Accumulation of Phenolic Compounds and Glucosinolates in Sprouts of Pale Green and Purple Kohlrabi (Brassica oleracea var. gongylodes) under Light and Dark Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Glucosinolates and HPLC Analysis
2.3. Extraction of PCs and HPLC Analysis
2.4. Statistical Analysis
3. Results and Discussion
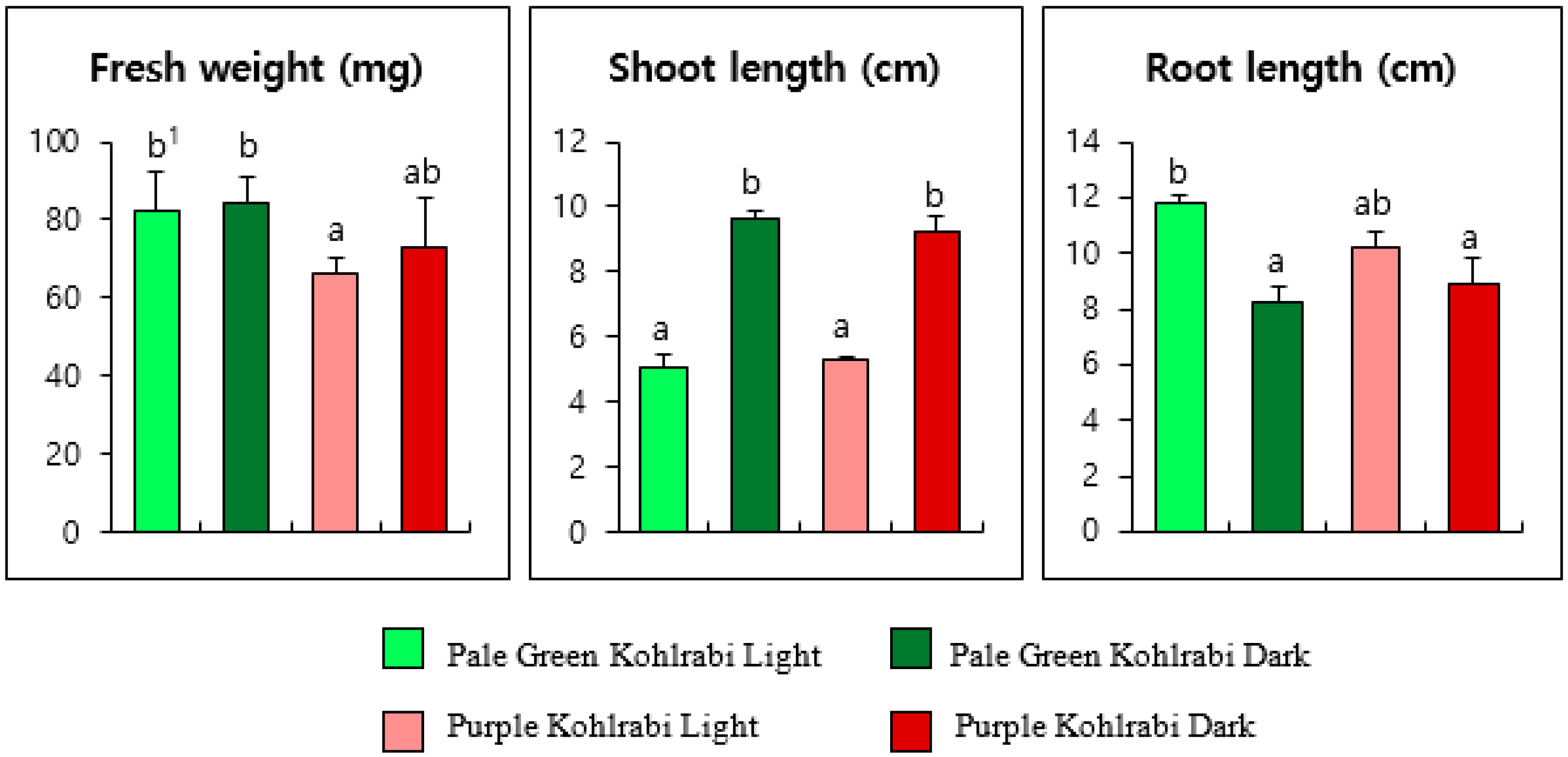
3.1. Effect of Light and Dark Treatment on the Growth of PGK and PK Sprouts
3.2. Accumulation of PCs during the Development of PGK and PK Sprouts under Dark and Light Conditions
3.3. Accumulation of Glucosinolates during the Development of PGK and PK Sprouts under Dark and Light Conditions
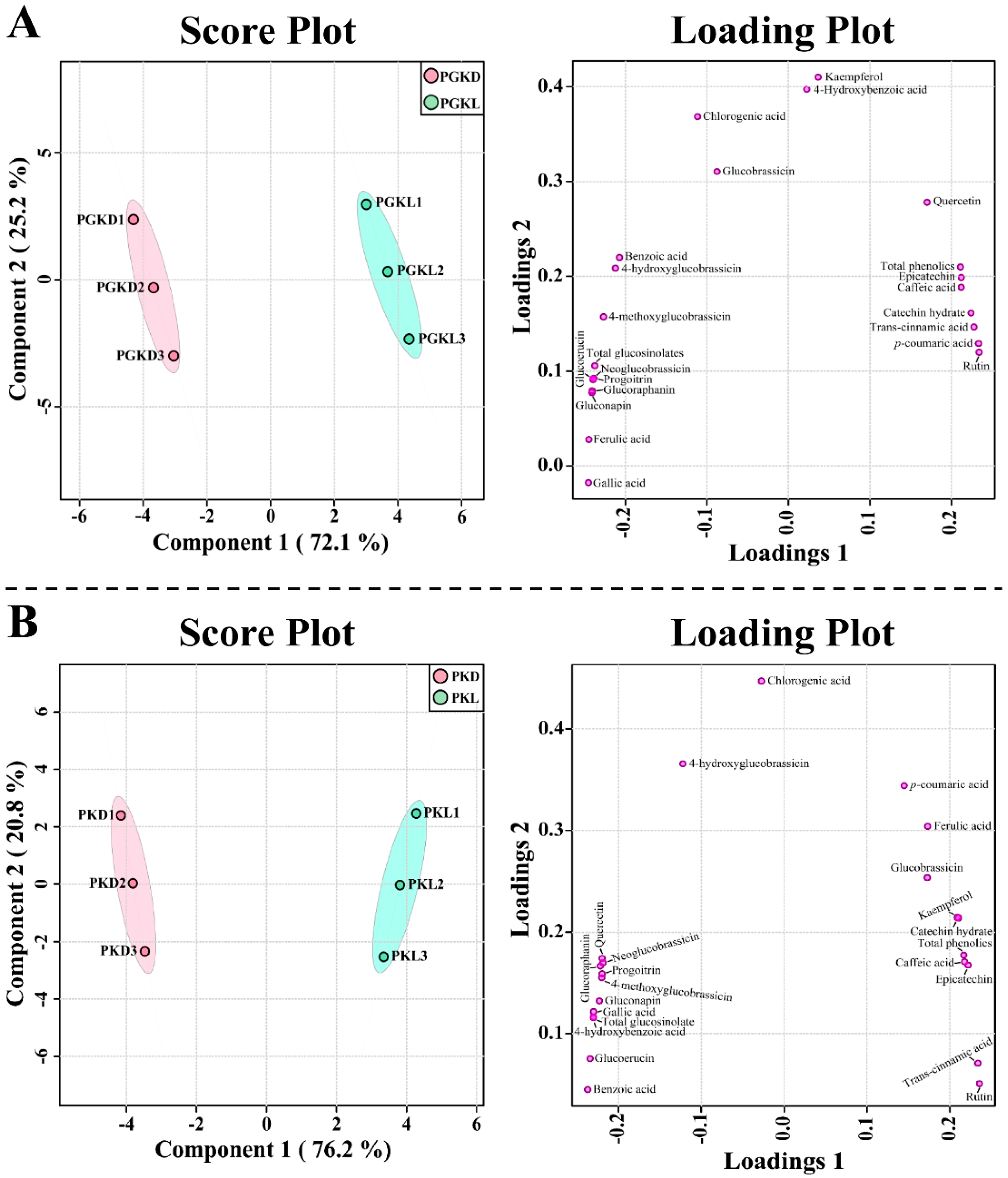
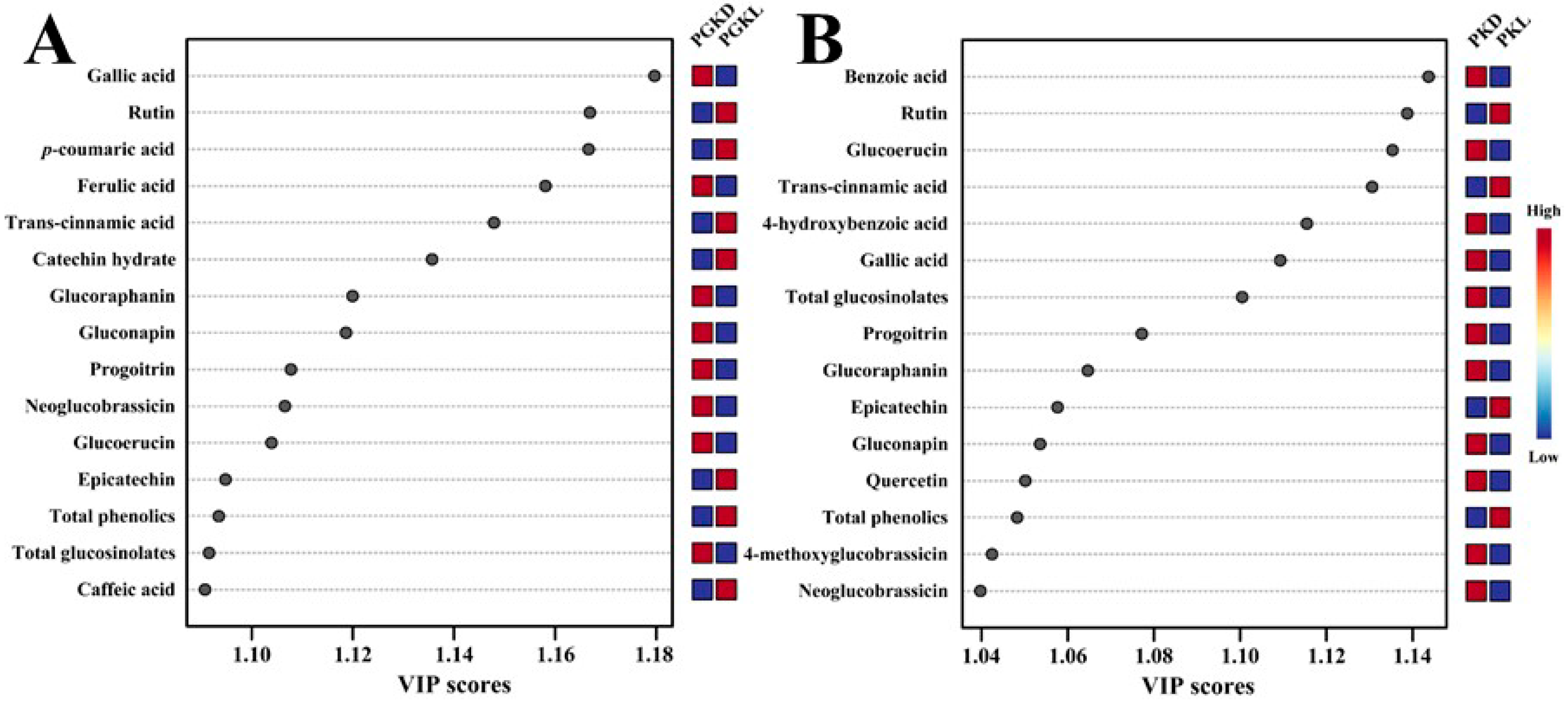
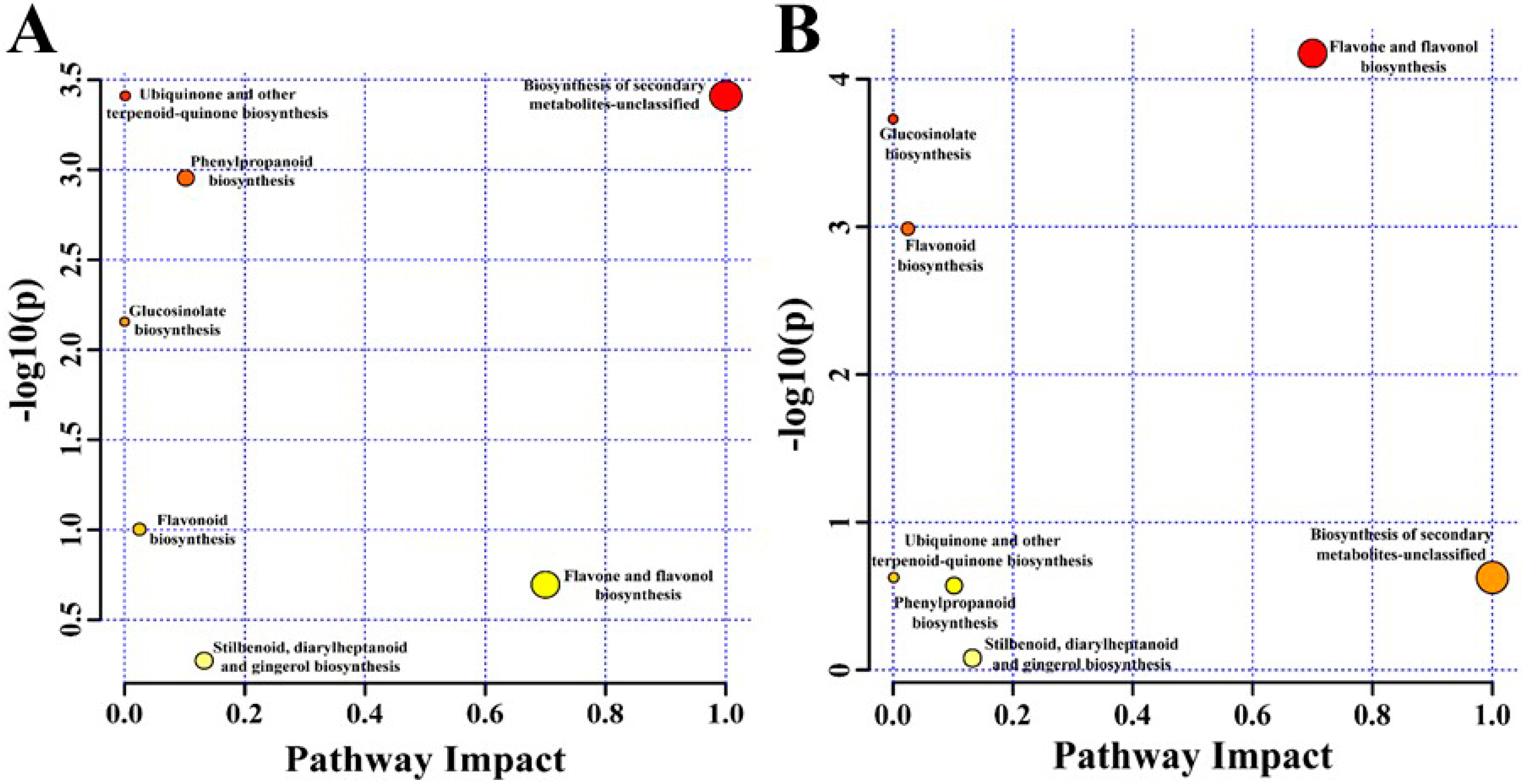
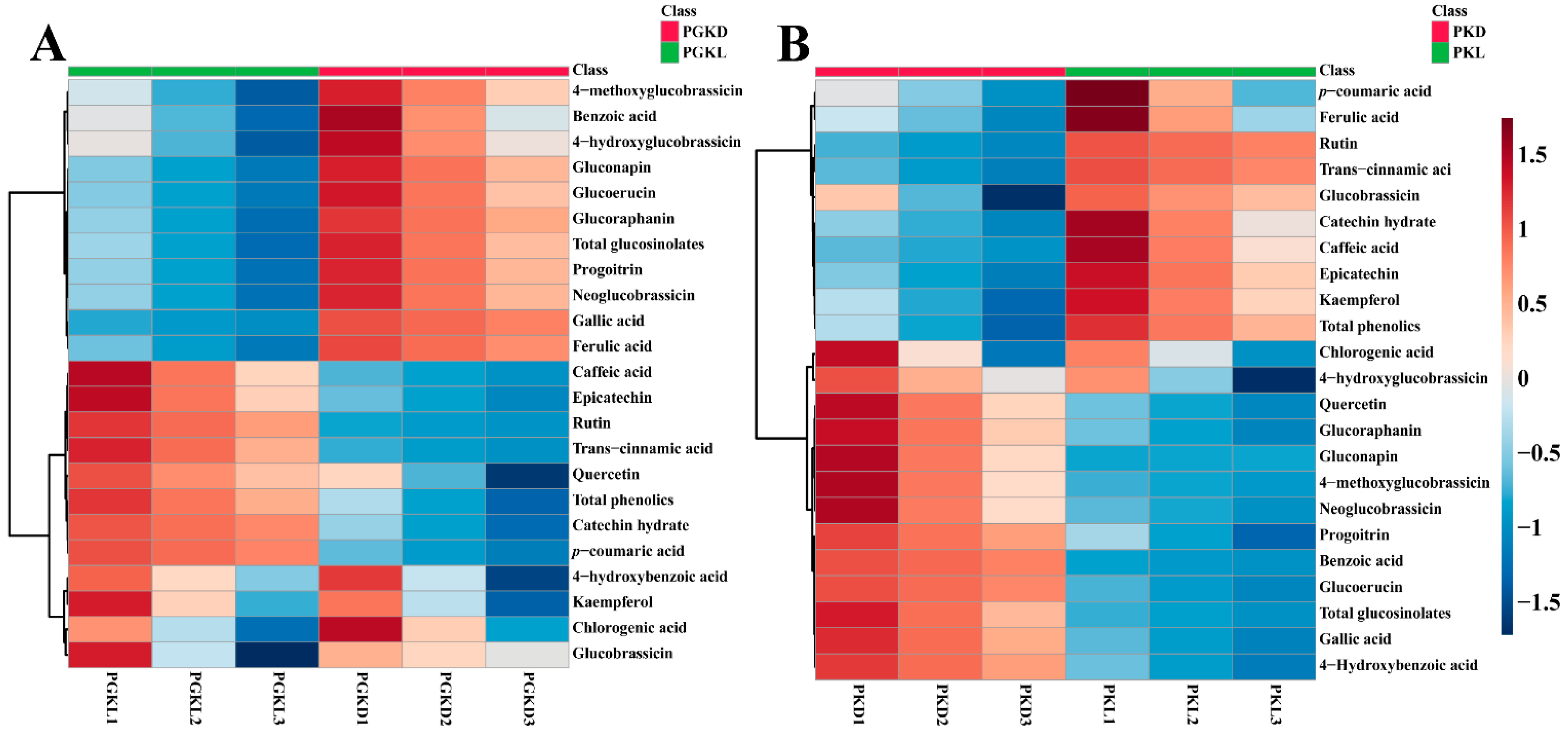
3.4. Profiles of PCs and Glucosinolates during the Development of PGK and PK Sprouts under Light and Dark Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.-W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.-J.; Park, S.U. Metabolic profiling of glucosinolates, anthocyanins, carotenoids, and other secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Karki, S.; Ehom, N.-Y.; Yoon, M.-H.; Kim, E.J.; Choi, J.S. Anti-diabetic and anti-inflammatory effects of green and red kohlrabi cultivars (Brassica oleracea var. gongylodes). Prev. Nutr. Food Sci. 2014, 19, 281. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [Green Version]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Lee, H.; Oh, I.