Physiological Basis of Combined Stress Tolerance to Low Phosphorus and Drought in a Diverse Set of Mungbean Germplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Screening in Hydroponics
2.2. Experiment 2: Evaluation of Selected Accessions in Soil
2.3. Water Relation Traits and Membrane Stability Index (MSI)
2.4. Tissue Phosphorus Concentration
2.5. Photosynthetic Pigment and Gas Exchange Measurements in Selected Accessions Grown in Soil
2.6. Characterization and Quantification of Organic Acid in Root Exudates in Selected Accessions
2.7. Grain Yield and Its Attributes in Selected Accessions
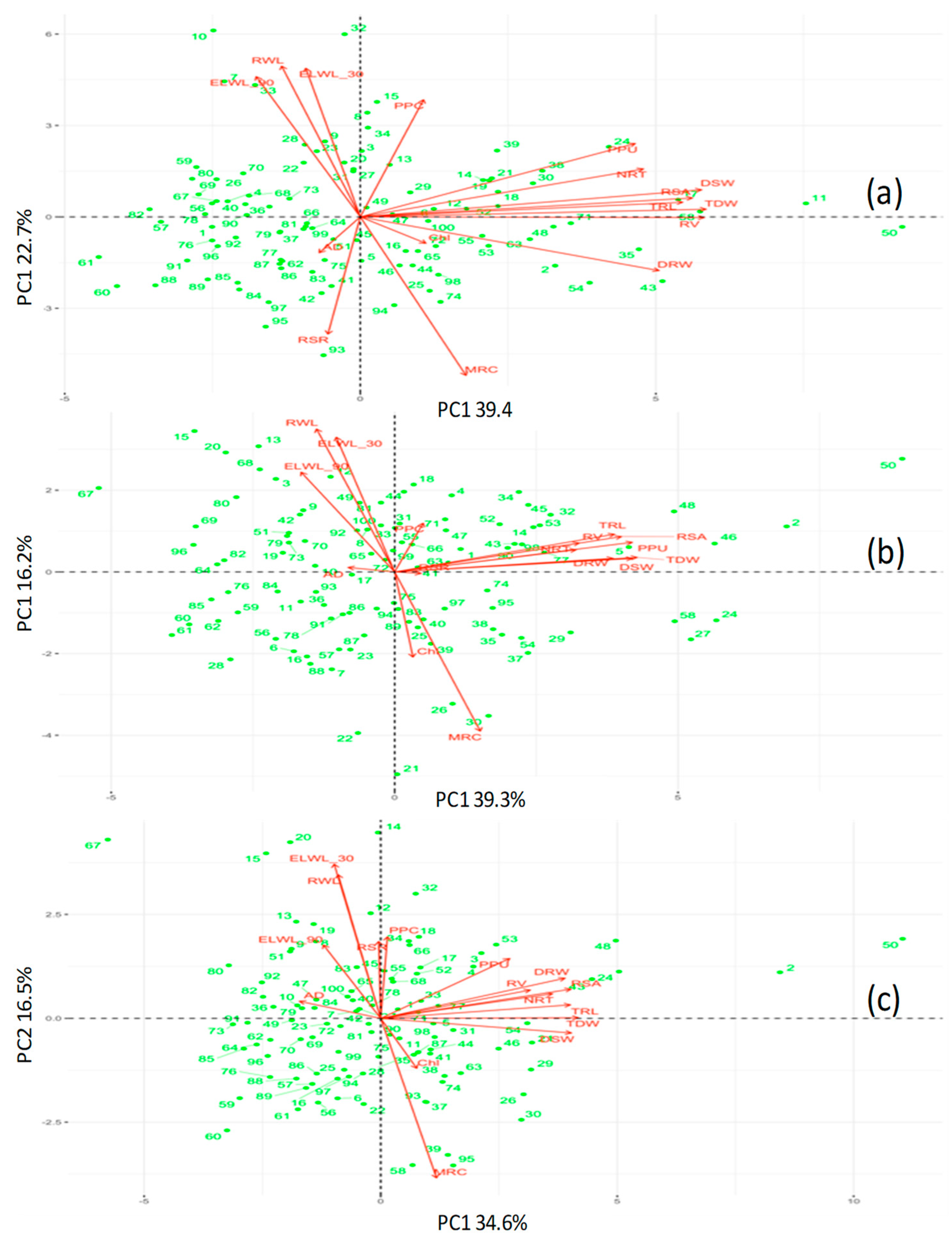
2.8. Stress Tolerance Evaluation in Hydroponics (PCA Ranking)
2.9. Statistical Analysis
3. Results
3.1. Evaluation of Diverse Mungbean Set in Hydroponics
3.2. Differential Response of Contrasting Groups to Low P, Drought and Combined Stress
3.3. Stress Susceptibility Index of Selected Mungbean Accessions Grown in Soil Culture
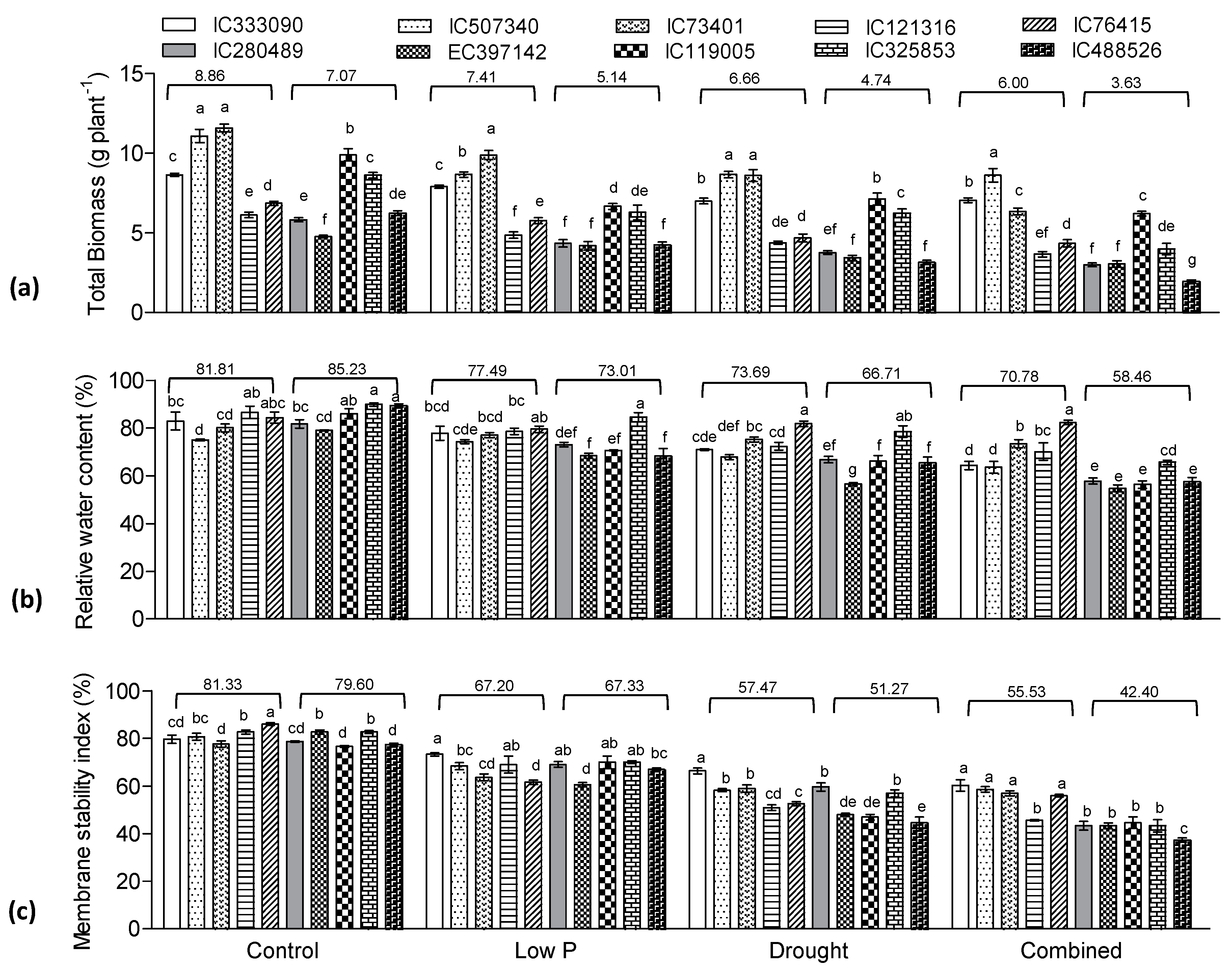
3.4. Biomass, RWC, and MSI Influenced by Low P, Drought and Combined Stress
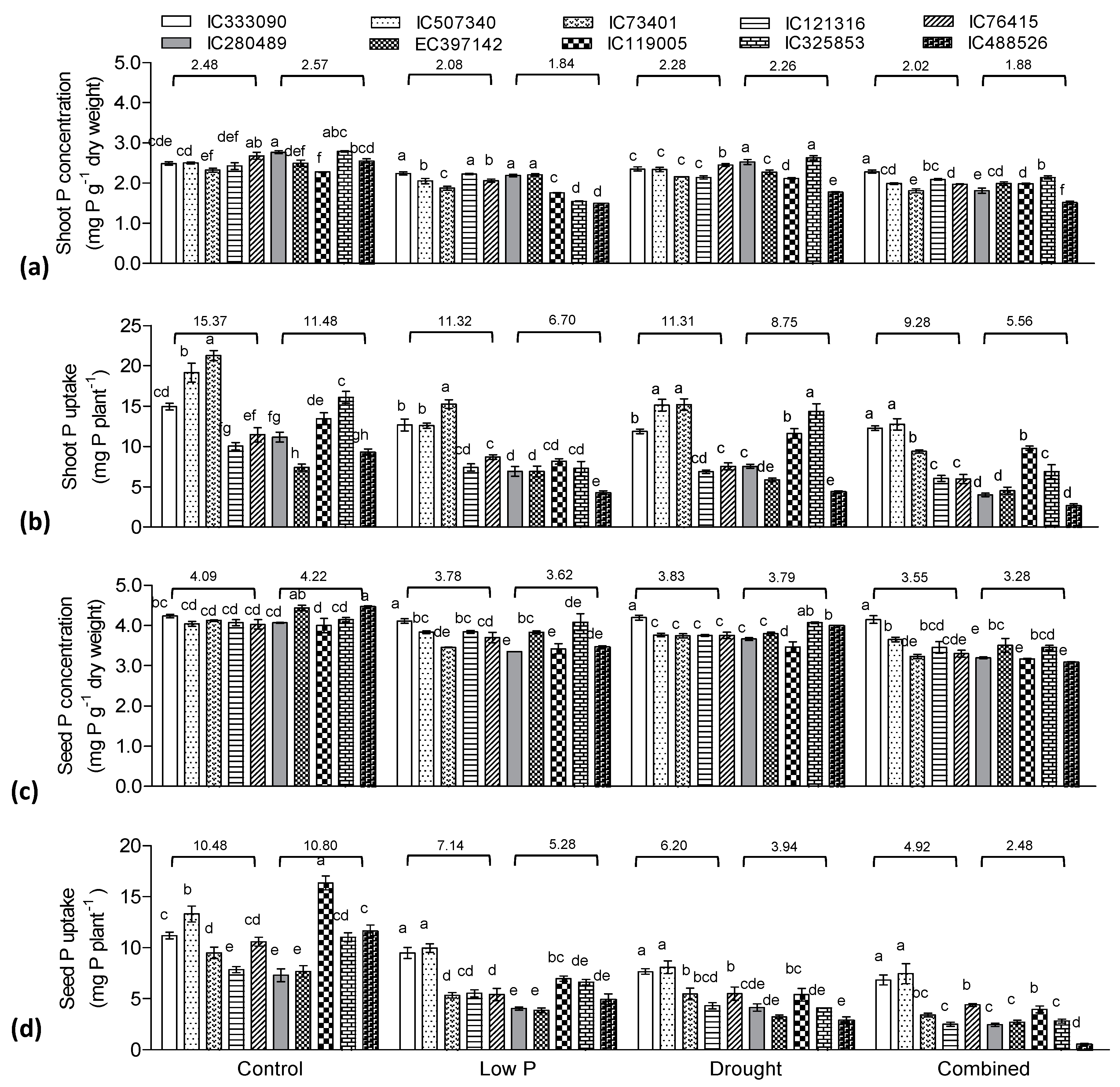
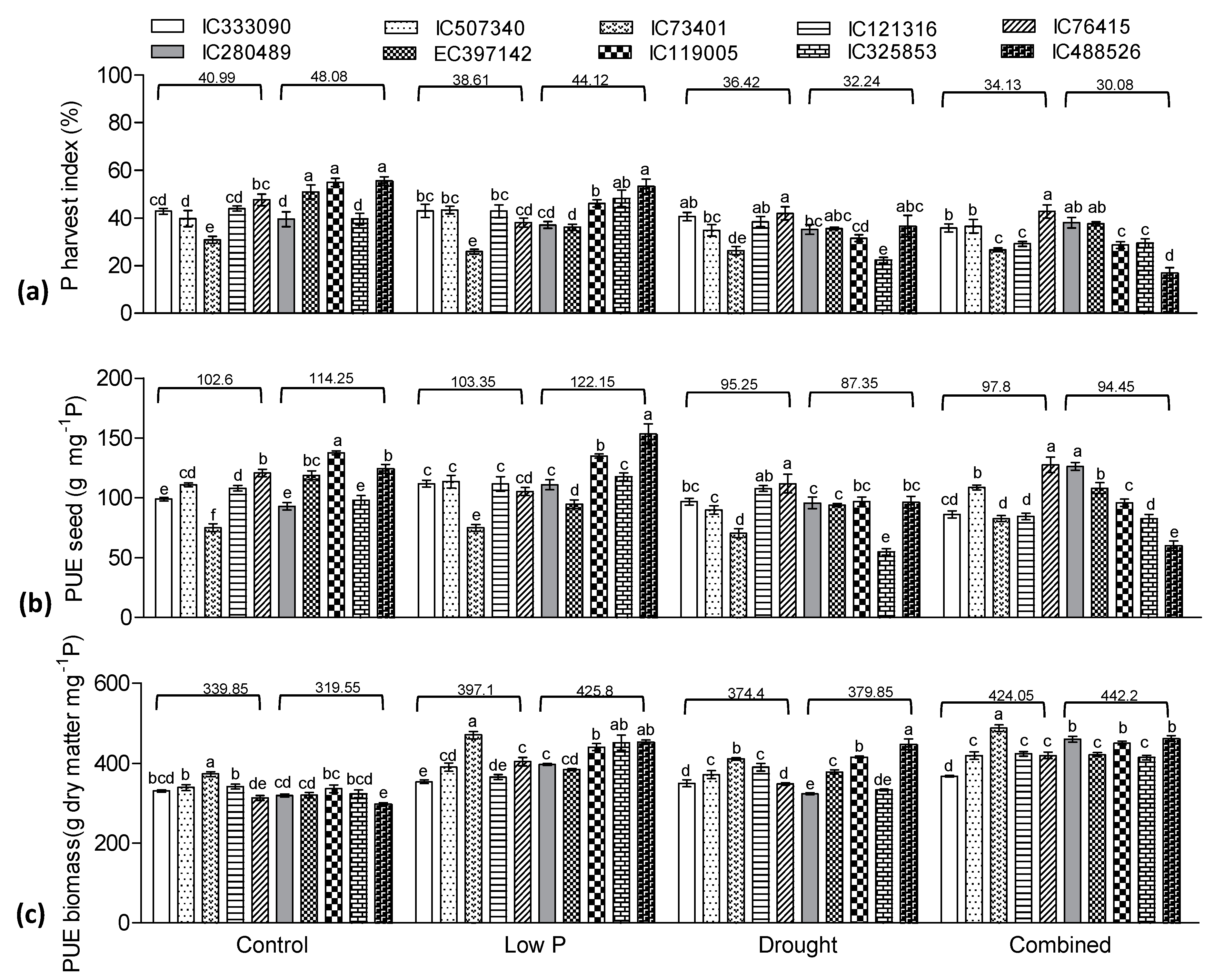
3.5. Effect of Low P, Drought, and Combined Stress on Phosphorus Indices
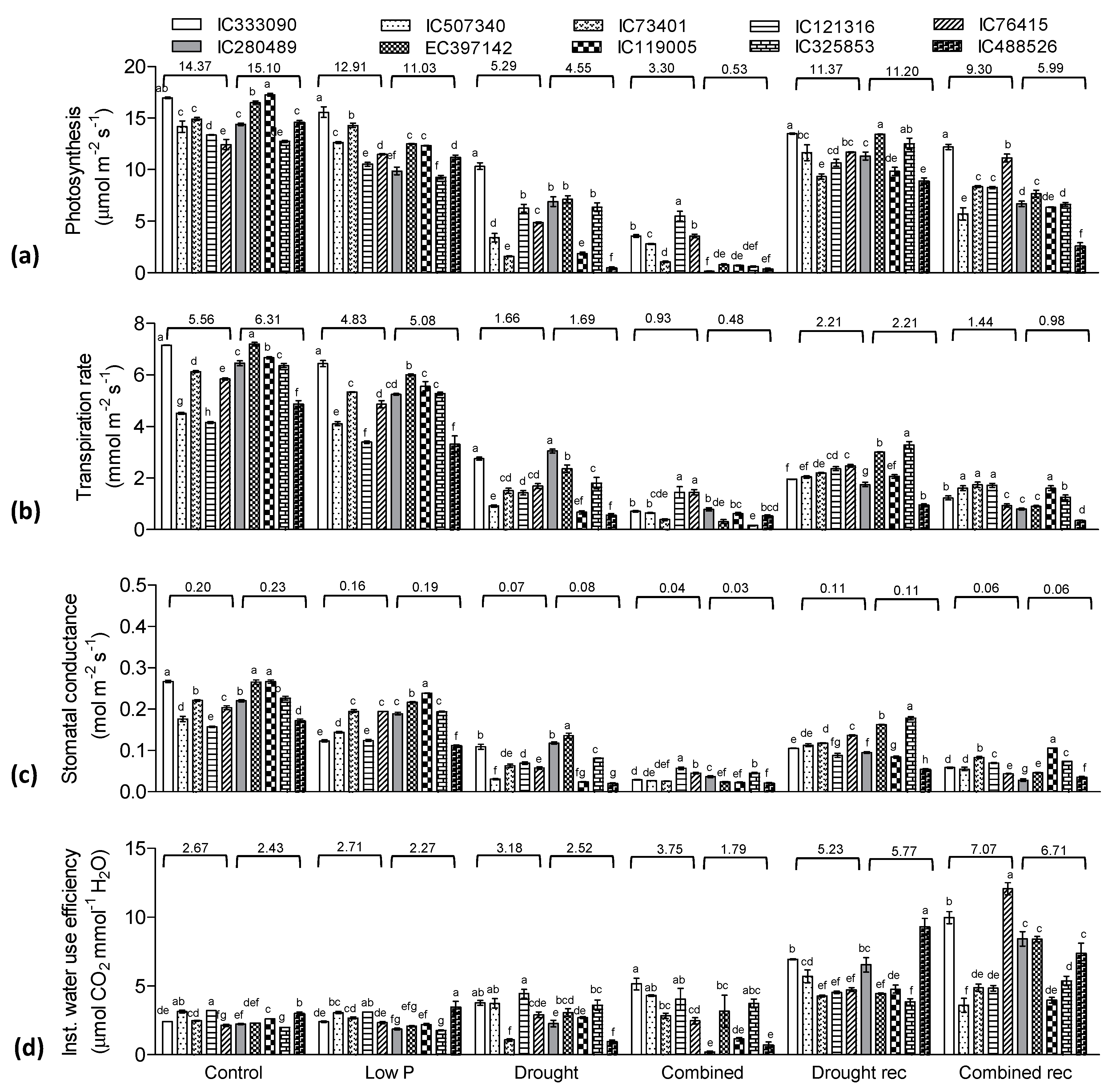
3.6. Gas Exchange Traits and Photosynthetic Pigments Influenced by Drought and Low P Stress
3.7. Organic Acid Exudation in Response to Low P, Drought and Combined Stress
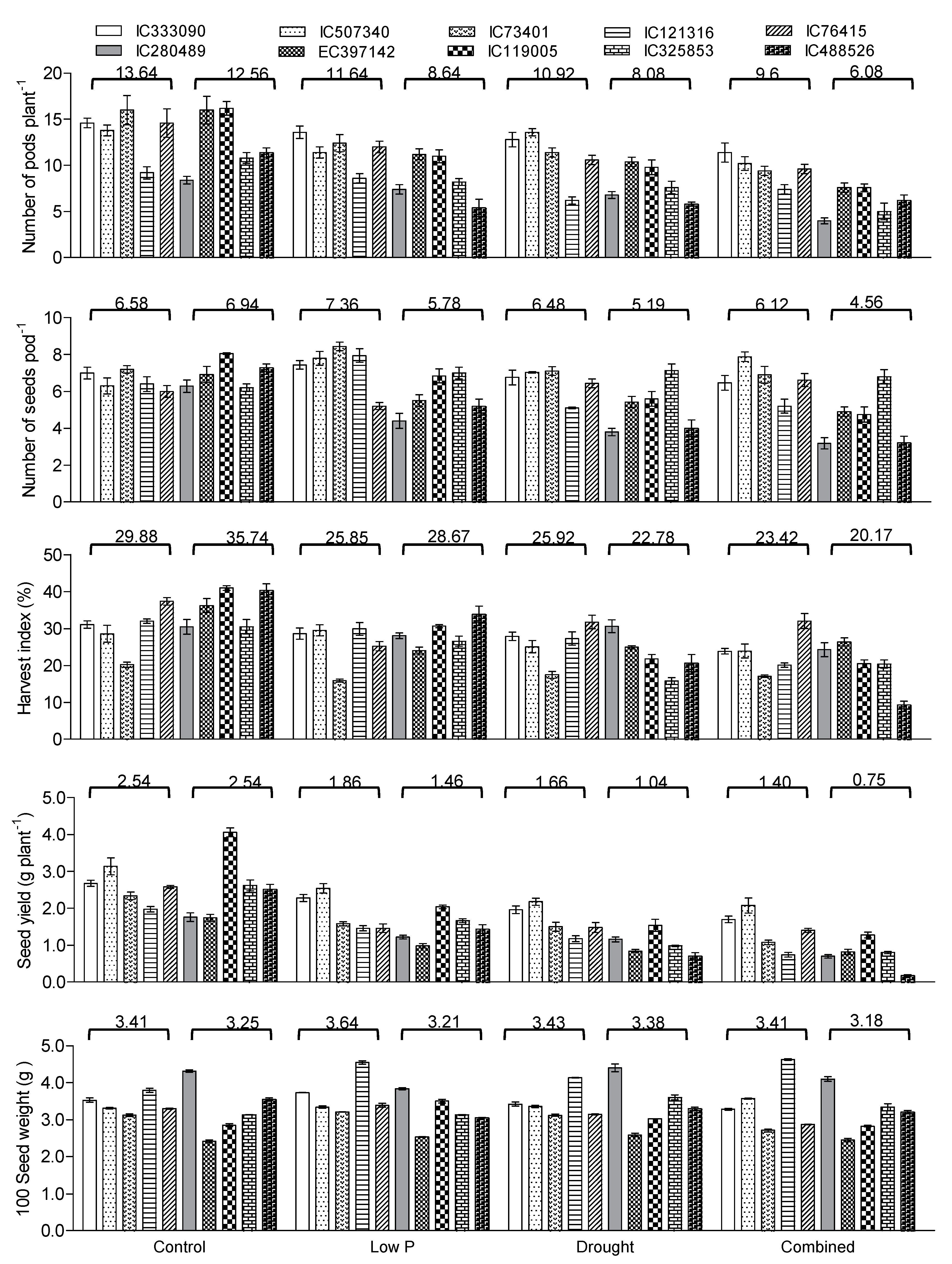
3.8. Yield Traits Influenced by Drought and Low P Stress
4. Discussion
4.1. Interaction of Low P and Drought on Growth and Root Traits in Hydroponics
