A Preliminary Investigation on Bacterial Diversity and Fermentation Quality of High-Moisture Alfalfa Silage Prepared with Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Analyses of Bacterial Populations, Organic Acids, and Chemical Composition
2.3. Analyses of Bacterial Communities
2.3.1. Bacterial DNA Isolation and 16S Amplicon Sequencing
2.3.2. Illumina Hiseq2500 Sequencing
2.4. Statistical Analyses
3. Results and Discussion
3.1. Characteristics of Alfalfa Material
3.2. Fermentation Quality of Alfalfa Silage
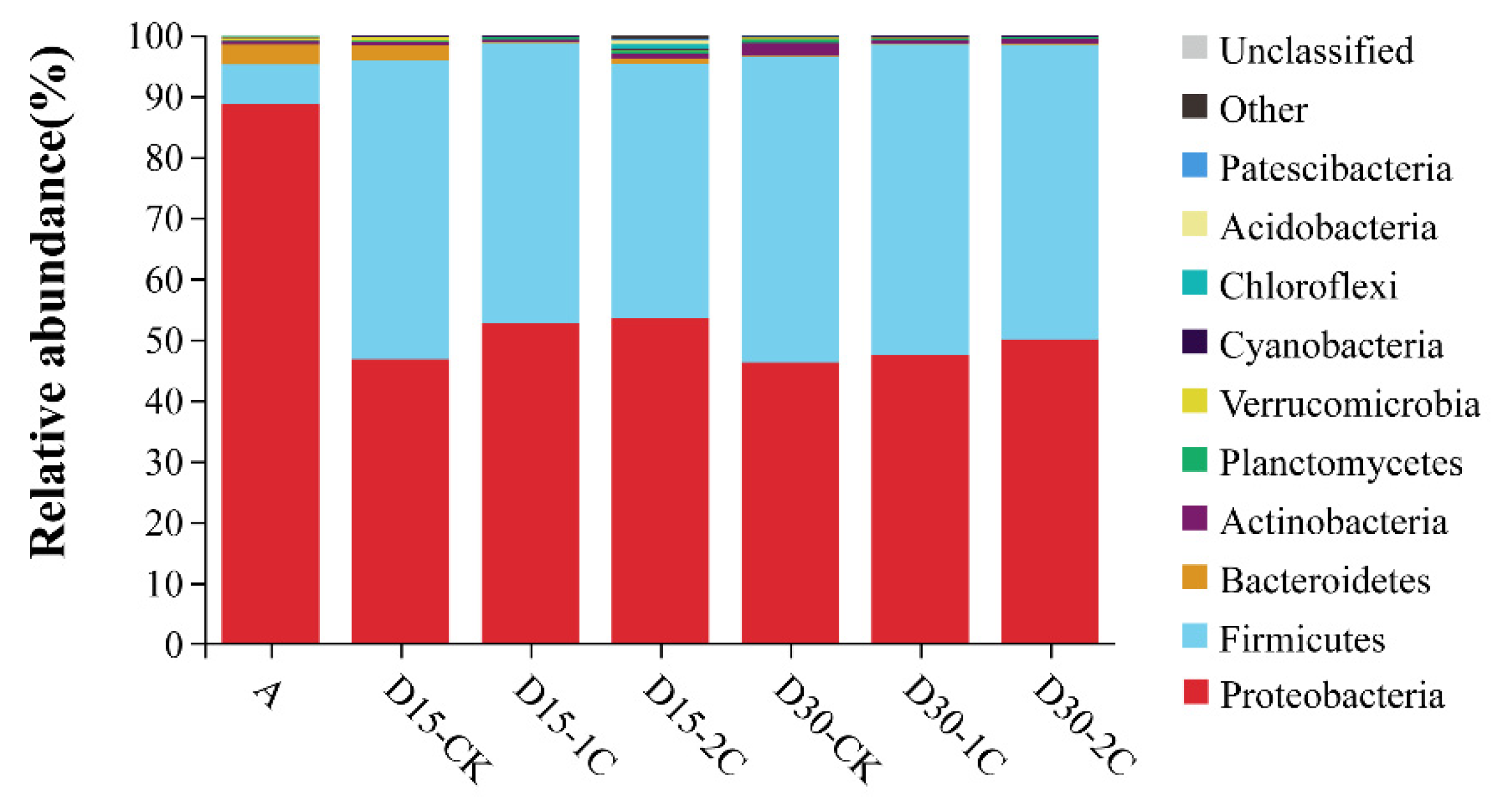
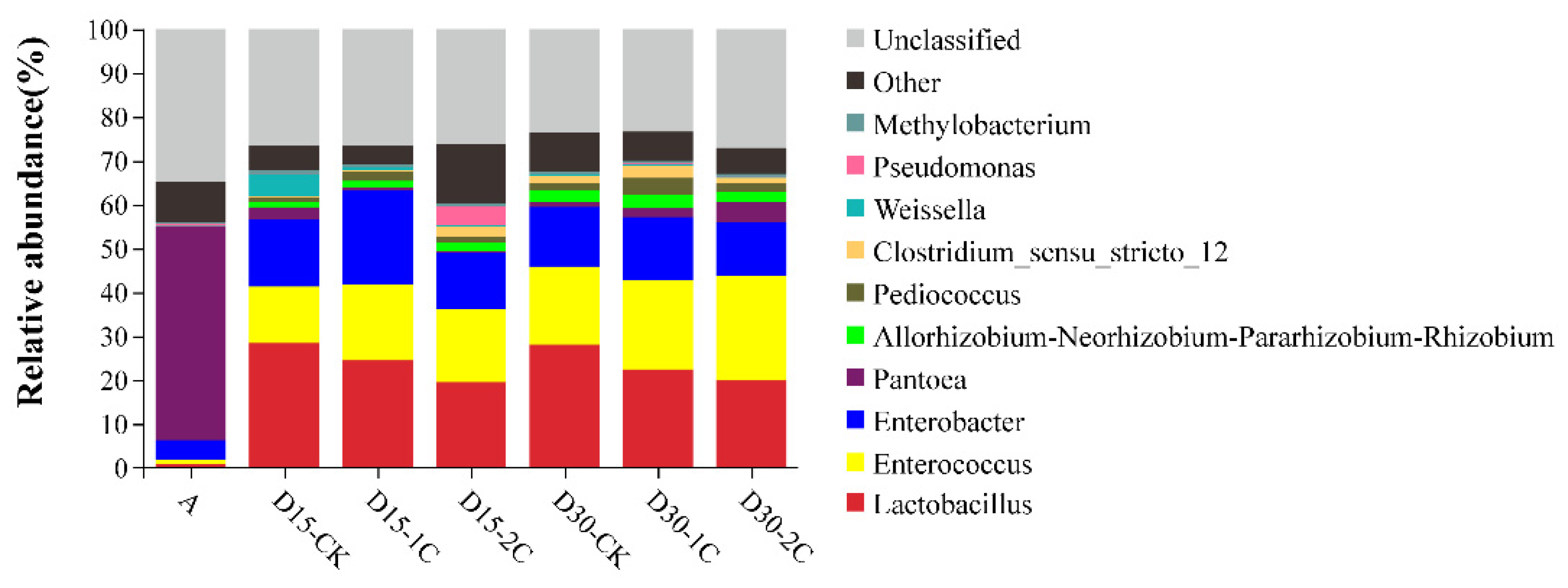
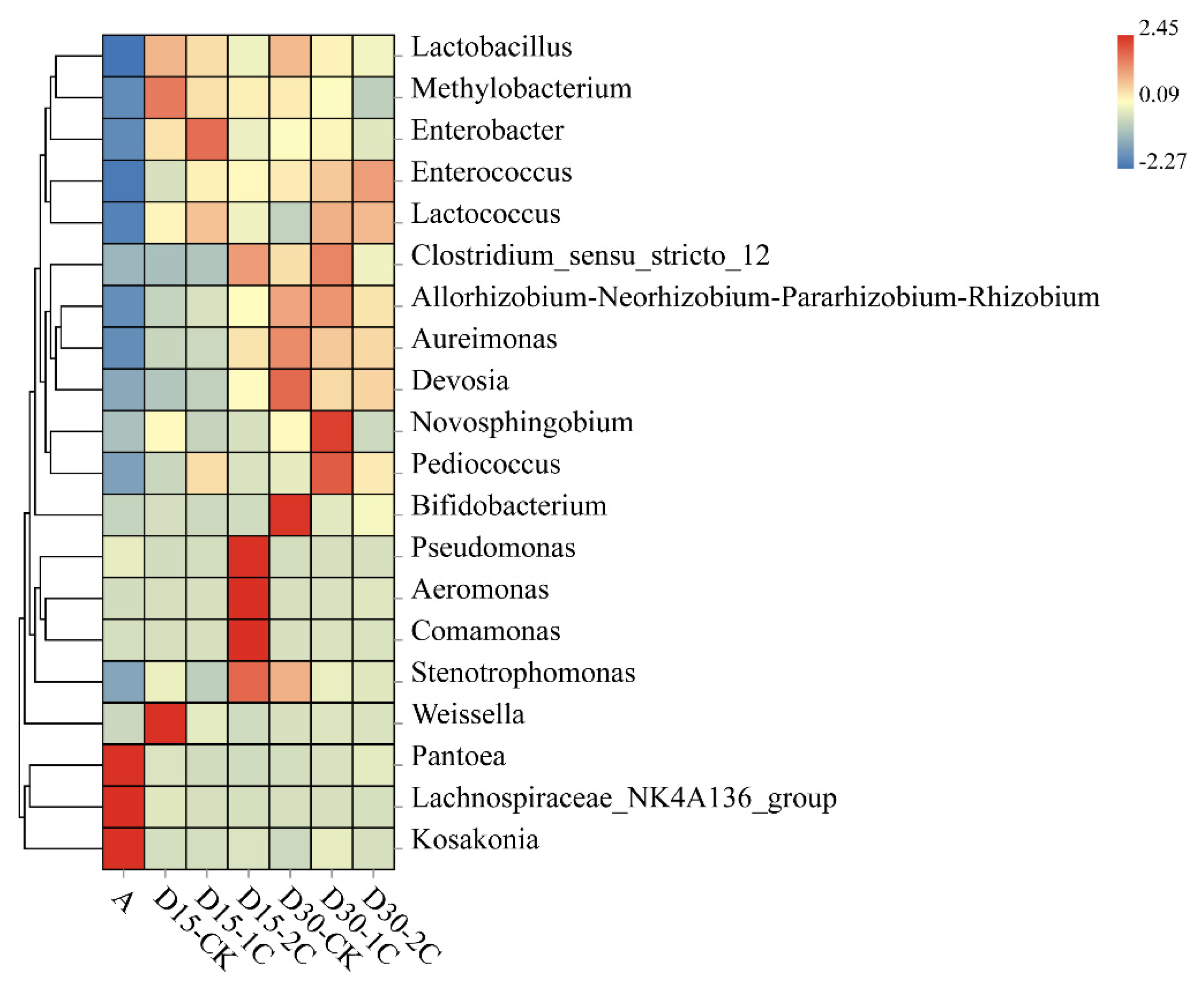
3.3. Bacterial Community of Alfalfa Silage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Yu, Z.; Wang, X.; Risu, N. Effects of chlorpyrifos and chlorantraniliprole on fermentation quality of alfalfa (Medicago sativa L.) silage inoculated with or without Lactobacillus plantarum LP. Anim. Sci. J. 2017, 88, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Marshall, S.A.; Campbell, C.P.; Buchanan-Smith, J.G. Proteolysis and rumen degradability of alfalfa silages preserved with a microbial inoculant, spent sulfite liquor, formic acid or formaldehyde. Can. J. Anim. Sci. 1993, 73, 559–570. [Google Scholar] [CrossRef]
- Ni, K.K.; Lu, Y.; Wang, X.K.; Guo, L.N.; Li, X.M.; Yang, F.Y. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresour. Technol. 2020, 297, 122249. [Google Scholar] [CrossRef]
- Calvelo, P.R.; Muetzel, S.; Camps, A.M.; Bishop, P.; Hina, K.; Hedley, M. Assessment of the influence of biochar on rumen and silage fermentation: A laboratory-scale experiment. Anim. Feed Sci. Technol. 2014, 196, 22–31. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhao, Z.J.; Chen, L.; Wang, L.Q.; Ji, L.Z.; Xiao, Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicol. Environ. Saf. 2020, 196, 110537. [Google Scholar] [CrossRef]
