A Research Method for Semi-Automated Large-Scale Cultivation of Maize to Full Maturity in an Artificial Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grow Room
2.2. Nutrients and Irrigation
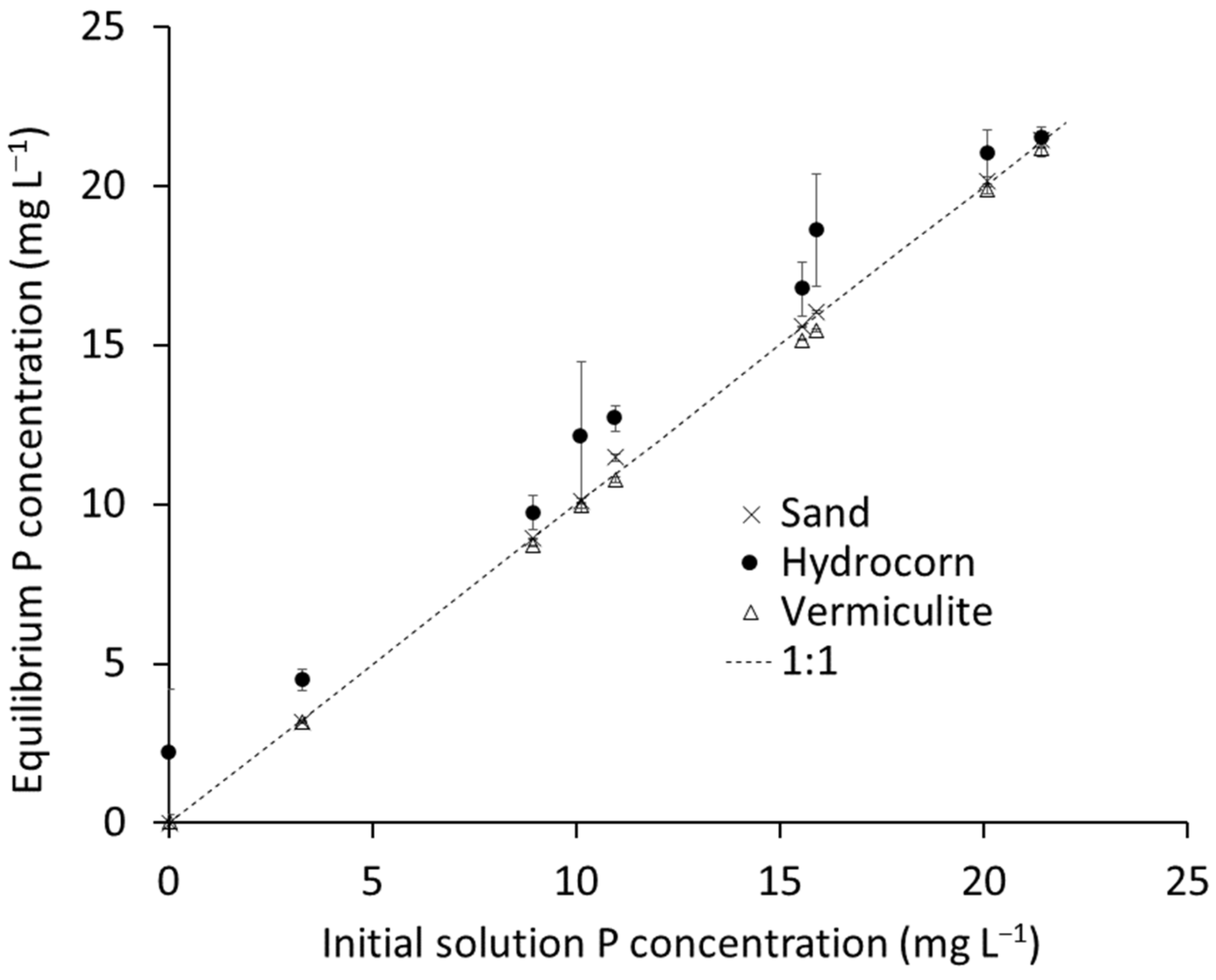
2.3. Growth Media
2.4. Plant Establishment and Management
2.5. Plant Harvest and Analysis
2.6. Statistical Analysis
3. Results and Discussion
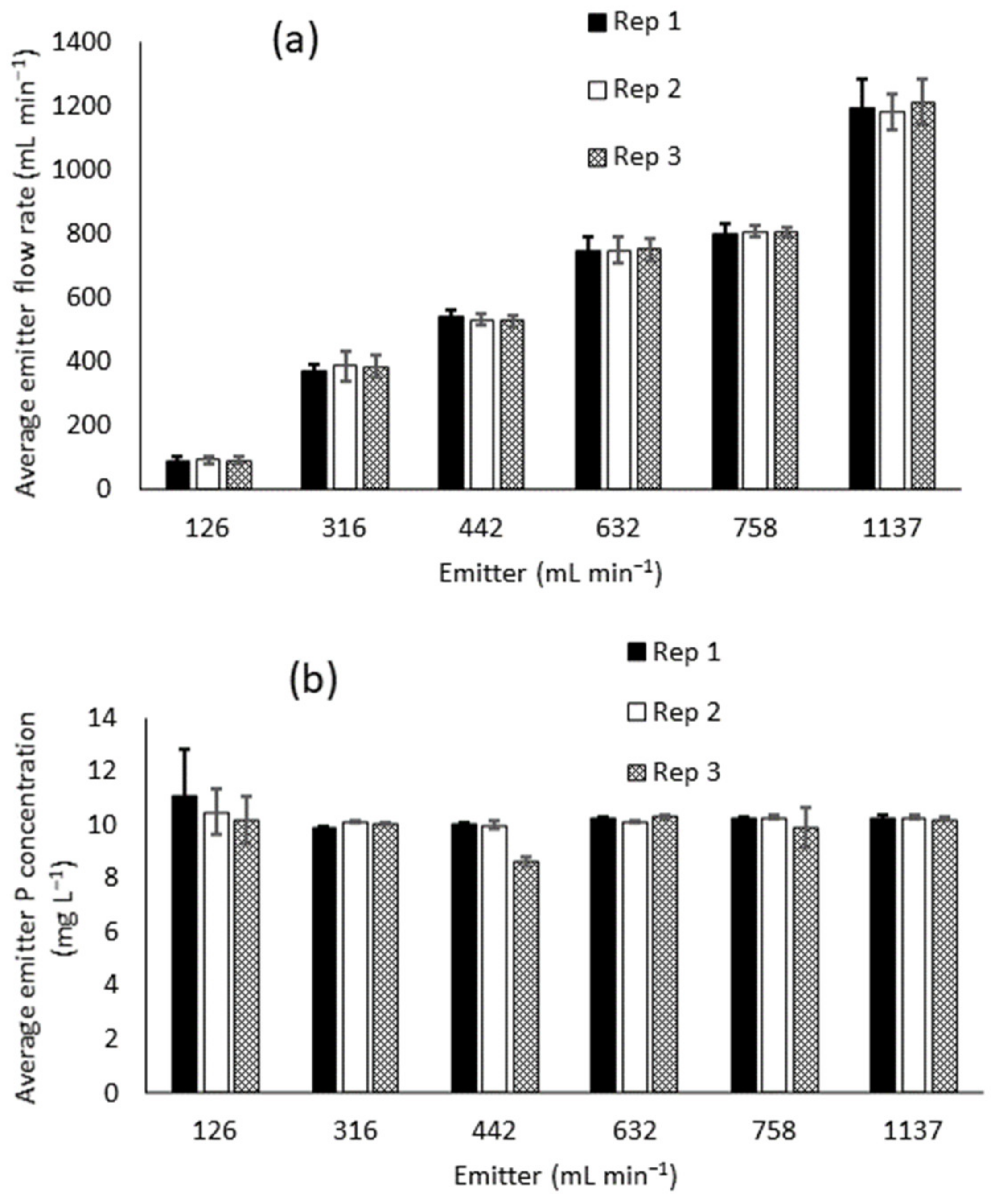
3.1. Emitter Performance
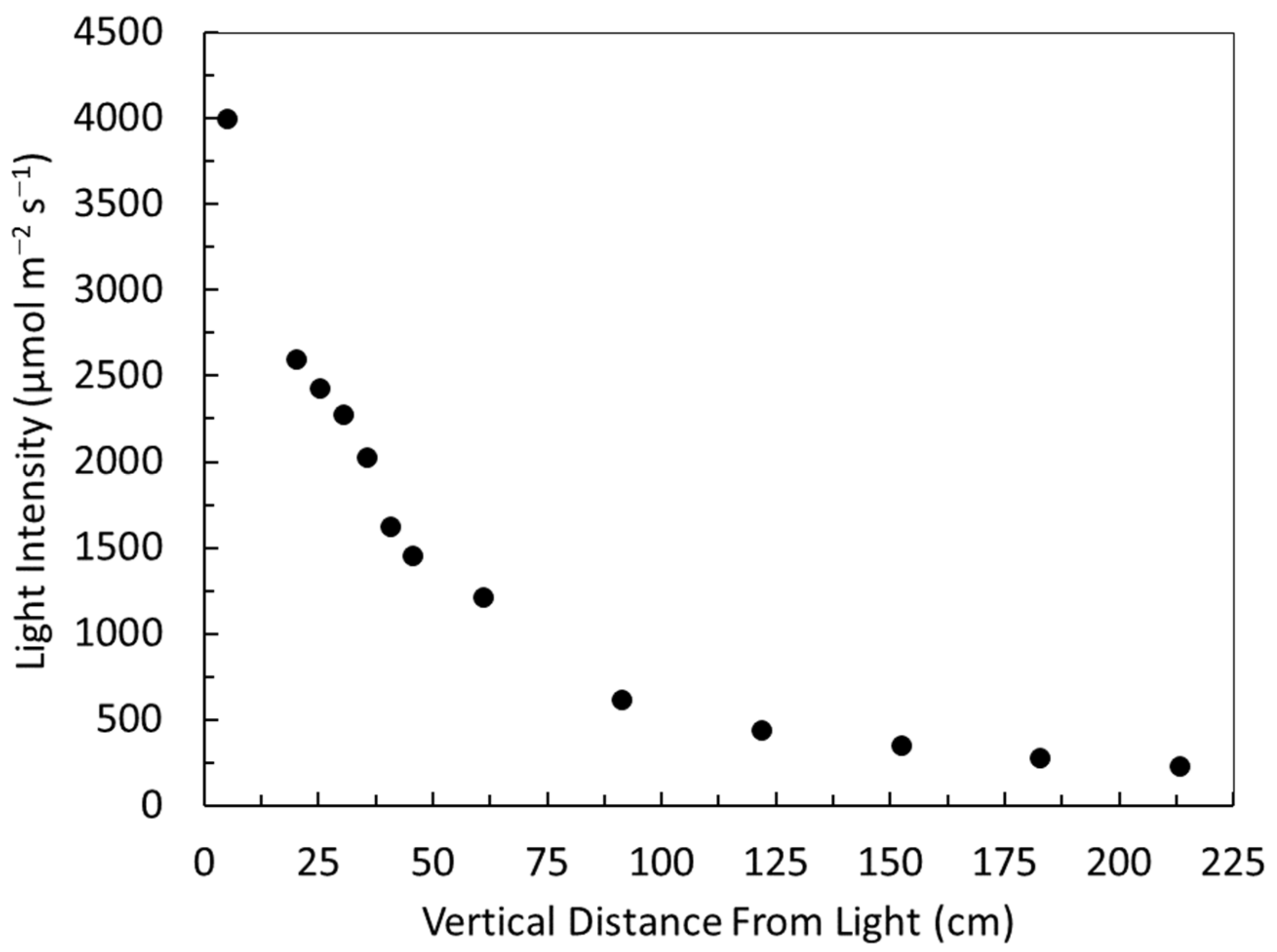
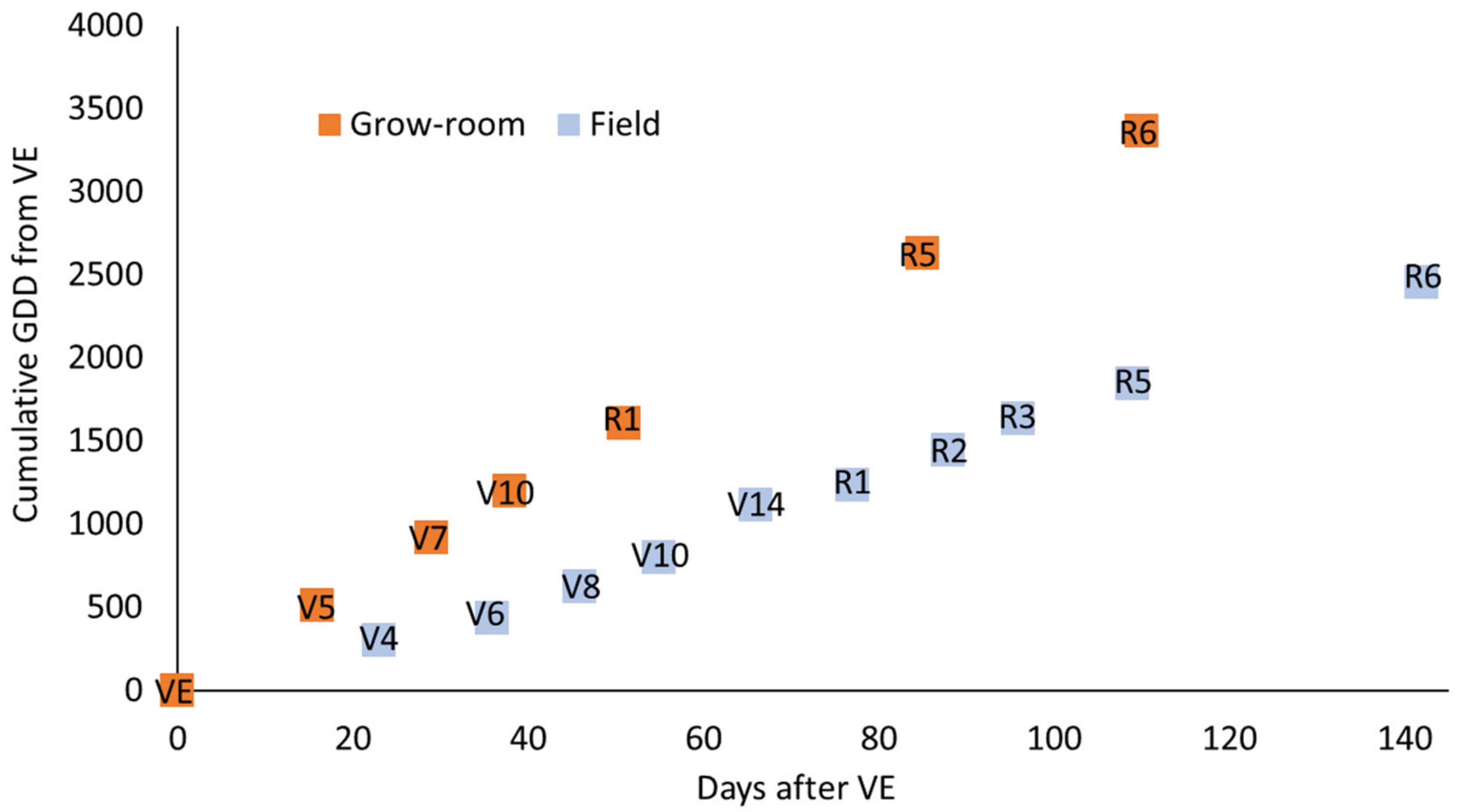
3.2. Light Quantity, Quality, Distribution and Duration
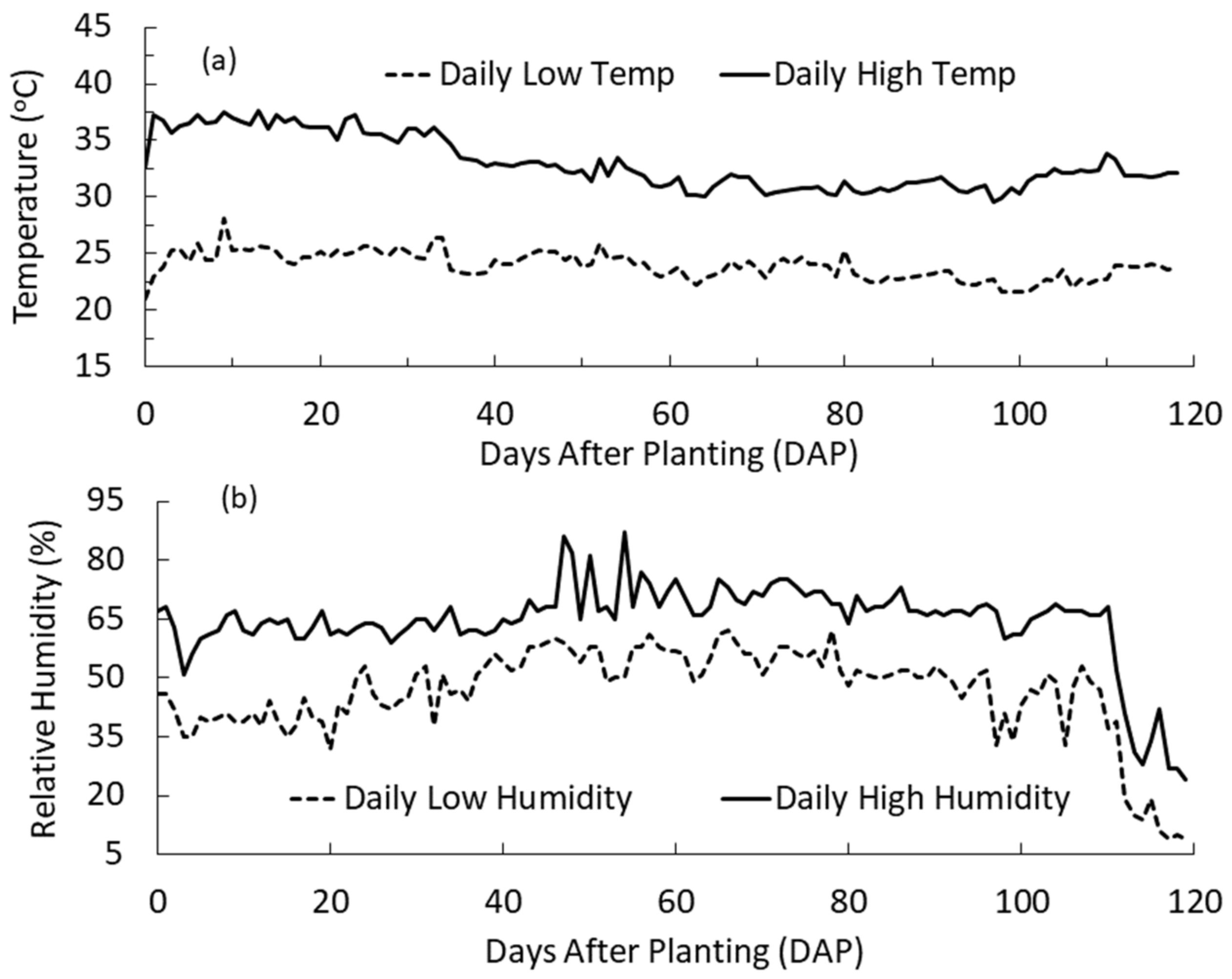
3.3. Temperature, Humidity and Air Exchange
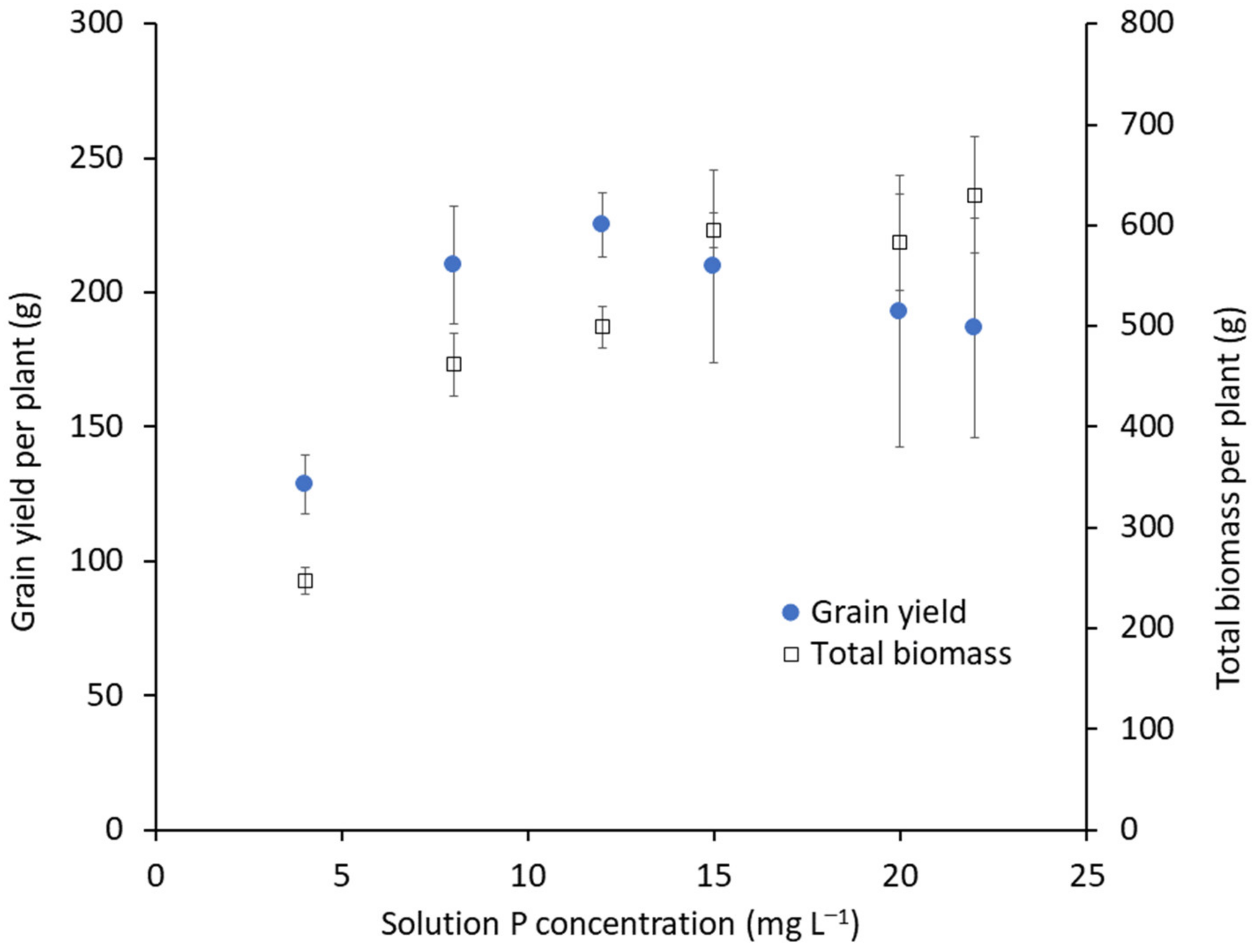
3.4. Plant Performance Data
4. Summary and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Disclaimer
Conflicts of Interest
References
- Wang, L.; Wang, B. Greenhouse microclimate environment adaptive control based on a wireless sensor network. Int. J. Agric. Biol. Eng. 2020, 13, 64–69. [Google Scholar]