4.2. Combined Low P and Drought Stress Has Additive Effect on Organic Acid Exudation
4.3. Combined Stress Influences Water Relation, Pigment Concentration and Gas Exchange
4.4. Influence of Combined Stress on Growth, Yield, P Uptake and P Use Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Singh, D.P.; Singh, B.B. Breeding for tolerance to abiotic stresses in mungbean. Food Legumes 2011, 24, 83–90. [Google Scholar]
- Kochian, L.V. Plant nutrition: Rooting for more phosphorus. Nature 2012, 488, 466. [Google Scholar] [CrossRef] [PubMed]
- Dunham, R.J.; Nye, P.H. The influence of soil water content on the uptake of ions by roots. III. Phosphate, potassium, calcium and magnesium uptake and concentration gradients in soil. J. Appl. Ecol. 1976, 13, 967–984. [Google Scholar] [CrossRef]
- Mackay, A.D.; Barber, S.A. Soil moisture effects on root growth and phosphorus uptake by corn. Agron. J. 1985, 77, 519–523. [Google Scholar] [CrossRef]
- Pinkerton, A.; Simpson, J.R. Interactions of surface drying and subsurface nutrients affecting plant growth on acidic soil profiles from an old pasture. Aust. J. Exp. Agric. 1986, 26, 681–689. [Google Scholar] [CrossRef]
- Zhang, D.; Lyu, Y.; Li, H.; Tang, X.; Hu, R.; Rengel, Z.; Zhang, F.; Whalley, W.R.; Davies, W.J.; Cahill, J.F., Jr.; et al. Neighbouring plants modify maize root foraging for phosphorus: Coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol. 2020, 226, 244–253. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Lucini, L.; Rea, E.; Colla, G. Nutrient solution concentration affects growth, mineral composition, phenolic acids, and flavonoids in leaves of artichoke and cardoon. Hort Sci. 2012, 47, 1424–1429. [Google Scholar] [CrossRef]