- Agnieszka, W.M.; Tomasz, P.; Alicja, N.; Adam, K.; Dariusz, K.; Aleksandra, G.; Agnieszka, A.P. The Effect of Biochar-Based Organic Amendments on the Structure of Soil Bacterial Community and Yield of Maize (Zea mays L.). Agronomy 2021, 11, 7. [Google Scholar]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Andrey, V.G.; Tatiana, M.M.; Saglara, S.M.; Leonid, V.P.; Gerhard, S.; Inna, V.Z.; Vishnu, D.R.; Svetlana, N.S.; Dinesh, M.; Jun, Y. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health Off. J. Soc. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Leng, R.; Preston, T.; Inthapanya, S. Biochar reduces enteric methane and improves growth and feed conversion in local “Yellow” cattle fed cassava root chips and fresh cassava foliage. Livest. Res. Rural Dev. 2012, 11, 24. [Google Scholar]
- Toth, J.D.; Dou, Z. Use and impact of biochar and charcoal in animal production systems. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 199–224. [Google Scholar]
- Joseph, S.; Pow, D.; Dawson, K.; Mitchell, D.R.G.; Rawal, A.; Hook, J.; Solaiman, Z.M. Feeding biochar to cows: An innovative solution for improving soil fertility and farm productivity. Pedosphere 2015, 25, 666–679. [Google Scholar] [CrossRef]
- Bu, J.; Wei, H.L.; Wang, Y.T.; Cheng, J.R.; Zhu, M.J. Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res. 2021, 202, 117440. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Wiseman, J.; Udén, P.; Mateos, G. Some experimental design and statistical criteria for analysis of studies in manuscripts submitted for consideration for publication. Anim. Feed Sci. Technol. 