- Katagiri, F.; Canelon-Suarez, D.; Griffin, K.; Petersen, J.; Meyer, R.K.; Siegle, M.; Mase, K. Design and construction of an inexpensive homemade plant growth chamber. PLoS ONE 2015, 10, e0126826. [Google Scholar]
- Potvin, C.; Tardif, S. Sources of variability and experimental designs in growth chambers. Funct. Ecol. 1988, 2, 123–130. [Google Scholar]
- Jones, P.; Jones, J.W.; Allen, L.H.; Mishoe, J.W. Dynamic computer control of closed environmental plant growth chambers. Design and verification. Trans. ASAE 1984, 27, 879–888. [Google Scholar]
- Eddy, R.; Hahn, D.T. Optimizing Greenhouse Corn Production: What Is the Best Lighting and Plant Density? Purdue University e-Pubs: West Lafayette, IN, USA, 2010. [Google Scholar]
- Fang, L.; Ma, Z.; Wang, Q.; Nian, H.; Ma, Q.; Huang, Q.; Mu, Y. Plant growth and photosynthetic characteristics of soybean seedlings under different led lighting quality conditions. J. Plant Growth Regul. 2021, 40, 668–678. [Google Scholar]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995; ISBN 0471587478. [Google Scholar]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Groleau-Renaud, V.; Plantureux, S.; Guckert, A. Influence of plant morphology on root exudation of maize subjected to mechanical impedance in hydroponic conditions. Plant Soil 1998, 201, 231–239. [Google Scholar]
- Nantawisarakul, T.; Newman, I.A. Growth and gravitropism of corn roots in solution. Plant Cell Environ. 1992, 15, 693–701. [Google Scholar]
- Abdalla, A.M.; Hettiaratchi, D.R.P.; Reece, A.R. The mechanics of root growth in granular media. J. Agric. Eng. Res. 1969, 14, 236–248. [Google Scholar]
- Ryan, P.R.; Newman, I.A.; Shields, B. Ion fluxes in corn roots measured by microelectrodes with ion-specific liquid membranes. J. Memb. Sci. 1990, 53, 59–69. [Google Scholar]
- Smith, G.S.; Johnston, C.M.; Cornforth, I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983, 94, 537–548. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar]
