey’s range test, Pearson correlation analysis | [38] |
| Variable | Unit | Control | BC | S | BC + S |
|---|---|---|---|---|---|
| Ca | [g·kg−1] | 1.15 c ± 0.08 | 3.17 b ± 0.28 | 1.67 c ± 0.10 | 4.87 a ± 0.38 |
| P | [g·kg−1] | 0.76 c ± 0.04 | 2.09 b ± 0.16 | 1.01 c ± 0.08 | 3.15 a ± 0.26 |
| Na | [g·kg−1] | 0.36 c ± 0.02 | 0.92 b ± 0.06 | 0.35 c ± 0.02 | 1.26 a ± 0.10 |
| K | [g·kg−1] | 1.35 c ± 0.11 | 3.63 b ± 0.23 | 2.11 c ± 0.16 | 5.65 a ± 0.43 |
| Mg | [g·kg−1] | 0.09 b ± 0.01 | 0.23 a ± 0.02 | 0.12 b ± 0.01 | 0.22 a ± 0.02 |
| Mn | [mg·kg−1] | 3.99 b ± 0.32 | 10.80 a ± 0.75 | 3.01 b ± 0.21 | 10.99 a ± 0.88 |
| B | [mg·kg−1] | 2.62 c ± 0.21 | 4.65 b ± 0.38 | 2.54 c ± 0.20 | 7.23 a ± 0.57 |
| DM | [%] | 5.96 b ± 0.11 | 15.00 a ± 0.36 | 5.78 b ± 0.13 | 15.05 a ± 0.40 |
| Ntotal | [g·kg−1] | 43.10 a ± 3.47 | 44.50 a ± 3.59 | 40.60 a ± 3.19 | 41.2 a ± 3.20 |
| N-NO3− | [g·kg−1] | 2.20 a ± 0.15 | 2.51 a ± 2.20 | 1.64 b ± 0.12 | 0.79 c ± 0.05 |
| N-NH4+ | [g·kg−1] | 5.17 a,b ± 0,42 | 4.02 b ± 0.32 | 5.45 a ± 0.39 | 5.96 a ± 0.45 |
| Nmin | [g·kg−1] | 7.37 a ± 0,55 | 6.53 a ± 0.53 | 7.09 a ± 0.57 | 6.75 a ± 0.52 |
| S-SO42− | [mg·kg−1] | 8.52 b ± 0.69 | 9.00 b ± 0.70 | 13.89 a ± 1.12 | 14.38 a ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammerschmiedt, T.; Holatko, J.; Sudoma, M.; Kintl, A.; Vopravil, J.; Ryant, P.; Skarpa, P.; Radziemska, M.; Latal, O.; Brtnicky, M. Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth. Agronomy 2021, 11, 2041. https://doi.org/10.3390/agronomy11102041
Hammerschmiedt T, Holatko J, Sudoma M, Kintl A, Vopravil J, Ryant P, Skarpa P, Radziemska M, Latal O, Brtnicky M. Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth. Agronomy. 2021; 11(10):2041. https://doi.org/10.3390/agronomy11102041
Chicago/Turabian StyleHammerschmiedt, Tereza, Jiri Holatko, Marek Sudoma, Antonin Kintl, Jan Vopravil, Pavel Ryant, Petr Skarpa, Maja Radziemska, Oldrich Latal, and Martin Brtnicky. 2021. "Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth" Agronomy 11, no. 10: 2041. https://doi.org/10.3390/agronomy11102041
APA StyleHammerschmiedt, T., Holatko, J., Sudoma, M., Kintl, A., Vopravil, J., Ryant, P., Skarpa, P., Radziemska, M., Latal, O., & Brtnicky, M. (2021). Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth. Agronomy, 11(10), 2041. https://doi.org/10.3390/agronomy11102041