-N.; Kim, J.; Jung, D.; Cuong, N.P.; Kim, Y.; Lee, J.; Kwon, O.; Park, S.U.; Lim, Y. Phenolic compound profiles and their seasonal variations in new red-phenotype head-forming Chinese cabbages. LWT 2018, 90, 433–439. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Kim, Y.B.; Li, X.; Kim, S.-J.; Kim, H.H.; Lee, J.; Kim, H.; Park, S.U. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef] [Green Version]
- Al-Gendy, A.; El-Gindi, O.; Hafez, A.S.; Ateya, A. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae). Food Chem. 2010, 118, 519–524. [Google Scholar] [CrossRef]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Folta, K.M. Environmentally modified organisms–expanding genetic potential with light. Crit. Rev. Plant Sci. 2014, 33, 486–508. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Folta, K.M. Sequential light programs shape kale (Brassica napus) sprout appearance and alter metabolic and nutrient content. Hortic. Res. 2014, 1, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, H.J.; Baek, S.-A.; Sathasivam, R.; Kim, J.K.; Park, S.U. Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa subsp. chinensis). Appl. Biol. Chem. 2021, 64, 1–16. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.-J.; Chung, E.; Lee, J.-H.; Park, S.U. Accumulation of anthocyanin and associated gene expression in radish sprouts exposed to light and methyl jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Thwe, A.A.; Park, N.I.; Suzuki, T.; Kim, S.J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression during the development of tartary buckwheat sprouts. J. Agric. Food Chem. 2012, 60, 5629–5635. [Google Scholar] [CrossRef]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Bellini, E.; Martelli, M. Anthocyanin synthesis in radish seedlings: Effects of continuous far red irradiation and phytochrome transformations. Z. Pflanzenphysiol. 1973, 70, 12–21. [Google Scholar] [CrossRef]
- Pizarro, L.; Stange, C. Light-dependent regulation of carotenoid biosynthesis in plants. Cienc. Investig. Agrar. 2009, 36, 143–162. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Kim, J.K.; Park, S.U. An update on biosynthesis and regulation of carotenoids in plants. S. Afr. J. Bot. 2021, 140, 290–302. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, J.K.; Kim, Y.B.; Lee, S.; Park, S.U. Molecular characterisation and the light–dark regulation of carotenoid biosynthesis in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Chem. 2013, 141, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, D.; Arakawa, O. Induction of phenolic compounds biosynthesis with light irradiation in the flesh of red and yellow apples. J. Appl. Hortic. 2006, 8, 101–104. [Google Scholar] [CrossRef]