- Kovar, J.L.; Barber, S.A. Reasons for differences among soils in placement of phosphorus for maximum predicted uptake. Soil Sci. Soc. Am. J. 1989, 53, 1733–1736. [Google Scholar]
- Kanai, H.; Sakai, A. An obligate-halophytic mangrove, Rhizophora mucronate, does not require Na+ for the uptake of nutrient ions in their roots. Aquat. Bot. 2021, 169, 103328. [Google Scholar]
- Timlin, D.J.; Naidu, T.C.M.; Fleisher, D.H.; Reddy, V.R. Quantitative effects of phosphorus on maize canopy photosynthesis and biomass. Crop Sci. 2017, 57, 3156–3169. [Google Scholar]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Special Report No. 48; Iowa State University: Ames, IA, USA, 1986. [Google Scholar]
- SAS/STAT. SAS/STAT User’s Guide, Version 9.1; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Barry, D.A.J.; Miller, M.H. Phosphorus nutritional requirement of maize seedlings for maximum yield. Agron. J. 1989, 81, 95–99. [Google Scholar]
- Katzin, D.; Marcelis, L.F.M.; van Mourik, S. Energy savings in greenhouses by transition from high-pressure sodium to LED lighting. Appl. Energy 2021, 281, 116019. [Google Scholar]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar]
