Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash
Abstract
1. Introduction
2. Soil Acidification
Aluminium Phytotoxicity
3. Phosphorus
3.1. Significance of Phosphorus in Plants
3.2. Dynamics of Phosphorus in Soil–Plant Systems
3.3. Phosphorus Forms in Soils
3.4. Phosphorus Pools
4. Phosphorus Retention and Release Mechanisms in Soils
4.1. Precipitation and Adsorption Reactions
4.2. Dissolution, Desorption, and Mineralisation Reactions
5. Factors Affecting Phosphorus Availability in Soils
5.1. Clay Content and Mineralogy
5.2. Organic Matter
5.3. Soil pH, Exchangeable Aluminium, Iron and Calcium Concentration
6. Potential of Using Organic Amendments to Mitigate Productivity of Phosphorus Fixing Soils
6.1. Compost
6.2. Animal Manure
6.3. Biochar
6.4. Crop Residues
7. Charcoal and Its Properties
Effects of Charcoal in Agriculture
8. Wood Ash and Its Properties
Effects of Wood Ash in Agriculture
9. Mechanisms of Improving Phosphorus Availability Using Charcoal and Wood Ash as Organic and Inorganic Soil Amendments
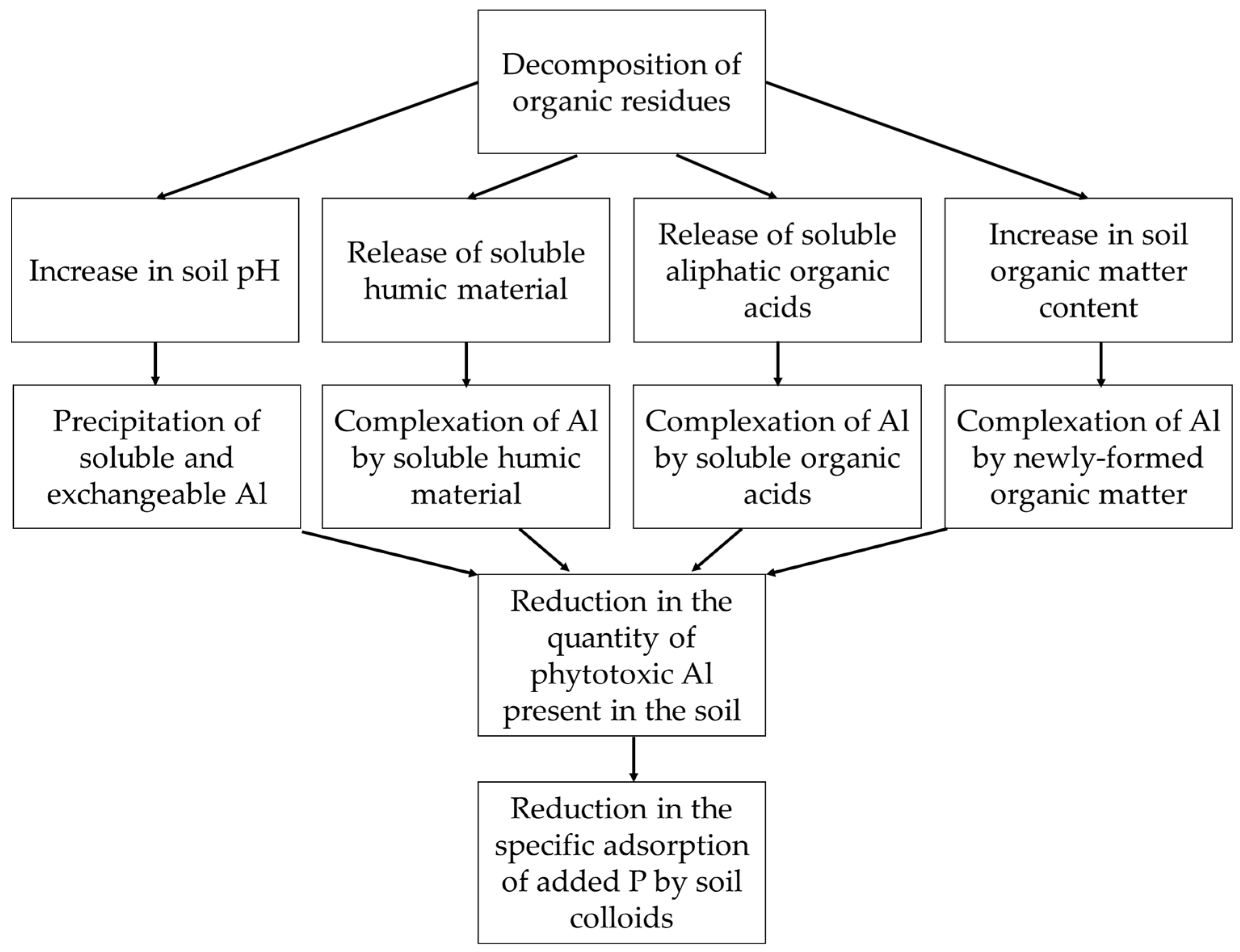
9.1. Amelioration of Soil Acidity upon Application of Charcoal and Wood Ash
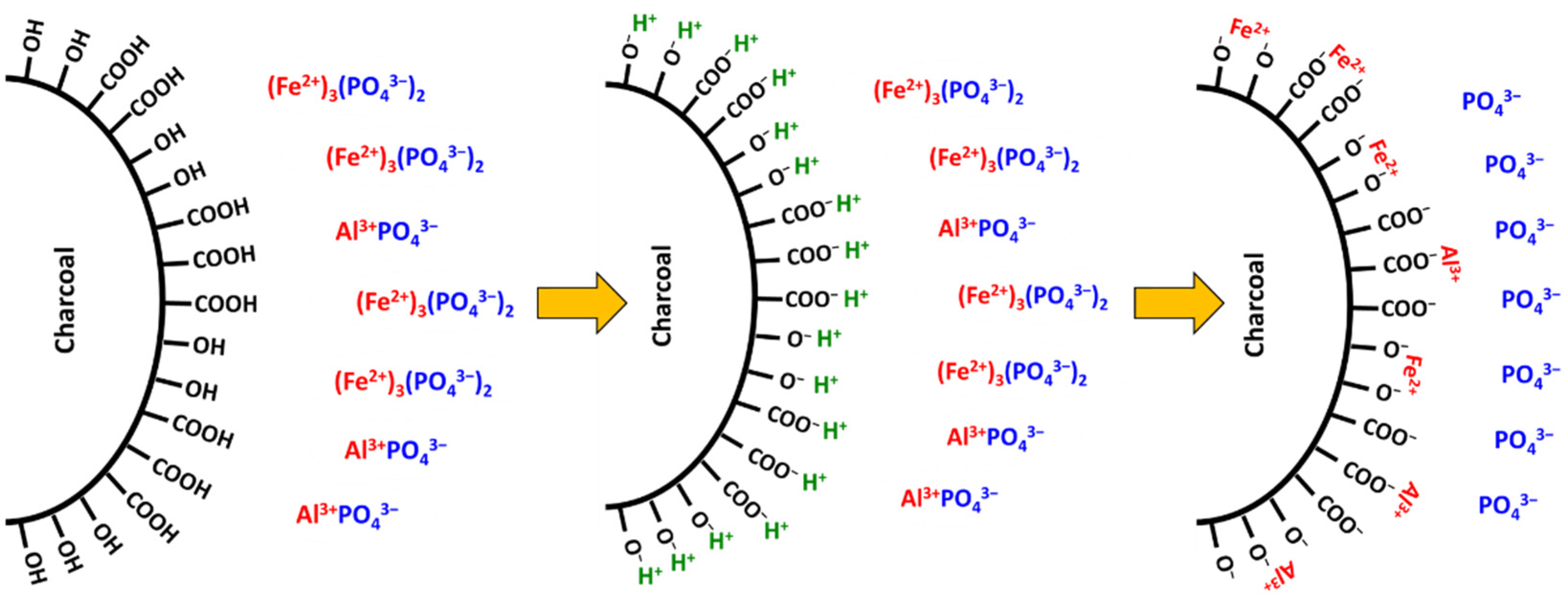
9.2. Complexation of Aluminium and Iron by Humic Substances and Blockage of Phosphorus Adsorption Sites by Organic Acids
9.3. Retention of Phosphorus-Rich Water by Porous Structure of Charcoal and Wood Ash
9.4. Direct Supply of Phosphorus from Charcoal and Wood Ash
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; USDA/NRCS; U.S. Government Printing Office: Washington, DC, USA, 2010.