- Feys, B.J.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saniewski, M.; Miyamoto, K.; Ueda, J. Methyl jasmonate induces gums and stimulates anthocyanin accumulation in peach shoots. J. Plant Growth Regul. 1998, 17, 121–124. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Grimes, H.D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc. Natl. Acad. Sci. USA 1991, 88, 6745–6749. [Google Scholar] [CrossRef] [Green Version]
- Saniewski, M.; Miszczak, A.; Kawa-Miszczak, L.; Wegrzynowicz-Lesiak, E.; Miyamoto, K.; Ueda, J. Effects of methyl jasmonate on anthocyanin accumulation, ethylene production, and CO2 evolution in uncooled and cooled tulip bulbs. J. Plant Growth Regul. 1998, 17, 33–37. [Google Scholar] [CrossRef]
- Francisco, M.; Velasco, P.; Moreno, D.A.; García-Viguera, C.; Cartea, M.E. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res. Int. 2010, 43, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of flavonoids and hydroxycinnamic acids in pak choi varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC–ESI-MS n and NMR and Their Quantification by HPLC–DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef]
- Fernandes, F.; Valentão, P.; Sousa, C.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem. 2007, 105, 1003–1010. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Vrchovská, V.; Valentão, P.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical composition and antioxidant activity of tronchuda cabbage internal leaves. Eur. Food Res. Technol. 2006, 222, 88–98. [Google Scholar] [CrossRef]