- Zheng, H.F.; Chen, L.D.; Yu, X.Y.; Zhao, X.F.; Ma, Y.; Ren, Z.B. Phosphorus control as an effective strategy to adapt soybean to drought at the reproductive stage: Evidence from field experiments across northeast China. Soil Use Manag. 2015, 31, 19–28. [Google Scholar] [CrossRef]
- Singh, D.K.; Sale, P.W.; Pallaghy, C.K.; McKenzie, B.M. Phosphorus concentrations in the leaves of defoliated white clover affect abscisic acid formation and transpiration in drying soil. New Phytol. 2000, 146, 249–259. [Google Scholar] [CrossRef]
- Garg, B.K.; Burman, U.; Kathju, S. The influence of phosphorus nutrition on the physiological response of moth bean genotypes to drought. J. Plant Nutr. Soil Sci. 2004, 167, 503–508. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764. [Google Scholar]
- Bruck, H.; Payne, W.A.; Sattelmacher, B. Effects of phosphorus and water supply on yield, transpirational water-use efficiency, and carbon isotope discrimination of pearl millet. Crop Sci. 2000, 40, 120–125. [Google Scholar] [CrossRef]
- Radin, J.W. Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol. 1984, 76, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Saneoka, H.; Fujita, K.; Ogata, S. Effect of phosphorus on drought tolerance in Chloris gayana Kunth and Coix lacryma-jobi L. Soil Sci. Plant Nutr. 1990, 36, 267–274. [Google Scholar] [CrossRef]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favourable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Cichy, K.A.; Snapp, S.S.; Blair, M.W. Plant growth habit, root architecture traits and tolerance to low soil phosphorus in an Andean bean population. Euphytica 2009, 165, 257–268. [Google Scholar] [CrossRef]