- Jusop, S.; Ishak, C.F. Weathered Tropical Soils the Ultisols and Oxisols; Universiti Putra Malaysia Press: Serdang, Malaysia, 2010. [Google Scholar]
- Shamshuddin, J.; Daud, N.W. Classification and management of highly weathered soils in Malaysia for production of plantation crops. In Principles, Application and Assessment in Soil Science; InTech: Rijeka, Croatia, 2011; pp. 75–86. [Google Scholar]
- Anda, M.; Shamshuddin, J.; Fauziah, C.I.; Syed Omar, S.R. Mineralogy and factors controlling charge development of three Oxisols developed from different parent materials. Geoderma 2008, 143, 153–167. [Google Scholar] [CrossRef]
- Zaharah, A.R.; Zulkifli, H.; Sharifuddin, H.A.H. Evaluating the efficacy of various phosphate fertilizer sources for oil palm seedlings. Nutr. Cycl. Agroecosyst. 1997, 47, 93–98. [Google Scholar] [CrossRef]
- Saleque, M.A.; Abedin, M.J.; Bhuiyan, N.I.; Zaman, S.K.; Panaullah, G.M. Long-term effects of inorganic and organic fertilizer sources on yield and nutrient accumulation of lowland rice. Field Crops Res. 2004, 86, 53–65. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Sanusi, S.; Othman, S.B. Effect of Christmas Island rock phosphate and rice straw compost application on soil phosphorus availability and maize (Zea mays L.) growth in a tropical acid soil of Kelantan, Malaysia. Open Agric. 2020, 5, 150–158. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Maru, A.; Haruna, A.O.; Asap, A.; Majid, N.M.A.; Maikol, N.; Jeffary, A.V. Reducing Acidity of Tropical Acid Soil to Improve Phosphorus Availability and Zea mays L. Productivity through Efficient Use of Chicken Litter Biochar and Triple Superphosphate. Appl. Sci. 2020, 10, 2127. [Google Scholar] [CrossRef]
- Asap, A.; Haruna, A.O.; Ab Majid, N.M.; Ali, M. Amending triple superphosphate with chicken litter biochar improves phosphorus availability. Eurasian J. Soil Sci. 2018, 7, 121–132. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving phosphorus availability in an acid soil using organic amendments produced from agroindustrial wastes. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Amirbahman, A. Phosphorus availability in boreal forest soils: A geochemical and nutrient uptake modeling approach. Geoderma 2010, 155, 46–54. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H. Charcoal and carbon storage in forest soils of the Rocky Mountain West. Front. Ecol. Environ. 2008, 6, 18–24. [Google Scholar] [CrossRef]
- Ogawa, M. Rehabilitation of Pine with Charcoal and Mycorrhiza; Chikushishokan Publishing: Tokyo, Japan, 2007. (In Japanese) [Google Scholar]
- Demeyer, A.; Voundi Nkana, J.; Verloo, M. Characteristics of wood ash influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Bramryd, T.; Frashman, B. Silvicultural use of wood ashes—Effects on the nutrient and heavy metal balance in a pine (Pinus sylvestris, L.) forest soil. Water, Air and Soil Pollution. In Proceedings of the Fifth International Conference on Acidic Deposition: Science and Policy, Goteborg, Sweden, 26–30 June 1995; Acid Reign ’95, Part 2. Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Nweke, I.A.; Mbah, C.N.; Oweremadu, E.O.; Dambaba, N.; Orji, E.C.; Ekesiobi, A.I.; Nnabuife, E.L.C. Soil pH, available P of an ultisol and castor performance as influenced by contrasting tillage methods and wood ash. Afr. J. Agric. Res. 2017, 12, 606–616. [Google Scholar] [CrossRef][Green Version]
- Scheepers, G.P.; du Toit, B. Potential use of wood ash in South African forestry: A review. South. For. A J. For. Sci. 2016, 78, 255–266. [Google Scholar] [CrossRef]
- Blake, L. Acid rain and soil acidification. In Encyclopedia of Soils in the Environment; Hillel, D., Rosenzweig, C., Powlson, D., Scow, K., Singer, M., Sparks, D., Eds.; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Wiklund, J. Effects of Wood Ash on Soil Fertility and Plant Performance in Southwestern Kenya. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2017. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Mcfarland, M.L.; Haby, V.A.; Redmon, L.; Bade, D. Managing Soil Acidity. Bulletin No. B-1720. 2001. Available online: http://overton.tamu.edu/soils (accessed on 16 May 2021).
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Sumner, M.E.; Noble, A.D. Soil acidification: The world story. In Handbook of Soil Acidity; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 1–28. [Google Scholar]
- Zhu, Q.; de Vries, W.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F. Enhanced acidification in Chinese croplands as derived from element budgets in the period 1980–2010. Sci. Total Environ. 2018, 618, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.C.B.; Bayer, C.; Mietniczuk, J.; Zanatta, J.; Bissani, C.A. Long-term acidification of a Brazilian Acrisol as affected by no till cropping systems and nitrogen fertilizer. Aust. J. Soil Res. 2008, 46, 7–26. [Google Scholar]
- McGivney, E.; Gustafsson, J.P.; Belyazid, S.; Zetterberg, T.; Löfgren, S. Assessing the impact of acid rain and forest harvest intensity with the HD-MINTEQ model–soil chemistry of three Swedish conifer sites from 1880 to 2080. Soil 2019, 5, 63–77. [Google Scholar] [CrossRef]
- Butler, T.J.; Likens, G.E. Acid rain. Encyclopedia Britannica, 19 March 2019. Available online: https://www.britannica.com/science/acid-rain (accessed on 10 June 2021).