- Kim, S.-J.; Zaidul, I.; Maeda, T.; Suzuki, T.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. A time-course study of flavonoids in the sprouts of tartary (Fagopyrum tataricum Gaertn.) buckwheats. Sci. Hortic. 2007, 115, 13–18. [Google Scholar] [CrossRef]
- Watanabe, M. An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Biosci. Biotechnol. Biochem. 2007, 71, 579–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Park, C.H.; Bong, S.J.; Lim, C.J.; Kim, J.K.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red mizuna (Brassica rapa L. var. japonica). Foods 2020, 9, 1079. [Google Scholar] [CrossRef]
- Park, C.H.; Kwon, S.-J.; Kim, N.S.; Baek, S.-A.; Yeo, H.J.; Park, Y.E.; Chung, Y.S.; Kim, J.K.; Park, S.U. Metabolic analysis of carotenoids and phenolic compounds found in green and purple kenaf. Nat. Prod. Commun. 2020, 15, 1934578X20971138. [Google Scholar]
- Rashid, A.; Ali, V.; Khajuria, M.; Faiz, S.; Gairola, S.; Vyas, D. GC–MS based metabolomic approach to understand nutraceutical potential of Cannabis seeds from two different environments. Food Chem. 2021, 339, 128076. [Google Scholar] [CrossRef]
- Raval, S.S.; Mahatma, M.K.; Chakraborty, K.; Bishi, S.K.; Singh, A.L.; Rathod, K.J.; Jadav, J.K.; Sanghani, J.M.; Mandavia, M.K.; Gajera, H.P. Metabolomics of groundnut (Arachis hypogaea L.) genotypes under varying temperature regimes. Plant Growth Regul. 2018, 84, 493–505. [Google Scholar] [CrossRef]
- Yan, S.; Huang, W.; Gao, J.; Fu, H.; Liu, J. Comparative metabolomic analysis of seed metabolites associated with seed storability in rice (Oryza sativa L.) during natural aging. Plant Physiol. Biochem. 2018, 127, 590–598. [Google Scholar] [CrossRef]