- Xu, H.X.; Weng, X.Y.; Yang, Y. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ. J. Plant Physiol. 2007, 5, 741–748. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y. Effects of drought stress on physiological and biochemical changes in Phaseolus vulgaris L. Legume Res. Int. J. 2017, 40, 55–62. [Google Scholar]
- Shubhra, D.J.; Goswami, C.L.; Munjal, R. Influence of phosphorus application on water relations, biochemical parameters and gum content in cluster bean under water deficit. Biol. Plant. 2004, 48, 445–448. [Google Scholar] [CrossRef]
- Baroowa, B.; Gogoi, N.; Paul, S.; Baruah, K.K. Response of leaf water status, stomatal characteristics, photosynthesis and yield in black gram and green gram genotypes to soil water deficit. Funct. Plant Biol. 2015, 42, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, L.P.; Guttikonda, S.K.; Phan Tran, L.S.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.K.; Mannan, M.A.; Haque, M.M.; Ahmed, J.U. Physiological basis of water stress tolerance in soybean. Bangladesh Agron. J. 2015, 18, 71–78. [Google Scholar] [CrossRef]
- Nunes, C.; de Sousa Araújo, S.; da Silva, J.M.; Fevereiro, M.P.S.; da Silva, A.B. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ. Exp. Bot. 2008, 63, 289–296. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.D. Physiological responses and dry matter partitioning of summer mungbean (Vigna radiata L.) genotypes subjected to drought conditions. J. Agron. Crop Sci. 2009, 195, 270–277. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean (Vigna radiata (L.) Wilczek) varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, N.J.; Vanaja, M.; Maheswari, M.; Yadav, S.K.; Reddy, P.R.; Venkateswarlu, B. Excised leaf water retention capacity (ELWRC) technique for screening blackgram genotypes for yield. J. Food Legumes 2009, 22, 211–212. [Google Scholar]