2006, 129, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative study of individual and Co-Application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front. Microbiol. 2018, 9, 1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, L.W.; Xing, Y.Q.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determinations of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertsom, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 4, 347–358. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 5, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.H. Theoretical Carbohydrates Requirement for Alfalfa Silage Production. Agron. J. 1962, 4, 291–293. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, P.K.; de Araujo, L.C.; Sanches, L.A.; Dos Santos Araujo, S.N.; de Lima, T.O.; Lino, A.D.A.; Ferreira, E.M. Effect of sward height on the fermentability coefficient and chemical composition of guinea grass silage. Grass Forage Sci. 2018, 3, 588–598. [Google Scholar] [CrossRef]
- He, L.W.; Chen, N.; Lv, H.J.; Wang, C.; Zhou, W.; Chen, X.Y.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 1, 951–960. [Google Scholar] [CrossRef]
- He, L.W.; Wang, C.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Dynamics of proteolysis, protease activity and bacterial community of Neolamarckia cadamba leaves silage and the effects of formic acid and Lactobacillus farciminis. Bioresour. Technol. 2019, 294, 122127. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef]
- Nishino, N.; Li, Y.; Wang, C.; Parvin, S. Effects of wilting and molasses addition on fermentation and bacterial community in guinea grass silage. Lett. Appl. Microbiol. 2010, 54, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Yuan, Z.H.; Sun, Y.M.; Kong, X.Y.; Dong, P.Y.; Zhang, J. A reused method for molasses-processed wastewater: Effect on silage quality and anaerobic digestion performance of Pennisetum. Bioresour. Technol. 2017, 241, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