- Jacob, A.; Malpathak, N. Green hairy root cultures of Solanum khasianum Clarke—A new route to in vitro solasodine production. Curr. Sci. 2004, 87, 1442–1447. [Google Scholar]
- Thwe, A.A.; Kim, Y.; Li, X.; Kim, Y.B.; Park, N.-I.; Kim, H.H.; Kim, S.-J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression in hairy roots of tartary buckwheat under light and dark conditions. Appl. Biochem. Biotechnol. 2014, 174, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]

| Trivial Name | Pale Green Kohlrabi | Purple Kohlrabi | ||
|---|---|---|---|---|
| Light | Dark | Light | Dark | |
| Gallic acid | 100.60 ± 3.73 c 1 | 168.70 ± 4.71 a | 121.00 ± 6.78 b | 176.31 ± 10.84 a |
| 4-hydroxybenzoic acid | 110.43 ± 6.51 b | 106.76 ± 12.06 c | 183.39 ± 8.32 b | 238.98 ± 8.28 a |
| Catechin hydrate | 262.03 ± 4.28 a | 205.25 ± 14.13 b | 243.94 ± 49.11 ab | 143.31 ± 19.16 c |
| Chlorogenic acid | 80.14 ± 20.62 a | 92.63 ± 24.15 a | 80.01 ± 13.19 a | 83.07 ± 19.40 a |
| Caffeic acid | 38.86 ± 7.01 a | 19.48 ± 1.43 b | 40.79 ± 6.95 a | 24.88 ± 1.35 b |
| Epicatechin | 930.39 ± 43.55 c | 800.69 ± 16.91 d | 1350.25 ± 76.97 a | 1107.11 ± 44.57 b |
| p-coumaric acid | 131.97 ± 2.92 b | 93.55 ± 5.30 c | 187.13 ± 19.45 a | 170.44 ± 7.36 a |
| Ferulic acid | 272.17 ± 52.26 b | 595.38 ± 32.53 a | 100.46 ± 19.42 c | 76.72 ± 8.32 c |