- Raina, S.K.; Govindasamy, V.; Kumar, M.; Singh, A.K.; Rane, J.; Minhas, P.S. Genetic variation in physiological responses of mung beans (Vigna radiata (L.) Wilczek) to drought. Acta Physiol. Plant. 2016, 38, 263. [Google Scholar] [CrossRef]
- Ramirez-Vallejo, P.; Kelly, J.D. Traits related to drought resistance in common bean. Euphytica 1998, 99, 127–136. [Google Scholar] [CrossRef]
- Clarke, J.M. Use of physiological and morphological traits in breeding programmes to improve drought resistance of cereals. In Proceedings of the An International Symposium on Improving Winter Cereals for Moisture-Limiting Areas, Capri, Italy, 27–31 October 1985. Chapter 14 (No. 94-096462. CIMMYT). [Google Scholar]
- Acosta-Gallegos, J.A.; Adams, M.W. Plant traits and yield stability of dry bean (Phaseolus vulgaris) cultivars under drought stress. J. Agric. Sci. 1991, 117, 213–219. [Google Scholar] [CrossRef]
- Zare, M.; Dehghani, B.; Alizadeh, O.; Azarpanah, A. The evaluation of various agronomic traits of mungbean (Vigna radiata L.) genotypes under drought stress and non-stress conditions. Int. J. Farming Allied Sci. 2013, 2, 764–770. [Google Scholar]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sukhlal, N.; Paliwal, K.K. Effect of phosphorus levels and microbial inoculants on symbiotic traits, N and P uptake, quality and yield of rainfed chickpea. All India Coordinated Project Improvement of Pulse. R.A.K. College of Agriculture Sehore. In Proceedings of the 3rd European Conference in Grain Legumes, Valladolid, Spain, 14–19 November 1998; pp. 418–419. [Google Scholar]