- Fageria, N.K.; Baligar, V.C. Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv. Agron. 2008, 99, 345–399. [Google Scholar]
- Kariuki, S.K.; Zhang, H.; Schroder, J.L.; Edwards, J.; Payton, M.; Carver, B.F.; Krenzer, E.G. Hard red winter wheat cultivar responses to a pH and aluminium concentration gradient. Agron. J. 2007, 99, 88–98. [Google Scholar] [CrossRef]
- Robarge, W.P. Environmental Soil and Water Chemistry: Principles and Applications. Soil Sci. 1999, 164, 609–610. [Google Scholar] [CrossRef]
- Stass, A.; Wang, Y.; Eticha, D.; Horst, W. Aluminium rhizotoxicity in maize grown in solutions with Al3+ or Al(OH)4− as predominant solution Al species. J. Exp. Bot. 2006, 57, 4033–4042. [Google Scholar] [CrossRef]
- Edmeades, D.C.; Ridley, A.M. Using lime to ameliorate topsoil and subsoil acidity. In Handbook of Soil Acidity; Marcel Dekker Inc.: New York, NY, USA, 2003; p. 336. [Google Scholar]
- Zdenko, R. Handbook of Soil Acidity; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Tsado, P.A.; Osunde, O.A.; Igwe, C.A.; Adeboye, M.K.A.; Lawal, B.A. Phosphorus sorption characteristics of some selected soil of the Nigerian Guinea Savanna. Int. J. Agri. Sci. 2012, 2, 613–618. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Reed, J.F. Visual plant symptoms as indicators of mineral nutrient deficiencies. In Detecting Mineral Nutrient Deficiencies in Tropical and Temperate Crops; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–12. [Google Scholar]
- Gichangi, E.M.; Mnkeni, P.N.S.; Muchaonyerwa, P. Phosphate sorption characteristics and external P requirements of selected South African soils. J. Agric. Rural Dev. Trop. Subtrop. 2008, 109, 139–149. [Google Scholar]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13rd ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Assuero, S.G.; Mollier, A.; Pellerin, S. The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ. 2004, 27, 887–895. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.L. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Soils around Us. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 1–31. [Google Scholar]
- Meyer, J.; Rein, P.; Turner, P.; Mathias, K.; McGregor, C. Good Management Practices for the Cane Sugar Industry (Final): Produced for the International Finance Corporation (IFC); PGBI Sugar & Bio-Energy (Pty) Ltd: Gauteng, South Africa, 2011; pp. 200–206. [Google Scholar]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Campbell, K.L.; Edwards, D.R. Phosphorus and water quality impacts. In Agricultural Nonpoint Source Pollution: Watershed Management and Hydrology; Ritter, W.F., Shirmohammadi, A., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Sims, J.T.; Maguire, R.O.; Leytem, A.B.; Gartly, K.L.; Pautler, M.C. Evaluation of Mehlich-3 as an agricultural and environmental soil P test for Mid-Atlantic United states of America. Soil Sci. Soc. Am. J. 2002, 70, 2016–2032. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Conklin, A.R. Introduction to Soil Chemistry: Analysis and Instrumentation, 1st ed.; John Wiley and Sons: New York, NY, USA, 2005; p. 256. [Google Scholar]
- Hiradate, S.; Ma, J.F.; Matsumoto, H. Strategies of plants to adapt to mineral stresses in problem soils. Adv. Agron. 2007, 96, 65–132. [Google Scholar]
- Menzies, N.; Lucia, S. The Science of Phosphorus Nutrition: Forms in the WSoil, Plant Uptake, and Plant Response. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2009/02/the-science-of-phosphorus-nutrition-forms-in-the-soil-plant-uptake-and-plant-response (accessed on 16 May 2021).
- Hansen, J.C.; Cade-Menun, B.J.; Strawn, D.G. Phosphorus speciation in manure-amended alkaline soils. J. Environ. Qual. 2004, 33, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Condron, L.M.; Richardson, S.J.; Peltzer, D.A.; Allison, V.J. Soil organic phosphorus transformations during pedogenesis. Ecosystems 2007, 10, 1166–1181. [Google Scholar] [CrossRef]
- Sharpley, A.N. Phosphorus availability. In Handbook of Soil Science; Summer, M.E., Ed.; CRC Press: New York, NY, USA, 2000; pp. 18–38. [Google Scholar]
- Pierzynski, G.M.; McDowell, R.W.; Thomas Sims, J. Chemistry, cycling, and potential movement of inorganic phosphorus in soils. Phosphorus Agric. Environ. 2005, 46, 51–86. [Google Scholar]
- Oelkers, E.H.; Valsami-Jones, E. Phosphate mineral reactivity and global sustainability. Elements 2008, 4, 83–87. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Plante, A.F. Soil biogeochemical cycling of inorganic nutrients and metals. In Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2007; pp. 389–432. [Google Scholar]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Turner, B.L.; McKelvie, I.D.; Haygarth, P.M. Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol. Biochem. 2002, 34, 27–35. [Google Scholar] [CrossRef]
- Condron, L.M.; Turner, B.L.; Cade-Menun, B.J. Chemistry and dynamics of soil organic phosphorus. Phosphorus Agric. Environ. 2005, 46, 87–121. [Google Scholar]
- Wang, X.X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front. Plant Sci. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Satyanarayana, T. Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol. Mol. Biol. 2011, 17, 93–103. [Google Scholar] [CrossRef]
- Lott, J.N.A.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic acid and phosphorus in crop seeds and fruits: A global estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar] [CrossRef]