- Xue, J.; Gou, L.; Zhao, Y.; Yao, M.; Yao, H.; Tian, J.; Zhang, W. Effects of light intensity within the canopy on maize lodging. Field Crop. Res. 2016, 188, 133–141. [Google Scholar]
- Ottman, M.J.; Welch, L.F. Supplemental radiation effects on senescence, plant nutrients, and yield of field-grown corn. Agron. J. 1988, 80, 619–626. [Google Scholar]
- He, P.; Zhou, W.; Jin, J. Effect of nitrogen application on redistribution and transformation of photosynthesized 14C during grain formation in two maize cultivars with different senescence appearance. J. Plant Nutr. 2002, 25, 2443–2456. [Google Scholar]
- Cliquet, J.-B.; Deleens, E.; Mariotti, A. C and N mobilization from stalk and leaves during kernel filling by 13C and 15N tracing in Zea mays L. Plant Physiol. 1990, 94, 1547–1553. [Google Scholar]
- Swank, J.C.; Below, F.E.; Lambert, R.J.; Hageman, R.H. Interaction of carbon and nitrogen metabolism in the productivity of maize. Plant Physiol. 1982, 70, 1185–1190. [Google Scholar] [PubMed] [Green Version]
- Goodman, A.M.; Ennos, A.R. The effects of mechanical stimulation on the morphology and mechanics of maize roots grown in an aerated nutrient solution. Int. J. Plant Sci. 2001, 162, 691–696. [Google Scholar]
- Goodman, A.M.; Ennos, A.R. A comparative study of the response of the roots and shoots of sunflower and maize to mechanical stimulation. J. Exp. Bot. 1996, 47, 1499–1507. [Google Scholar]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development; Iowa State University Extension Publication PMR1009: Ames, IA, USA, 2011. [Google Scholar]
- NASS, U. Quick Stats; USDA National Agricultural Statistics Service: Washington, DC, USA, 2017.
- Nielsen, R.L.; Camberato, J.; Lee, J. Yield Response of Corn to Plant Population in Indiana. Purdue University Department of Agronomy Applied Crop Production Research Update. 2019. Available online: http://www.kingcorn.org/news/timeless/CornPopulations.pdf (accessed on 19 June 2021).