- Burman, U.; Garg, B.K.; Kathju, S. Interactive effects of thiourea and phosphorus on cluster bean under water stress. Biol. Plant. 2004, 48, 61–65. [Google Scholar] [CrossRef]
- Burman, U.; Garg, B.K.; Kathju, S. Effect of phosphorus application on cluster bean under different intensities of water stress. J. Plant Nutr. 2009, 32, 668–680. [Google Scholar] [CrossRef]
- Kwapata, M.B.; Hall, A.E. Effects of moisture regime and phosphorus on mycorrhizal infection, nutrient uptake, and growth of cowpeas (Vigna unguiculata (L.) Walp.). Field Crop. Res. 1985, 12, 241–250. [Google Scholar] [CrossRef]
- Neenu, S.; Ramesh, K.; Ramana, S.; Biswas, A.K.; Rao, A.S. Growth and yield of different varieties of chickpea (Cicer arietinum L.) as influenced by the phosphorus nutrition under rainfed conditions on Vertisols. Int. J. Bio-Resour. Stress Manag. 2014, 5, 53–57. [Google Scholar] [CrossRef]
- Gutierrez-Boem, F.H.; Thomas, G.W. Phosphorus nutrition and water deficits in field-grown soybeans. Plant Soil 1999, 207, 87–96. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- Gayacharan; Tripathi, K.; Meena, S.; Panwar, B.S.; Lal, H.; Rana, J.C.; Singh, K. Understanding genetic variability in the mungbean (Vigna radiata L.) gene pool. Ann. Appl. Biol. 2020. [Google Scholar] [CrossRef]
- Singh, D.; Dikshit, H.K.; Singh, R. A new phenotyping technique for screening for drought tolerance in lentil (Lens culinaris M edik.). Plant Breed. 2013, 132, 185–190. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular: Washington, DC, USA, 1954; p. 939.