- Raboy, V. Seed phosphorus and the development of low-phytate crops. In Inositol Phosphates: Linking Agriculture and the Environment; Turner, B.L., Richardson, A.E.M., Eds.; CAB International: Wallingford, UK, 2007; pp. 111–132. [Google Scholar]
- Gerke, J. Phytate (inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- George, T.S.; Richardson, A.E.; Li, S.S.; Gregory, P.J.; Daniell, T.J. Extracellular release of a heterologous phytase from roots of transgenic plants: Does manipulation of rhizosphere biochemistry impact microbial community structure? FEMS Microbiol. Ecol. 2009, 70, 433–445. [Google Scholar] [CrossRef]
- Wasaki, J.; Maruyama, H.; Tanaka, M.; Yamamura, T.; Dateki, H.; Shinano, T.; Ito, S.; Osaki, M. Overexpression of the LASAP2 gene for secretory acid phosphatase in white lupin improves the phosphorus uptake and growth of tobacco plants. Soil Sci. Plant Nutr. 2009, 55, 107–113. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Hesterberg, D.; Klysubun, W.; Gustafsson, J.P. Phosphorus dynamics in Swedish agricultural soils as influenced by fertilization and mineralogical properties: Insights gained from batch experiments and XANES spectroscopy. Sci. Total Environ. 2016, 566, 1410–1419. [Google Scholar] [CrossRef]
- Laakso, J.; Uusitalo, R.; Yli-Halla, M. Phosphorus speciation in agricultural catchment soils and in fresh and dried sediments of five constructed wetlands. Geoderma 2016, 271, 18–26. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Ch’ng, H.Y.; Majid, N.M.A. Improving Phosphorus Availability for Plant Uptake in Tropical Acid Soils Using Organic Amendments Derived from Agro-Industrial Waste; Universiti Putra Malaysia Press: Serdang, Malaysia, 2015. [Google Scholar]
- Hocking, P.J. Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Adv. Agron. 2001, 74, 63–97. [Google Scholar]
- Daly, K.; Styles, D.; Lalor, S.; Wall, D.P. Phosphorus sorption, supply potential and availability in soils with contrasting parent material and soil chemical properties. Eur. J. Soil Sci. 2015, 66, 792–801. [Google Scholar] [CrossRef]
- Pearson, A.J.; Gaw, S.; Hermanspahn, N.; Glover, C.N.; Anderson, C.W. Radium in New Zealand agricultural soils: Phosphate fertiliser inputs, soil activity concentrations and fractionation profiles. J. Environ. Radioact. 2019, 205, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gerard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Whalen, J.K.; Sampedro, L. Soil Ecology and Management; CABI: Wallingford, Oxford, UK, 2010. [Google Scholar]
- Power, J.F.; Prasad, R. Soil Fertility Management for Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Pearson Inc.: London, UK, 2013. [Google Scholar]
- Idris, O.A.; Ahmed, H.S. Phosphorus sorption capacity as a guide for phosphorus availability of selected Sudanese soil series. Afr. Crop Sci. J. 2012, 20, 59–65. [Google Scholar]
- Tening, A.S.; Foba-Tendo, J.N.; Yakum-Ntaw, S.Y.; Tchuenteu, F. Phosphorus fixing capacity of a volcanic soil on the slope of mount Cameroon. Agric. Biol. J. N. Am. 2013, 4, 166–174. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Hassan, G.I.; Hussain, A.; Rasool, F. Phosphorus availability issue- its fixation and role of phosphate solubilizing bacteria in phosphate solubilization. Res. J. Agric. Sci. 2011, 2, 174–179. [Google Scholar]
- Syers, J.K.; Cornforth, I.S. Chemistry of soil fertility. In Proceedings of the New Zealand Institute of Chemistry Annual Conference, Hamilton, OH, USA, 1983. [Google Scholar]
- Lindsay, W.L.; Vlek, P.L.; Chien, S.H. Phosphate minerals. Miner. Soil Environ. 1989, 1, 1089–1130. [Google Scholar]
- Larsen, S. Soil phosphorus. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1967; pp. 151–210. [Google Scholar]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Gerard, F. A mechanistic model for understanding root-induced chemical changes controlling phosphorus availability. Ann. Bot. 2010, 105, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D.L. Phosphate reaction dynamics in soils and soil components: A multiscale approach. Adv. Agron. 2007, 94, 135–179. [Google Scholar]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Troeh, F.R.; Thompson, L.M. Soils and Soil Fertility; Blackwell: New York, NY, USA, 2005. [Google Scholar]
- Asmare, M.; Heluf, G.; Markku, Y.; Birru, Y. Phosphorus Status, Inorganic Phosphorus Forms, and Other Physicochemical Properties of Acid Soils of Farta District, Northwestern Highlands of Ethiopia. Appl. Environ. Soil Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Ayaga, G.; Todd, A.; Brookes, P.C. Enhanced biological cycling of phosphorus increases its availability to crops in low-input sub-Saharan farming systems. Soil Biol. Biochem. 2006, 38, 81–90. [Google Scholar] [CrossRef]