| Benzoic acid | 1856.83 ± 102.68 b | 2083.99 ± 133.45 a | 523.71 ± 23.32 d | 1221.26 ± 47.93 c |
| Rutin | 2166.00 ± 177.71 c | 1002.35 ± 39.62 d | 6852.39 ± 75.22 a | 5717.95 ± 101.61 b |
| Trans-cinnamic acid | 65.65 ± 9.14 b | 23.41 ± 2.48 c | 78.38 ± 4.11 a | 29.35 ± 6.28 c |
| Quercetin | 197.01 ± 10.90 a | 148.95 ± 31.60 b | 152.83 ± 10.96 b | 232.77 ± 28.56 a |
| Kaempferol | 150.03 ± 31.56 ab | 133.91 ± 34.00 b | 201.49 ± 21.03 a | 139.61 ± 20.27 b |
| Total | 6362.13 ± 171.73 c | 5475.04 ± 272.56 d | 10,115.76 ± 168.17 a | 9361.74 ± 238.39 b |
| Trivial Name | Pale Green Kohlrabi | Purple Kohlrabi | ||
|---|---|---|---|---|
| Light | Dark | Light | Dark | |
| Progoitrin | 5194.60 ± 804.86 b 1 | 11,249.77 ± 829.59 a | 124.61 ± 13.49 c | 206.38 ± 6.74 c |
| Glucoraphanin | 385.00 ± 50.52 c | 748.13 ± 37.89 b | 700.00 ± 68.20 b | 1500.63 ± 146.50 a |
| Gluconapin | 350.06 ± 55.90 b | 863.97 ± 70.95 a | 26.07 ± 0.00 c | 55.86 ± 6.45 c |
| 4-hydroxyglucobrassicin | 339.09 ± 45.59 c | 501.66 ± 45.59 bc | 664.24 ± 96.54 ab | 803.59 ± 42.91 a |
| Glucoerucin | 1605.92 ± 245.79 b | 3789.29 ± 350.43 a | 1327.73 ± 114.38 b | 3300.35 ± 94.91 a |
| Glucobrassicin | 832.28 ± 131.75 a | 899.39 ± 23.25 a | 689.09 ± 15.50 ab | 550.38 ± 59.42 b |
| 4-methoxyglucobrassicin | 2330.30 ± 356.38 b | 3851.93 ± 279.02 a | 1272.81 ± 24.86 c | 2153.25 ± 201.67 b |
| Neoglucobrassicin | 2373.36 ± 375.72 b | 5143.88 ± 378.48 a | 894.80 ± 46.96 c | 1756.10 ± 196.15 bc |