- Yang, R.C.; Jana, S.; Clarke, J.M. Phenotypic diversity and associations of some potentially drought-responsive characters in durum wheat. Crop Sci. 1991, 31, 1484–1491. [Google Scholar] [CrossRef]
- Clarke, J.M.; McCAIG, T.N. Excised-leaf water retention capability as an indicator of drought resistance of Triticum genotypes. Can. J. Plant Sci. 1982, 62, 571–578. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–177. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Krishnapriya, V.; Pandey, R. Root exudation index: Screening organic acid exudation and phosphorus acquisition efficiency in soybean genotypes. Crop Pasture Sci. 2016, 67, 1096–1109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tran, H.; Shan, L.; Kim, J.; Childs, K.; Ervin, E.H.; Frazier, T.; Zhao, B. Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol. Biofuels 2015, 8, 152. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Sebastien, L.; Julie, J.; Francois, H. FactoMineR: An R Package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Nazari, L.; Pakniyat, H. Assessment of drought tolerance in barley genotypes. J. Appl. Sci. 2010, 10, 151–156. [Google Scholar] [CrossRef]
- Dehbalaei, S.; Farshadfar, E.; Farshadfar, M. Assessment of drought tolerance in bread wheat genotypes based on resistance/tolerance indices. Int. J. Agric. Crop Sci. 2013, 5, 2352–2358. [Google Scholar]
- Alloush, G.A. Responses of hydroponically-grown chickpea to low phosphorus: pH changes, nutrient uptake rates, and root morphological changes. Agronomie 2003, 23, 123–133. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Ali, O.; Sarker, A.; Nielsen, N.E.; Rahman, M.M. Genetic variation in root traits and nutrient acquisition of lentil genotypes. J. Plant Nutr. 2006, 29, 643–655. [Google Scholar] [CrossRef]
- Pandey, R.; Meena, S.K.; Krishnapriya, V.; Ahmad, A.; Kishora, N. Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 2014, 33, 919–928. [Google Scholar] [CrossRef]
- Reddy, V.R.P.; Aski, M.S.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Pandey, R.; Singh, M.P.; Ramtekey, V.; Rai, N.; Nair, R.M.; et al. Genetic variation for root architectural traits in response to phosphorus deficiency in mungbean at the seedling stage. PLoS ONE 2020, 15, e0221008. [Google Scholar] [CrossRef]
- Pan, X.W.; Li, W.B.; Zhang, Q.Y.; Li, Y.H.; Liu, M.S. Assessment on phosphorus efficiency characteristics of soybean genotypes in phosphorus-deficient soils. Agric. Sci. China 2008, 7, 958–969. [Google Scholar] [CrossRef]
- Soumya, P.R.; Sharma, S.; Meena, M.K.; Pandey, R. Response of diverse bread wheat genotypes in terms of root architectural traits at seedling stage in response to low phosphorus stress. Plant Physiol. Rep. 2020. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Kaur, J.; Singh, S.; Grewal, S.K.; Singh, M. Variation of morpho-physiological traits in geographically diverse pigeonpea [Cajanus cajan (L.) Millsp] germplasm under different phosphorus conditions. J. Plant Nutr. 2018, 41, 1321–1332. [Google Scholar] [CrossRef]
- Sarker, B.C.; Rashid, P.; Jarmoker, J.L. Anatomical changes of lentil (Lens culinaris medik.) under phosphorus deficiency stress. Bangladesh J. Bot. 2015, 44, 73–78. [Google Scholar] [CrossRef]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Chen, Z.; Liu, H.Y.; Wang, X.L.; Siddique, K.H.; Li, F.M. Phosphorus application increases root growth, improves daily water use during the reproductive stage, and increases grain yield in soybean subjected to water shortage. Environ. Exp. Bot. 2019, 166, 103816. [Google Scholar] [CrossRef]
- Lambers, H.; Hayes, P.E.; Laliberte, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Ali, R.; Malhotra, R.S.; Jahoor, A.; Rahman, M.M. Variation in root morphological and physiological traits and nutrient uptake of chickpea genotypes. J. Plant Nutr. 2007, 30, 829–841. [Google Scholar] [CrossRef]
- Pandey, R.; Krishnapriya, V.; Kishora, N.; Singh, S.B.; Singh, B. Shoot labelling with 14CO2: A technique for assessing total root carbon exudation under phosphorus stress. Indian J. Plant Physiol. 2013, 18, 250–262. [Google Scholar] [CrossRef]
- Pang, J.; Zhao, H.; Bansal, R.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Siddique, K.H. Leaf transpiration plays a role in phosphorus acquisition among a large set of chickpea genotypes. Plant Cell Environ. 2018, 41, 2069–2079. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef]
- Song, F.; Han, X.; Zhu, X.; Herbert, S.J. Response to water stress of soil enzymes and root exudates from drought and non-drought tolerant corn hybrids at different growth stages. Can. J. Soil Sci. 2012, 92, 501–507. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ludlow, M.M. Who taught plants thermodynamics? The unfulfilled potential of plant water potential. Funct. Plant Biol. 1985, 12, 213–217. [Google Scholar] [CrossRef]
- Sawwan, J.; Shibli, R.A.; Swaidat, I.; Tahat, M. Phosphorus regulates osmotic potential and growth of African violet under in vitro-induced water deficit. J. Plant Nutr. 2000, 23, 759–771. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Fujita, K.; Ogata, S. Cell membrane stability and leaf water relations as affected by phosphorus nutrition under water stress in maize. Soil Sci. Plant Nutr. 1990, 36, 661–666. [Google Scholar] [CrossRef]
- Hao, L.; Wang, Y.; Zhang, J.; Xie, Y.; Zhang, M.; Duan, L.; Li, Z. Coronatine enhances drought tolerance via improving antioxidative capacity to maintaining higher photosynthetic performance in soybean. Plant Sci. 2013, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580. [Google Scholar]