- Mengesha, A.T. Characterizing Phosphate Desorption Kinetics from Soil: An Approach to Predicting Plant Available Phosphorus. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2009. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Turan, M.; Atao ˘glu, N.; Sahιn, F. Evaluation of the Capacity of Phosphate Solubilizing Bacteria and Fungi on Different Forms of Phosphorus in Liquid Culture. J. Sustain. Agr. 2006, 28, 99–108. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahemad, M.; Oves, M.; Ahmad, E.; Khan, M.S. Role of Phosphate-Solubilizing Bacteria in Legume Improvement. In Microbes for Legume Improvement; Springer: Cham, Switzerland, 2010; pp. 273–292. [Google Scholar]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Baumann, K.; Jung, P.; Samolov, E.; Lehnert, L.W.; Büdel, B.; Karsten, U.; Bendix, J.; Achilles, S.; Schermer, M.; Matus, F.; et al. Biological soil crusts along a climatic gradient in Chile: Richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol. Biochem. 2018, 127, 286–300. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barron, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. Agrotecnologia 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Bulmer, D.; Kar, G.; Hamilton, J.; Siciliano, S.; Peak, D. Extent and mechanism of interaction between phosphate and citrate in a calcareous soil. Soil Sci. Soc. Am. J. 2018, 82, 315–322. [Google Scholar] [CrossRef]
- Nayereh, Y.; Kalbasi, M.; Shariatmadari, H. Cumulative and residual effects of organic and chemical fertilizers on chemical properties and P sorption-desorption reactions in a calcareous soil: Ii. Phosphorus desorption kinetics. World Appl. Sci. J. 2010, 11, 462–469. [Google Scholar]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Harlin, J.L. Soil Fertil Fertilizers; Maxwell Publishing Company: New York, NY, USA, 1993. [Google Scholar]
- Ano, A.O.; Ubochi, C.I. Neutralization of soil acidity by animal manures: Mechanism of reaction. Afr. J. Biotechnol. 2007, 6, 364–368. [Google Scholar]
- Jen, H.C.; Wu, J.T.; Huang, W.T. Effects of Compost on the Availability of Nitrogen and Phosphorus in Strongly Acidic Soils; Taiwan Agricultural Research Institute: Wufeng, Taiwan, 2008.
- Motavalli, P.; Miles, R. Soil phosphorus fractions after 111 years of animal manure and fertilizer applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar]
- Nthejane, M.M.; du Preez, C.C.; van Huyssteen, C.W. Relationships between agronomic and environmental phosphorus analyses of selected soils. Water SA 2021, 47, 97–105. [Google Scholar] [CrossRef]
- Nader, R.H.; Amal, W.; Abou, E.K.; Raafat, N.Z. Effect of organic and bio-fertilizers on phosphorus and some micronutrients availability in a calcareous soil, Soil, Water and Environment Research Institute, Agriculture Research Center, Giza, Egypt. Res. J. Agric. Biol. Sci. 2008, 4, 545–552. [Google Scholar]
- Paulo, S.P.; Merlin, A.; Rosolem, C.A. Organic Compounds from Plant Extracts and Their Effect on Soil Phosphorus Availability. Pesqui. Agropecu. Bras. 2008, 43, 1379–1388. [Google Scholar]
- Hariprasad, P.; Niranjana, S.R. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil 2009, 316, 13–24. [Google Scholar] [CrossRef]
- Martins, M.A.; Santos, C.; Almeida, M.M.; Costa, M.E.V. Hydroxyapatite micro-and nanoparticles: Nucleation and growth mechanisms in the presence of citrate species. J. Colloid Interface Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef]
- Gerke, J.; Beißner, L.; Römer, W. The quantitative effect of chemical phosphate mobilization by carboxyxlate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. J. Plant Nutr. Soil Sci. 2000, 163, 207–212. [Google Scholar] [CrossRef]
- Gerke, J. Humic (organic matter)-Al (Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Iyamuremye, F.; Dick, R.P.; Baham, J. Organic amendments and phosphorus dynamics: I. Phosphorus chemistry and sorption. Soil Sci. 1996, 161, 426–435. [Google Scholar] [CrossRef]
- Haynes, R.J.; Mokolobate, M.S. Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the mechanisms involved. Nutr. Cycl. Agroecosyst. 2001, 59, 47–63. [Google Scholar] [CrossRef]
- Erich, M.S.; Fitzgerald, C.B.; Porter, G.A. The effect of organic amendments on phosphorus chemistry in a potato cropping system. Agric. Ecosyst. Environ. 2002, 88, 79–88. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, G.; Shen, J.; Bergstrom, L.; Zhang, F. Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 2015, 44, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Sikora, F.J.; Gilkes, R.J.; McLaughlin, M.J. Comparing of the difference and balance methods to calculate percent recovery of fertilizer phosphorus applied to soils: A critical discussion. Nutr. Cycl. Agroecosyst. 2012, 92, 1–8. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Siebielec, G.; Ukalska-Jaruga, A.; Kidd, P. Bioavailability of Trace Elements in Soils Amended with High-Phosphate Materials. PHOSPHATE in Soils: Interaction with Micronutrients, Radionuclides and Heavy Metals; CRC Press: Boca Raton, FL, USA, 2014; pp. 237–268. [Google Scholar]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv. Agron. 2000, 69, 99–151. [Google Scholar]
- Palm, C.A.; Giller, K.E.; Mafongoya, P.L.; Swift, M.J. Management of organic matter in the tropics: Translating theory into practice. In Managing Organic Matter in Tropical Soils: Scope and Limitations; Springer: Dordrecht, The Netherlands, 2001; pp. 63–75. [Google Scholar]
- Gichangi, E.M. Effects of organic amendments on the transformations and bioavailability of phosphorus in soils: A review. Discov. Agric. 2019, 5, 41–50. [Google Scholar]