| Items | Alfalfa |
|---|---|
| DM (%) | 30.0 ± 0.23 |
| Crude protein (%DM) | 14.1 ± 0.09 |
| Neutral detergent fiber (%DM) | 47.1 ± 2.02 |
| Acid detergent fiber (%DM) | 31.5 ± 0.73 |
| Water soluble carbohydrate (%DM) | 3.32 ± 0.40 |
| Lactic acid bacteria (log10 cfu/g FM) | 6.68 ± 0.39 |
| Yeasts (log10 cfu/g FM) | 5.06 ± 0.06 |
| Molds (log10 cfu/g FM) | 4.37 ± 0.06 |
| Coliform bacteria (log10 cfu/g FM) | 6.25 ± 0.79 |
| Items | Treatments | SEM | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| Ensiling Days | CK | 1%C | 2%C | D | C | D*C | ||
| DM (%) | 15 | 28.4 c | 29.7 bB | 31.3 a | 0.43 | NS | ** | ** |
| 30 | 29.1 b | 30.7 aA | 30.5 a | 0.26 | ||||
| pH | 15 | 5.09 a | 4.93 b | 4.85 c | 0.04 | NS | ** | NS |
| 30 | 5.08 a | 5.03 a | 4.85 b | 0.04 | ||||
| Lactic acid (%DM) | 15 | 1.27 | 1.27 | 1.26 B | 0.01 | NS | * | * |
| 30 | 1.19 b | 1.21 b | 1.38 aA | 0.04 | ||||
| Acetic acid (%DM) | 15 | 0.82 | 0.78 | 0.81 | 0.03 | NS | NS | NS |
| 30 | 0.78 | 0.84 | 0.84 | 0.04 | ||||
| Propionic acid (%DM) | 15 | ND | ND | ND | - | - | - | - |
| 30 | ND | ND | ND | - | ||||
| Butyric acid (%DM) | 15 | ND | ND | ND | - | - | NS | - |
| 30 | 0.51 | 0.84 | ND | 0.10 | ||||
| Lactic acid bacteria (log10 cfu/g FM) | 15 | 8.54 A | 8.54 A | 8.68 A | 0.05 | ** | NS | NS |
| 30 | 8.07 B | 8.18 B | 8.12 B | 0.03 | ||||
| Yeasts (log10 cfu/g FM) | 15 | <2.00 | <2.00 | <2.00 | - | - | - | - |
| 30 | <2.00 | <2.00 | <2.00 | - | ||||
| Molds (log10 cfu/g FM) | 15 | <2.00 | <2.00 | <2.00 | - | - | - | - |
| 30 | <2.00 | <2.00 | <2.00 | - | ||||
| Coliform bacteria (log10 cfu/g FM) | 15 | 5.73 a | 4.79 b | 4.02 b | 0.27 | - | ** | - |