| Total | 13,410.58 ± 2066.50 b | 27,048.00 ± 2015.20 a | 5699.33 ± 379.94 c | 10,326.52 ± 754.75 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathasivam, R.; Kim, M.C.; Yeo, H.J.; Nguyen, B.V.; Sohn, S.I.; Park, S.U.; Kim, J. Accumulation of Phenolic Compounds and Glucosinolates in Sprouts of Pale Green and Purple Kohlrabi (Brassica oleracea var. gongylodes) under Light and Dark Conditions. Agronomy 2021, 11, 1939. https://doi.org/10.3390/agronomy11101939
Sathasivam R, Kim MC, Yeo HJ, Nguyen BV, Sohn SI, Park SU, Kim J. Accumulation of Phenolic Compounds and Glucosinolates in Sprouts of Pale Green and Purple Kohlrabi (Brassica oleracea var. gongylodes) under Light and Dark Conditions. Agronomy. 2021; 11(10):1939. https://doi.org/10.3390/agronomy11101939
Chicago/Turabian StyleSathasivam, Ramaraj, Min Cheol Kim, Hyeon Ji Yeo, Bao Van Nguyen, Soo In Sohn, Sang Un Park, and Joonyup Kim. 2021. "Accumulation of Phenolic Compounds and Glucosinolates in Sprouts of Pale Green and Purple Kohlrabi (Brassica oleracea var. gongylodes) under Light and Dark Conditions" Agronomy 11, no. 10: 1939. https://doi.org/10.3390/agronomy11101939
APA StyleSathasivam, R., Kim, M. C., Yeo, H. J., Nguyen, B. V., Sohn, S. I., Park, S. U., & Kim, J. (2021). Accumulation of Phenolic Compounds and Glucosinolates in Sprouts of Pale Green and Purple Kohlrabi (Brassica oleracea var. gongylodes) under Light and Dark Conditions. Agronomy, 11(10), 1939. https://doi.org/10.3390/agronomy11101939