- Ye, H.; Chen, F.; Sheng, Y.; Sheng, G.; Fu, J. Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep. Purif. Technol. 2006, 50, 283–290. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Effects of exogenous rare earth elements on phosphorus adsorption and desorption in different types of soils. Chemosphere 2014, 103, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vanlauwe, B.; Diels, J.; Aihou, K.; Iwuafor, E.N.O.; Lyasse, O.; Sanginga, N.; Merckx, R. Direct Interactions between N Fertilizer and Organic Matter: Evidence from Trials with 15N-Labelled Fertilizer. Integrated Plant Nutrient Management in Sub-Saharan Africa: From Concept to Practice; CAB International: Wallingford, UK, 2002; pp. 173–184. [Google Scholar]
- Kumari, P.; Nema, A.K. Effect of different fertilizer treatment and soil texture on the emission of CO2 in the atmosphere from the soil. Clim. Chang. 2018, 4, 1–11. [Google Scholar]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH increase due to biological decarboxylation of organic anions. Soil Biol. Biochem. 1996, 28, 617–624. [Google Scholar] [CrossRef]
- Sanusi, S.; Ch’ng, H.Y.; Othman, S. Effects of incubation period and Christmas Island rock phosphate with different rate of rice straw compost on phosphorus availability in acid soil. AIMS Agric. Food 2018, 3, 384–396. [Google Scholar] [CrossRef]
- Mensah, A.K.; Frimpong, K.A. Biochar and/or compost applications improve soil properties, growth, and yield of maize grown in acidic rainforest and coastal savannah soils in Ghana. Int. J. Agron. 2018, 2018. [Google Scholar] [CrossRef]
- Ikerra, T.W.D.; MnKeni, P.N.S.; Singh, B.R. Effects of added composts and farmyard manure on phosphorus release from Minjingu phosphate rock and its uptake by maize. Nor. J. Agric. Sci. 1994, 8, 13–23. [Google Scholar]
- Lata Verma, S.; Marschner, P. Compost effects on microbial biomass and soil P pools as affected by particle size and soil properties. J. Plant Nutr. Soil Sci. 2013, 13, 313–328. [Google Scholar] [CrossRef]
- Horta, C. Fertilisation with Compost: Effects on Soil Phosphorus Sorption and on Phosphorus Availability in Acid Soils. Open J. Soil Sci. 2019, 9, 255–268. [Google Scholar] [CrossRef]
- Gichangi, E.M.; Mnkeni, P.N.; Brookes, P.C. Effects of goat manure and inorganic phosphate addition on soil inorganic and microbial biomass phosphorus fractions under laboratory incubation conditions. Soil Sci. Plant Nutr. 2009, 55, 764–771. [Google Scholar] [CrossRef]
- Turner, B.L.; Leytem, A.B. Phosphorus compounds in sequential extracts of animal manures: Chemical speciation and a novel fractionation procedure. Environ. Sci. Technol. 2004, 38, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Parham, J.A.; Deng, S.P.; Da, H.N.; Sun, H.Y.; Raun, W.R. Long-term cattle manure application in soil. II. Effect on soil microbial populations and community structure. Biol. Fertil. Soils 2003, 38, 209–215. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus Sorption and Availability from Biochars and Soil/Biochar Mixtures. CLEAN–Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Dume, B.; Ayele, D.; Regassa, A.; Berecha, G. Improving available phosphorus in acidic soil using biochar. J. Soil Sci. Environ. 2017, 8, 87–94. [Google Scholar]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management; Routledge: Oxford, UK, 2015; pp. 453–486. [Google Scholar]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Breuning-Madsen, H.; Andersen, M.N. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Bai, S.H.; Xu, C.Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Wallace, H.; Reverchon, F.; Van Zwieten, L. Soil and foliar nutrient and nitrogen isotope composition (δ 15 N) at 5 years after poultry litter and green waste biochar amendment in a macadamia orchard. Environ. Sci. Pollut. Res. 2015, 22, 3803–3809. [Google Scholar]
- Rafique, M.; Ortas, I.; Rizwan, M.; Chaudhary, H.J.; Gurmani, A.R.; Munis, M.F.H. Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 2020, 238, 124710. [Google Scholar] [CrossRef]
- Trippe, K.M.; Manning, V.A.; Reardon, C.L.; Klein, A.M.; Weidman, C.; Ducey, T.F.; Novak, J.M.; Watts, D.W.; Rushmiller, H.; Spokas, K.A.; et al. Phytostabilization of acidic mine tailings with biochar, biosolids, lime, and locally-sourced microbial inoculum: Do amendment mixtures influence plant growth, tailing chemistry, and microbial composition? Appl. Soil Ecol. 2021, 165, 103962. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Ott, M.R.; Strawn, D.G.; Tirocke, J.M. Using organic amendments to restore soil physical and chemical properties of a mine site in northeastern Oregon, USA. Appl. Eng. Agric. 2018, 34, 43–55. [Google Scholar] [CrossRef]
- Noack, S.R.; McLaughlin, M.J.; Smernik, R.J.; McBeath, T.M.; Armstrong, R.D. Crop residue phosphorus: Speciation and potential bioavailability. Plant Soil 2012, 359, 375–385. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 1999, 68, 197–319. [Google Scholar]
- Abdala, D.B.; da Silva, I.R.; Vergutz, L.; Sparks, D.L. Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils. Chemosphere 2015, 119, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.R.; Brancheriau, L.; Napoli, A.; Trugilho, P.F. Factors affecting the mechanics of carbonized wood: Literature review. Wood Sci. Technol. 2016, 50, 519–536. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The future of food and agriculture—Trends and challenges. Annual Report. 2017. [Google Scholar]
- Mathieson, J.G.; Somerville, M.A.; Deev, A.; Jahanshahi, S. Utilization of biomass as an alternative fuel in ironmaking. In Iron Ore; Woodhead Publishing Limited: Sawston, Cambridge, UK, 2015; pp. 581–613. [Google Scholar]