- Boomsma, C.R.; Santini, J.B.; Tollenaar, M.; Vyn, T.J. Maize morphophysiological responses to intense crowding and low nitrogen availability: An analysis and review. Agron. J. 2009, 101, 1426–1452. [Google Scholar]
- Greveniotis, V.; Zotis, S.; Sioki, E.; Ipsilandis, C. Field population density effects on field yield and morphological characteristics of maize. Agriculture 2019, 9, 160. [Google Scholar]
- Karlen, D.L.; Flannery, R.L.; Sadler, E.J. Aerial accumulation and partitioning of nutrients by corn. Agron. J. 1988, 80, 232–242. [Google Scholar]
- Woli, K.P.; Sawyer, J.E.; Boyer, M.J.; Abendroth, L.J.; Elmore, R.W. Corn era hybrid macronutrient and dry matter accumulation in plant components. Agron. J. 2018, 110, 1648–1658. [Google Scholar]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013, 105, 161–170. [Google Scholar]
- Robles, M.; Ciampitti, I.A.; Vyn, T.J. Responses of maize hybrids to twin-row spatial arrangement at multiple plant densities. Agron. J. 2012, 104, 1747–1756. [Google Scholar]
- Swanson, S.; Wilhelm, W. Planting date and residue rate effects on growth, partitioning, and yield of corn. Agron. J. 1996, 88, 205–210. [Google Scholar]
- Overman, A.R.; Wilson, D.M.; Vidak, W.; Allhands, M.N.; Perry, T.C., Jr. Model for partitioning of dry matter and nutrients in corn. J. Plant Nutr. 1995, 18, 959–968. [Google Scholar]
- Mengel, D.B.; Barber, S.A. Development and distribution of the corn root system under field conditions. Agron. J. 1974, 66, 341–344. [Google Scholar]
- Ren, X.; Chen, X.; Jia, Z. Effect of rainfall collecting with ridge and furrow on soil moisture and root growth of corn in semiarid northwest China. J. Agron. Crop Sci. 2010, 196, 109–122. [Google Scholar]
- Eghball, B.; Maranville, J.W. Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agron. J. 1993, 85, 147–152. [Google Scholar]
- Ordóñez, R.A.; Castellano, M.J.; Danalatos, G.N.; Wright, E.E.; Hatfield, J.L.; Burras, L.; Archontoulis, S.V. Insufficient and excessive N fertilizer input reduces maize root mass across soil types. Field Crop. Res. 2021, 267, 108142. [Google Scholar]
- Heckman, J.R.; Sims, J.T.; Beegle, D.B.; Coale, F.J.; Herbert, S.J.; Bruulsema, T.W.; Bamka, W.J. Nutrient removal by corn grain harvest. Agron. J. 2003, 95, 587–591. [Google Scholar]
- A & L Laboratories; Ankerman, D.; Large, R. Agronomy Handbook: Soil and Plant Analysis; A & L Laboratories: Modesto, CA, USA, 2001. [Google Scholar]
- Zublena, J.P. Nutrient Removal by Crops in North Carolina; AG-North Carolina Agriculture Extension Services North Carolina State University: Raleigh, NC, USA, 1991. [Google Scholar]
- Codling, E.E.; Mulchi, C.L.; Chaney, R.L. Grain yield and mineral element composition of maize grown on high phosphorus soils amended with water treatment residual. J. Plant Nutr. 2007, 30, 225–240. [Google Scholar]
- Avila-Segura, M.; Barak, P.; Hedtcke, J.L.; Posner, J.L. Nutrient and alkalinity removal by corn grain, stover and cob harvest in Upper Midwest USA. Biomass Bioenergy 2011, 35, 1190–1195. [Google Scholar]
- Duarte, A.P.; Abreu, M.F.D.; Francisco, E.A.B.; Gitti, D.D.C.; Barth, G.; Kappes, C. Reference values of grain nutrient content and removal for corn. Rev. Bras. Ciênc. Solo 2019, 43, e0180102. [Google Scholar]
- Campbell, C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin #34; Food and Agriculture Organization: Raleigh, NC, USA, 2000. [Google Scholar]


| Nutrient | Solution Concentration (mg L−1) | Primary Source(s) |
|---|---|---|
| N | 180.00 | NH4NO3 & Ca(NO3)2 ● 4H2O |
| P | 4.00 to 22.00 | KH2PO4 |
| K | 120.00 | KCl |
| S | 73.90 | (NH4)2SO4 |
| Mg | 35.00 | MgSO4 ● 7H2O |
| Ca | 80.00 | Ca(NO3)2 ● 4H2O |
| Fe | 2.00 | Fe DTPA |
| Zn | 0.05 | ZnSO4 ● 7H2O |
| B | 0.25 | H3BO3 |
| Mn | 0.25 | MnCl2 ● 4H2O |
| Cu | 0.02 | CuSO4 ● 5H2O |
| Mo | 0.01 | (NH4)6Mo7O24 ● 4H2O |
| Item | Cost |
|---|---|
| Electric bill (monthly) | USD 550 |
| Lights (USD 1000) × 24 | USD 24,000 |
| Astronomical timer—lights | USD 500 |
| Timer—main exhaust fan | USD 200 |
| Timer-for AC and pedestal fans × 4 | USD 200 |