| 30 | <2.00 | <2.00 | <2.00 | - | ||||
| Items | Treatments | SEM | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| Ensiling Days | CK | 1%C | 2%C | D | C | D*C | ||
| Crude protein (%DM) | 15 | 14.1 | 13.8 | 13.6 | 0.11 | NS | ** | NS |
| 30 | 14.2 a | 13.7 ab | 13.5 b | 0.12 | ||||
| True protein (%TN) | 15 | 36.0 c | 37.4 b | 40.0 aA | 0.60 | ** | ** | ** |
| 30 | 34.9 b | 37.6 a | 37.7 aB | 0.49 | ||||
| Nonprotein-N (%TN) | 15 | 64.0 a | 62.6 b | 60.0 cB | 0.60 | ** | ** | ** |
| 30 | 65.1 a | 62.4 b | 62.3 bA | 0.49 | ||||
| Ammonia–N (%TN) | 15 | 6.60 aB | 6.20 aB | 5.20 bB | 0.22 | ** | ** | NS |
| 30 | 8.10 aA | 7.44 bA | 6.68 cA | 0.22 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Zhang, Q.; Wang, M. A Preliminary Investigation on Bacterial Diversity and Fermentation Quality of High-Moisture Alfalfa Silage Prepared with Biochar. Agronomy 2021, 11, 1971. https://doi.org/10.3390/agronomy11101971
Guo X, Zheng M, Wu S, Zou X, Chen X, Zhang Q, Wang M. A Preliminary Investigation on Bacterial Diversity and Fermentation Quality of High-Moisture Alfalfa Silage Prepared with Biochar. Agronomy. 2021; 11(10):1971. https://doi.org/10.3390/agronomy11101971
Chicago/Turabian StyleGuo, Xiang, Mingyang Zheng, Shuo Wu, Xuan Zou, Xiaoyang Chen, Qing Zhang, and Mingya Wang. 2021. "A Preliminary Investigation on Bacterial Diversity and Fermentation Quality of High-Moisture Alfalfa Silage Prepared with Biochar" Agronomy 11, no. 10: 1971. https://doi.org/10.3390/agronomy11101971
APA StyleGuo, X., Zheng, M., Wu, S., Zou, X., Chen, X., Zhang, Q., & Wang, M. (2021). A Preliminary Investigation on Bacterial Diversity and Fermentation Quality of High-Moisture Alfalfa Silage Prepared with Biochar. Agronomy, 11(10), 1971. https://doi.org/10.3390/agronomy11101971