- Phonphuak, N.; Thiansem, S. Effects of charcoal on physical and mechanical properties of fired test briquettes. Sci. Asia 2011, 37, 120–124. [Google Scholar] [CrossRef]
- Boateng, A.A. Characterization and thermal conversion of charcoal derived from fluidized-bed fast pyrolysis oil production of switchgrass. Ind. Eng. Chem. Res. 2007, 46, 8857–8862. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 33–52. [Google Scholar]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Simple Technologies for Charcoal Making; FAO: Rome, Italy, 1987. [Google Scholar]
- Food and Agriculture Organization (FAO). Industrial Charcoal Making; FAO: Rome, Italy, 1985. [Google Scholar]
- Tryon, E.H. Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Mbagwu, J.S.C.; Piccolo, A.; Spallacci, P. Effects of field applications of organic wastes from different sources on chemical, rheological and structural properties of some Italian surface soils. Biores. Tech. 1991, 37, 71–78. [Google Scholar] [CrossRef]
- Rose, D.A. The effect of long-continued organic manuring on some physical properties of soils. In Advances in Soil Organic Matter Research: The Impact on Agriculture and the Environment; Woodhead Publishing Limited: Sawston, Cambridge, UK, 1991; pp. 197–205. [Google Scholar]
- Glaser, B.; Balashov, E.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in density fractions of anthropogenic soils of the Brazilian Amazon region. Org. Geochem. 2000, 31, 669–678. [Google Scholar] [CrossRef]
- Mbagwu, J.S.; Piccolo, A. Effects of Humic Substances from Oxidized Coal on Soil Chemical Properties and Maize Yield. The Role of Humic Substances in the Ecosystems and in Environmental Protection; IHSS, Polish Society of Humic Substances: Wroclaw, Poland, 1997; pp. 921–925. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Qiu, Y.; Cheng, H.; Xu, C.; Sheng, G.D. Surface characteristics of crop-residue-derived black carbon and lead (II) adsorption. Water Res. 2008, 42, 567–574. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva Jr, J.P.; Rondon, M.; Cravo, M.D.S.; Greenwood, J.; Nehls, T.; Steiner, C.; Glaser, B. Slash-and-char-a feasible alternative for soil fertility management in the central Amazon. In Proceedings of the 17th World Congress of Soil Science, Bangkok, Thailand, 14–21 August 2002; pp. 1–12. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of non-polar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of wood ash in concrete manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. Forestry 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Knapp, B.A.; Insam, H. Recycling of biomass ashes: Current technologies and future research needs. In Recycling of Biomass Ashes; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–16. [Google Scholar]
- Ozolincius, R.; Buozyte, R.; Varnagiryte-Kabasinskiene, I. Wood ash and nitrogen influence on ground vegetation cover and chemical composition. Biomass Bioenergy 2007, 31, 710–716. [Google Scholar] [CrossRef]
- Risse, M. Best Management Practices for Wood Ash as Agricultural Soil Amendment; Bulletin, 1142; University of Georgia: Athens, GA, USA, 2013. [Google Scholar]
- Werkelin, J.; Skrifvars, B.J.; Hupa, M. Ash-forming elements in four Scandinavian wood species. Part 1: Summer harvest. Biomass Bioenergy 2005, 29, 451–466. [Google Scholar] [CrossRef]
- Etiegni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Naik, T.R. Tests of Wood ash as a Potential Source for Construction Materials. UWM Center for By-product Utilisation; Report No. CBU-1999-09; Department of Civil Engineering and Mechanics, University of Wisconsin-Milwauke: Milwauke, WI, USA, 1999. [Google Scholar]
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolincius, R.; Varnagiryte-Kabasinskiene, I. Wood ash recycling–possibilities and risks. In Sustainable Use of Forest Biomass for Energy; Springer: Dordrecht, The Netherlands, 2008; pp. 79–108. [Google Scholar]
- Campbell, A.G. Recycling and disposing of wood ash. Tappi. J. 1990, 73, 141–146. [Google Scholar]
- Odlare, M.; Pell, M. Effect of wood fly ash and compost on nitrification and denitrification in agricultural soil. Appl. Energy 2009, 86, 74–80. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Ballard, B.D.; Abrahamson, L.P. Wood ash effects on soil solution and nutrient budgets in a willow bioenergy plantation. Water Air Soil Pollut. 2004, 159, 209–224. [Google Scholar] [CrossRef]
- Ohno, T. Neutralization of soil acidity and release of phosphorus and potassium by wood ash. J. Environ. Qual. 1992, 21, 433–438. [Google Scholar] [CrossRef]
- Mandre, M.; Parn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Goodwin, E.J.; Burrow, A.M. Effects of Application of Mill-Generated Primary Sludge and Boiler Ash on Loblolly Pine Survival and Growth; Gen. Tech. Rep. SRS-92; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 135–138.
- Santalla, M.; Omil, B.; Rodriguez-Soalleiro, R.; Merino, A. Effectiveness of wood ash containing charcoal as a fertilizer for a forest plantation in a temperate region. Plant Soil 2011, 346, 63–78. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Smolander, A.; Kukkola, M.; Moilanen, M.; Saramaki, J. 30-Year effects of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes and stand growth in a Scots pine stand. For. Ecol. Manag. 2012, 278, 63–70. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Mbah, C.N.; Nwite, J.N.; Njoku, C.; Nweke, I.A. Response of maize (Zea mays L.) to different rates of wood-ash application in acid ultisol in Southeast Niger. Afr. J. Agric. Res. 2010, 5, 580–583. [Google Scholar]