| Saturn 6 controller | USD 500 |
| Electrician labor | USD 1900 |
| Irrigation Controller | USD 300 |
| Dosatron injectors D25F1 1% (USD 280) × 8 | USD 2240 |
| Dosatron adjustable injector D14MZ520 | USD 1960 |
| Exhaust fan | USD 1100 |
| AC Unit (USD 600) × 2 | USD 1200 |
| Humidifier (USD 300) × 2 | USD 600 |
| Pedestal fans 30″ dia. (USD 250) × 2 | USD 500 |
| Pots | USD 240 |
| Silica sand | USD 865 |
| Plumbing supplies (PEX, Fittings, hardware, misc.) | USD 1200 |
| Wood, Aluminum sheeting (drainage trays) | USD 1950 |
| Materials for light mount system: galvanized struts, pulleys, wire rope, worm gear winches | USD 1515 |
| TOTAL (not including monthly electric) | USD 40,970 |
| Yield Component | Units | Min. | Max. | Mean | Std. Dev. | Median |
|---|---|---|---|---|---|---|
| Grain yield † | g plant−1 | 149.9 | 251.8 | 210.2 | 32.1 | 212.3 |
| Leaf biomass | g plant−1 | 67.6 | 99.4 | 81.8 | 10.7 | 79.9 |
| Stem biomass | g plant−1 | 77.9 | 164.9 | 108.7 | 29.6 | 93.1 |
| Root biomass | g plant−1 | 35.9 | 233.3 | 93.8 | 53.6 | 84.1 |
| Total biomass | g plant−1 | 364.5 | 619.0 | 461.8 | 67.2 | 445.1 |
| Harvest index | 0.35 | 0.55 | 0.48 | 0.07 | 0.51 | |
| Plant height | cm | 195.0 | 267.0 | 230.0 | 24.1 | 223.0 |
| Ear height | cm | 64.0 | 102.0 | 80.7 | 10.7 | 78.5 |
| Stem diameter | mm | 21.3 | 30.9 | 26.1 | 2.9 | 25.8 |
| Leaf | Stem | Root | Grain | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | Units | Min | Max | Mean | Med | Min | Max | Mean | Med | Min | Max | Mean | Med | Min | Max | Mean | Med |
| N | g kg−1 | 10.1 | 14.2 | 11.5 | 11.4 | 9.2 | 17.4 | 12.3 | 11.9 | 5.6 | 16.6 | 9.7 | 9.1 | 17.4 | 21.7 | 19.5 | 19.6 |
| P | g kg−1 | 0.51 | 0.76 | 0.62 | 0.60 | 0.24 | 0.66 | 0.43 | 0.40 | 0.25 | 0.53 | 0.38 | 0.36 | 1.90 | 3.03 | 2.30 | 2.24 |
| K | g kg−1 | 11.72 | 24.18 | 16.18 | 14.00 | 7.53 | 28.90 | 17.15 | 16.84 | 2.38 | 11.29 | 7.45 | 8.60 | 2.86 | 4.25 | 3.38 | 3.35 |
| S | g kg−1 | 3.47 | 6.39 | 4.44 | 4.28 | 0.92 | 2.14 | 1.46 | 1.43 | 1.46 | 3.86 | 2.76 | 2.66 | 1.21 | 1.55 | 1.39 | 1.40 |
| Mg | g kg−1 | 2.83 | 4.42 | 3.48 | 3.40 | 0.91 | 2.13 | 1.38 | 1.37 | 0.07 | 1.27 | 0.75 | 0.73 | 0.71 | 0.95 | 0.82 | 0.83 |
| Ca | g kg−1 | 5.72 | 10.18 | 7.05 | 6.78 | 0.73 | 2.35 | 1.54 | 1.59 | 0.74 | 1.72 | 1.32 | 1.38 | 0.00 | 0.04 | 0.02 | 0.03 |
| Fe | mg kg−1 | 31.4 | 70.7 | 46.2 | 45.3 | 17.8 | 39.2 | 26.0 | 22.3 | 202.9 | 670.6 | 350.0 | 312.9 | 12.9 | 22.9 | 18.3 | 18.2 |
| Zn | mg kg−1 | 25.7 | 76.8 | 45.0 | 45.5 | 7.0 | 42.3 | 14.3 | 11.7 | 9.7 | 26.5 | 16.9 | 15.0 | 14.1 | 19.6 | 16.4 | 15.8 |
| B | mg kg−1 | 73.8 | 106.5 | 87.2 | 85.4 | 11.2 | 63.1 | 29.3 | 21.0 | 2.8 | 12.0 | 7.4 | 7.0 | 4.9 | 15.2 | 7.8 | 7.4 |
| Mn | mg kg−1 | 25.6 | 44.7 | 33.7 | 32.6 | 3.5 | 12.9 | 6.2 | 5.5 | 2.8 | 10.6 | 6.2 | 6.2 | 2.7 | 5.3 | 3.8 | 3.6 |
| Cu | mg kg−1 | 13.5 | 34.4 | 25.0 | 25.9 | 3.2 | 34.9 | 7.8 | 5.6 | 9.5 | 25.8 | 16.8 | 14.6 | 0.8 | 10.6 | 2.7 | 1.7 |
| Mo | mg kg−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.52 | 1.06 | 0.58 | 0.00 | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiethorn, M.; Penn, C.; Camberato, J. A Research Method for Semi-Automated Large-Scale Cultivation of Maize to Full Maturity in an Artificial Environment. Agronomy 2021, 11, 1898. https://doi.org/10.3390/agronomy11101898
Wiethorn M, Penn C, Camberato J. A Research Method for Semi-Automated Large-Scale Cultivation of Maize to Full Maturity in an Artificial Environment. Agronomy. 2021; 11(10):1898. https://doi.org/10.3390/agronomy11101898
Chicago/Turabian StyleWiethorn, Matthew, Chad Penn, and James Camberato. 2021. "A Research Method for Semi-Automated Large-Scale Cultivation of Maize to Full Maturity in an Artificial Environment" Agronomy 11, no. 10: 1898. https://doi.org/10.3390/agronomy11101898
APA StyleWiethorn, M., Penn, C., & Camberato, J. (2021). A Research Method for Semi-Automated Large-Scale Cultivation of Maize to Full Maturity in an Artificial Environment. Agronomy, 11(10), 1898. https://doi.org/10.3390/agronomy11101898