- Nottidge, D.O.; Balogun, R.B.; Njoku, N.R. Effect of rice-husk ash on exchange acidity, growth and yield of groundnut (Arachis hypogaea L.) in an acid ultisol. Global J. Agric. Sci. 2009, 8, 1–6. [Google Scholar] [CrossRef][Green Version]
- Nweke, I.A. Contrasting tillage systems and wood ash effect on soil chemical properties. British J. Environ. Sci. 2018, 7, 8–25. [Google Scholar]
- Hagerberg, D.; Wallander, H. The impact of forest residue removal and wood ash amendment on the growth of the ectomycorrhizal external mycelium. FEMS Microbiol. Ecol. 2002, 39, 139–146. [Google Scholar] [CrossRef]
- Rukshana, F.; Butterly, C.R.; Baldock, J.A.; Xu, J.M.; Tang, C. Model organic compounds differ in priming effects on alkalinity release in soils through carbon and nitrogen mineralisation. Soil Biol. Biochem. 2012, 51, 35–43. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, M.; Wang, B.; Zhang, L.; Wen, S.; Gao, S. Effectiveness of crop straws, and swine manure in ameliorating acidic red soils: A laboratory study. J. Soils Sediments 2018, 18, 2893–2903. [Google Scholar] [CrossRef]
- Risse, M.; Harris, G. Biopower. In Biomass Energy Data Book; Boundy, B., Diegel, S.W., Wright, L., Davis, S.C., Eds.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011. [Google Scholar]
- Lin, D.H.; Tian, X.L.; Li, T.T.; Zhang, Z.Y.; He, X.; Xing, B.S. Surface-bound humic acid increased Pb2+ sorption on carbon nanotubes. Environ. Pollut. 2012, 167, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.J.; Pan, B.C.; Zhang, W.M.; Lv, L.; Zhang, Q.X. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Von Wandruszka, R. Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochem. Trans. 2006, 7, 1–8. [Google Scholar] [CrossRef]
- Hinsinger, P.; Brauman, A.; Devau, N.; Gerard, F.; Jourdan, C.; Laclau, J.P.; Le Cadre, E.; Jaillard, B.; Plassard, C. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 2011, 348, 29–61. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Raben-Lange, B.; Gimsing, A.L.; Strobel, B.W. Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma 2005, 127, 270–279. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Palanivell, P. Organic and Mineral Amendments on Rice (Oryza sativa L.) Yield and Nutrients Recovery Efficiency. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2016. [Google Scholar]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J. Biochar effects on nutrient leaching. Biochar Environ. Manag. Sci. Technol. 2009, 271, 303–320. [Google Scholar]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Choo, L.N.L.K.; Ahmed, O.H.; Majid, N.M.B.N.; Ab Aziz, Z.F.B. Improving Nitrogen Availability on a Tropical Peat Soil Cultivated with Ananas comosus L. Merr. Using Pineapple Residue Ash. J. Plant Nutr. Soil Sci. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhao, D.; Cao, Y.; Huot, H.; Tang, Y.T.; Zhang, H.; Xiao, T. Phosphorous Retention and Release by Sludge-Derived Hydrochar for Potential Use as a Soil Amendment. J. Environ. Qual. 2019, 48, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Qian, T.; Zhang, X.; Hu, J.; Jiang, H. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93, 2069–2075. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Joseph, S.; Sohi, S.P.; Heal, K.V. Biochar and enhanced phosphate capture: Mapping mechanisms to functional properties. Chemosphere 2017, 179, 57–74. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Pugliese, S.; Jones, T.; Preston, M.D.; Hazlett, P.; Tran, H.; Basiliko, N. Wood ash as a forest soil amendment: The role of boiler and soil type on soil property response. Can. J. Soil Sci. 2014, 94, 621–634. [Google Scholar] [CrossRef]
- Omil, B.; Pineiro, V.; Merino, A. Soil and tree responses to the application of wood ash containing charcoal in two soils with contrasting properties. For. Ecol. Manag. 2013, 295, 199–212. [Google Scholar] [CrossRef]
- Gomez-Rey, M.X.; Madeira, M.; Coutinho, J. Soil C and N dynamics, nutrient leaching and fertility in a pine plantation amended with wood ash under Mediterranean climate. Eur. J. For. Res. 2013, 132, 281–295. [Google Scholar] [CrossRef]
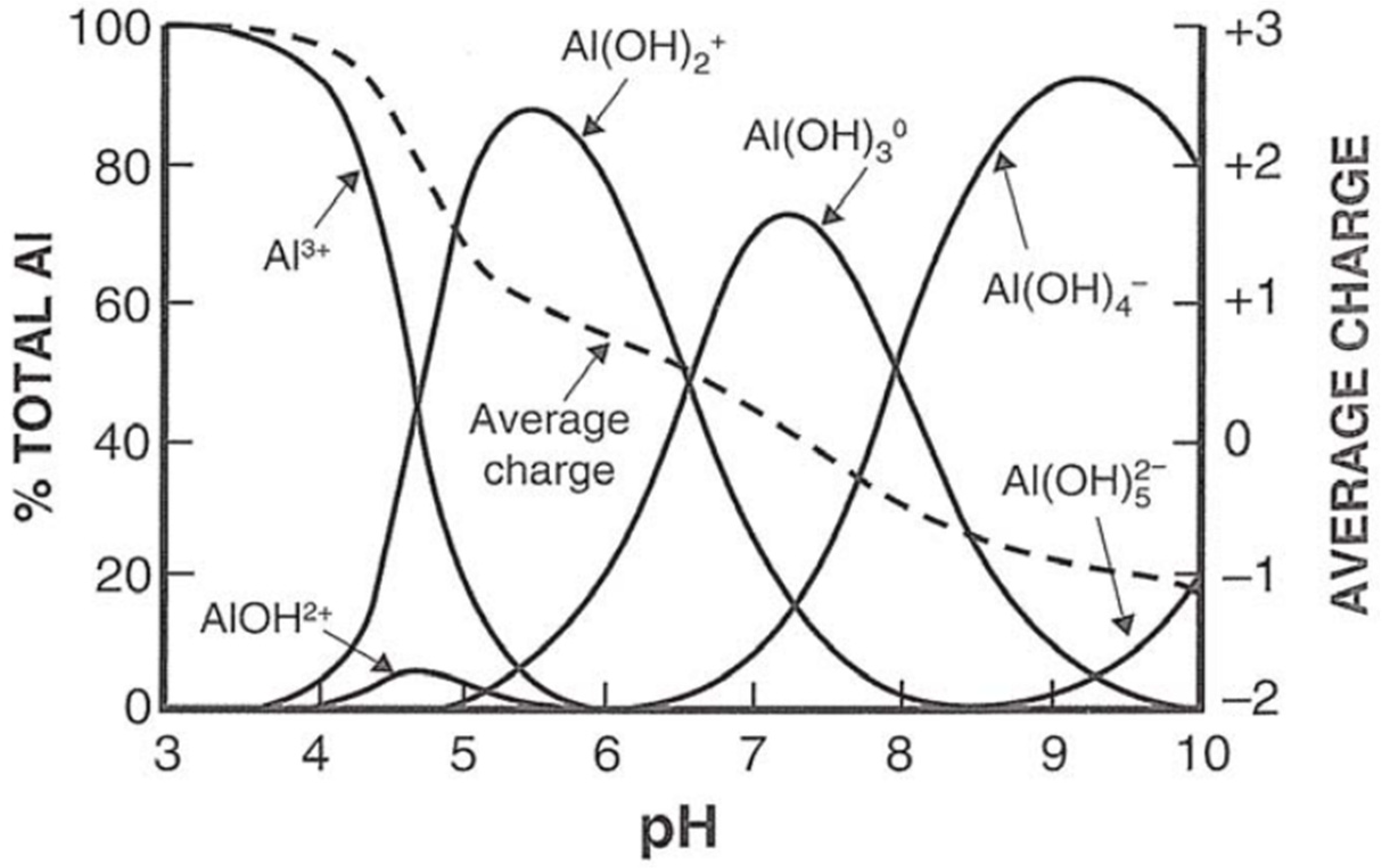
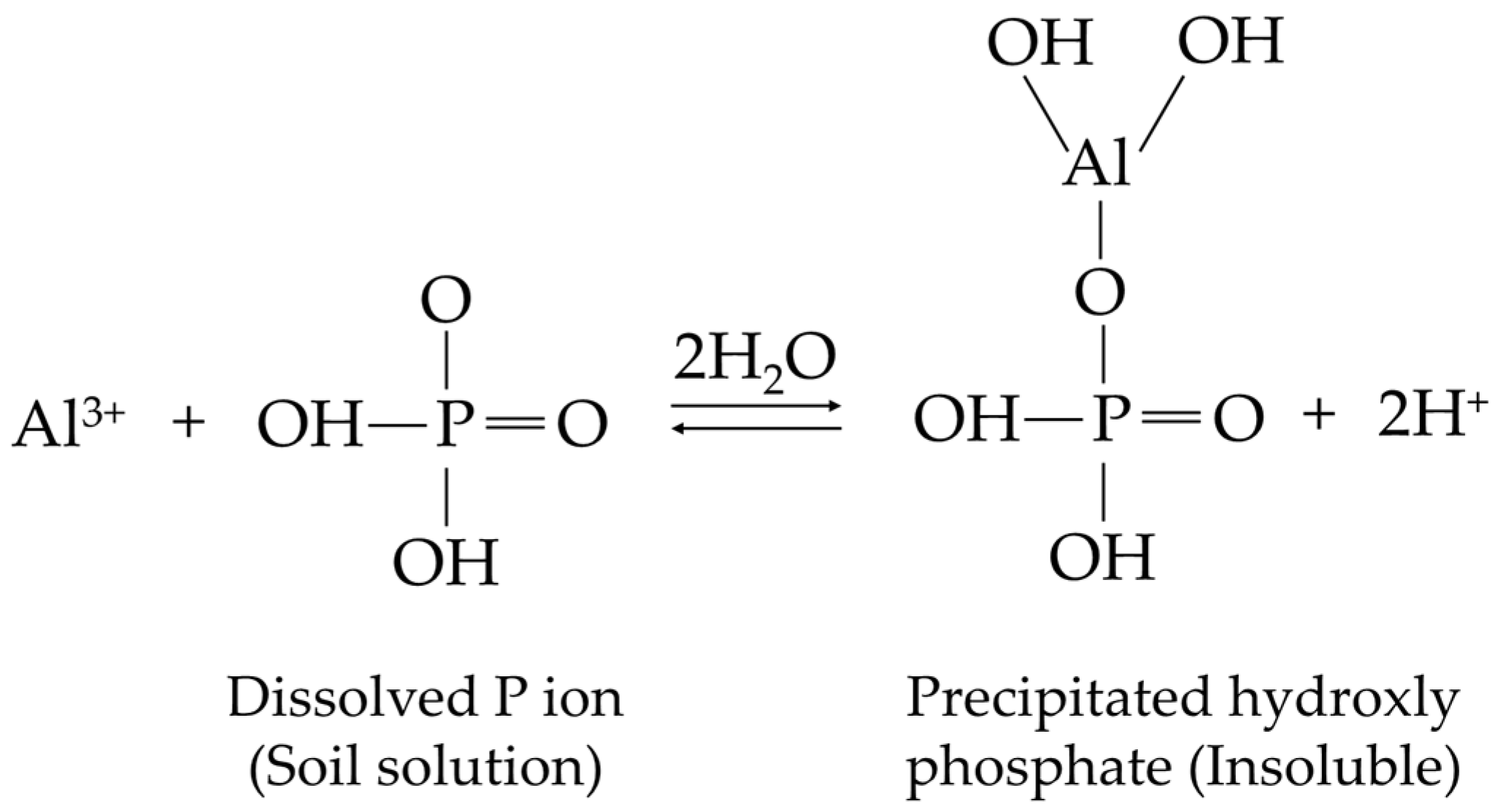

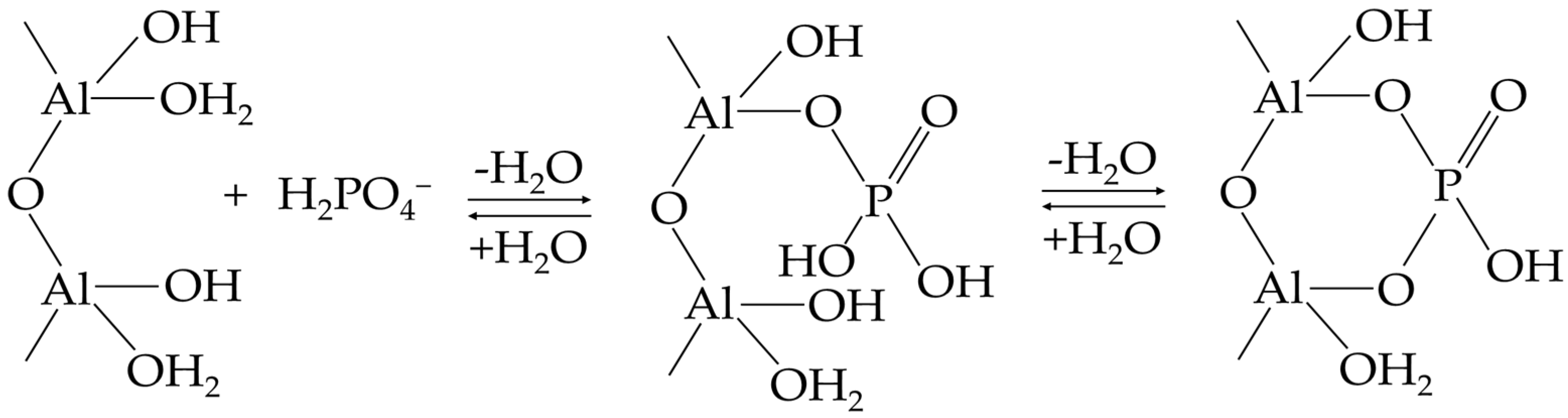
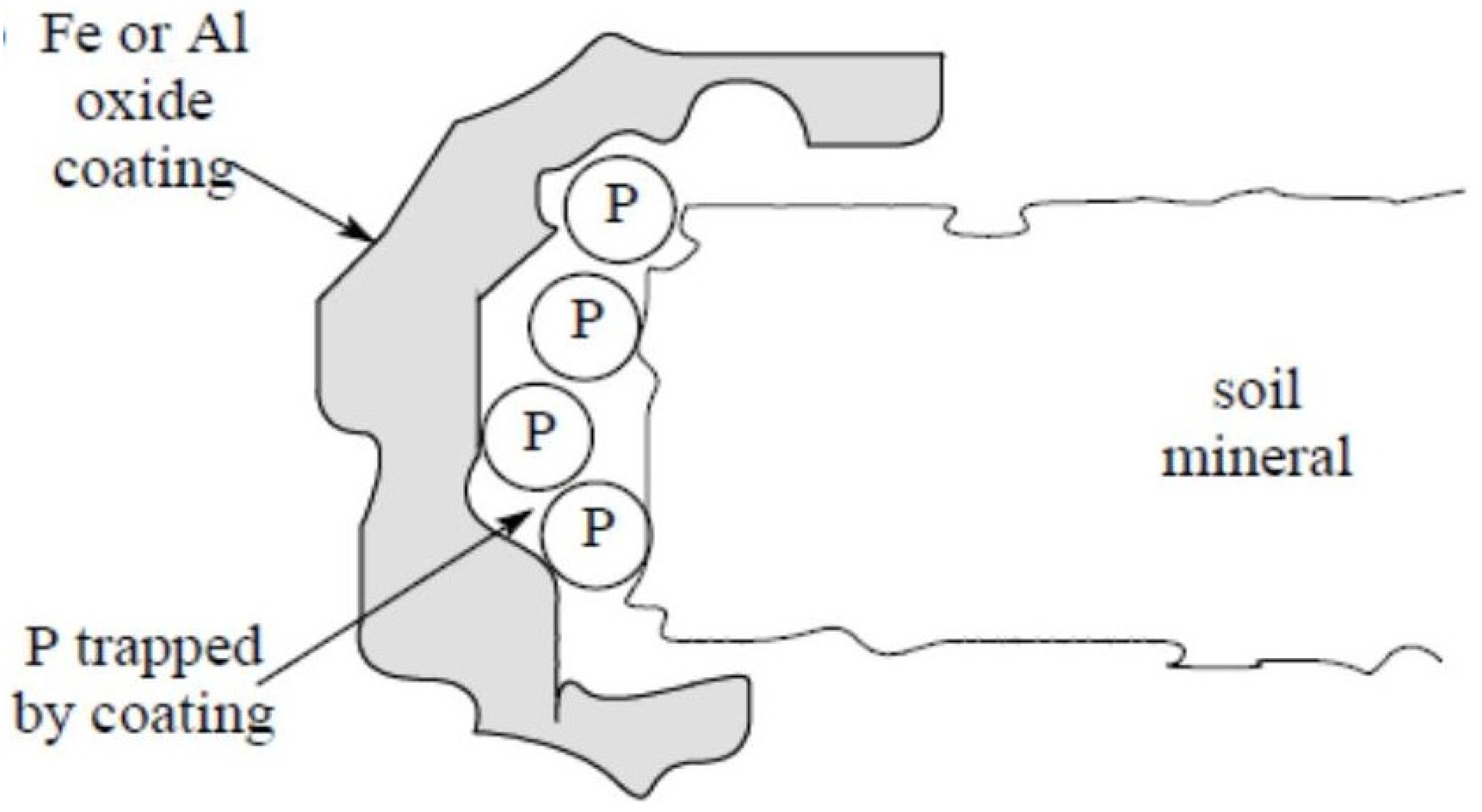
| Level of Acidity | Soil pH |
|---|---|
| Slightly acid | 6.6–6.1 |
| Moderately acid | 6.0–5.5 |
| Strongly acid | 5.5–5.1 |
| Very strongly acid | 5.0–4.5 |
| Extremely acid | <4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy 2021, 11, 2010. https://doi.org/10.3390/agronomy11102010
Johan PD, Ahmed OH, Omar L, Hasbullah NA. Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy. 2021; 11(10):2010. https://doi.org/10.3390/agronomy11102010
Chicago/Turabian StyleJohan, Prisca Divra, Osumanu Haruna Ahmed, Latifah Omar, and Nur Aainaa Hasbullah. 2021. "Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash" Agronomy 11, no. 10: 2010. https://doi.org/10.3390/agronomy11102010
APA StyleJohan, P. D., Ahmed, O. H., Omar, L., & Hasbullah, N. A. (2021). Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy, 11(10), 2010. https://doi.org/10.3390/agronomy11102010