- Lima, J.D.; Mosquim, P.R.; Da Matta, F.M. Leaf gas exchange and chlorophyll fluorescence parameters in Phaseolus vulgaris as affected by nitrogen and phosphorus deficiency. Photosynthetica 1999, 37, 113–121. [Google Scholar] [CrossRef]
- Abid, G.; Hessini, K.; Aouida, M.; Aroua, I.; Baudoin, J.P.; Muhovski, Y.; Mergeai, G.; Sassi, K.; Machraoui, M.; Souissi, F.; et al. Agro-physiological and biochemical responses of Faba bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress. Biotechnol. Agron. Soc. Environ. 2017, 21, 1–14. [Google Scholar]
- Zlatev, Z.S.; Yordanov, I.T. Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol. 2004, 30, 3–18. [Google Scholar]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Nazran, A.; Ahmed, J.U.; Karim, A.J.M.S.; Ghosh, T.K. Physiological responses of mungbean (Vigna radiata) varieties to drought stress. Bangladesh J. Agric. Res. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Siddique, K.H. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol. 2018, 219, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Jacobsen, J.S.; Wraith1, J.M. Response of malt barley to phosphorus fertilization under drought conditions. J. Plant Nutr. 2005, 28, 1605–1617. [Google Scholar] [CrossRef]
- Masle, J.; Farquhar, G.D.; Wong, S.C. Transpiration ratio and plant mineral content are related among genotypes of a range of species. Funct. Plant Biol. 1992, 19, 709–721. [Google Scholar] [CrossRef]
- Mourice, S.K.; Tryphone, G.M. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for adaptation to low phosphorus. Isrn Agron. 2012, 2012. [Google Scholar] [CrossRef]
- Zhou, T.; Du, Y.; Ahmed, S.; Liu, T.; Ren, M.; Liu, W.; Yang, W. Genotypic differences in phosphorus efficiency and the performance of physiological characteristics in response to low phosphorus stress of soybean in southwest of China. Front. Plant Sci. 2016, 7, 1776. [Google Scholar] [CrossRef]
- Santos, M.G.D.; Ribeiro, R.V.; Oliveira, R.F.D.; Pimentel, C. Gas exchange and yield response to foliar phosphorus application in Phaseolus vulgaris L. under drought. Braz. J. Plant Physiol. 2004, 16, 171–179. [Google Scholar] [CrossRef]
- Raza, S.; Farrukh Saleem, M.; Mustafa Shah, G.; Jamil, M.; Haider Khan, I. Potassium applied under drought improves physiological and nutrient uptake performances of wheat (Triticum aestivun L.). J. Soil Sci. Plant Nutr. 2013, 13, 175–185. [Google Scholar]
- Baroowa, B.; Gogoi, N. Biochemical changes in two Vigna spp. during drought and subsequent recovery. Indian J. Plant Physiol. 2013, 18, 319–325. [Google Scholar] [CrossRef]
- Baroowa, B.; Gogoi, N. Biochemical changes in black gram and green gram genotypes after imposition of drought stress. J. Food Legumes 2014, 27, 350–353. [Google Scholar]
- Malik, M.A.; Hussain, S.; Warraich, E.A.; Habib, A.; Ullah, S. Effect of seed inoculation and phosphorus application on growth, seed yield and quality of mungbean (Vigna radiata L.) cv. NM-98. Int. J. Agric. Biol. 2002, 4, 515–516. [Google Scholar]
- Singh, D.K.; Sale, P.W.; McKenzie, B.M. Water relations of white clover (Trifolium repens L.) in a drying soil, as a function of phosphorus supply and defoliation frequency. Aust. J. Agric. Res. 1997, 48, 675–682. [Google Scholar] [CrossRef]
- Malik, A.M.J.A.D.; Waheed, A.; Qadir, G.; Asghar, R. Interactive effects of irrigation and phosphorus on green gram (Vigna radiata L.). Pak. J. Bot. 2006, 38, 1119. [Google Scholar]
- Uarrota, V.G. Response of cowpea (Vigna unguiculata L. Walp.) to water stress and phosphorus fertilization. J. Agron. 2010, 9, 87–91. [Google Scholar] [CrossRef][Green Version]
- Rose, T.J.; Pariasca-Tanaka, J.; Rose, M.T.; Fukuta, Y.; Wissuwa, M. Genotypic variation in grain phosphorus concentration, and opportunities to improve P-use efficiency in rice. Field Crop. Res. 2010, 119, 154–160. [Google Scholar] [CrossRef]
- Rose, T.J.; Wissuwa, M. Rethinking internal phosphorus utilization efficiency: A new approach is needed to improve PUE in grain crops. Adv. Agron. 2012, 116, 185–217. [Google Scholar]
| Low P | Drought | Combined | |||
|---|---|---|---|---|---|
| Tolerant | Sensitive | Tolerant | Sensitive | Tolerant | Sensitive |
| IC314606 | EC398916 | EC398916 | EC398907 | IC280489 | EC398907 |
| EC396156 | EC398414 | IC314606 | IC565301 | IC76468 | EC396156 |
| IC257571 | IC252008 | IC507276 | IC73112 | IC489015 | IC489004 |
| IC401586 | IC305250 | IC507418 | IC76370 | IC76414 | IC148401 |
| IC305222 | IC507526 | IC257571 | IC357035 | IC507321 | IC565301 |
| IC565301 | IC76422 | IC401586 | IC305250 | EC397142 | IC73112 |
| IC148442 | IC488849 | IC305222 | IC507526 | EC397141 | EC398131 |
| IC280489 | IC488808 | IC148442 | IC488582 | IC76466 | IC488904 |
| EC398131 | IC118993 | IC280489 | IC76346 | IC76499 | IC507504 |
| IC76468 | IC119005 | IC507527 | IC398746 | IC76415 | IC76451 |
| IC507527 | IC405261 | IC76414 | IC488808 | IC333090 | IC488582 |
| EC397142 | IC73401 | IC507321 | IC118993 | IC507340 | IC76346 |
| EC397141 | EC396413 | EC397142 | EC398880 | IC76422 | IC489093 |
| IC285532 | IC488875 | EC397141 | IC119005 | IC488808 | IC121249 |
| IC488813 | IC488526 | IC507320 | IC754464 | EC398937 | EC398880 |
| IC507504 | IC119018 | IC76415 | IC73401 | EC398886 | IC119005 |
| IC76415 | IC325853 | IC507483 | EC314285 | IC405261 | IC73401 |
| IC333090 | IC76581 | IC333090 | IC488526 | IC305249 | EC314285 |
| IC507340 | IC507296 | IC507340 | IC119018 | IC121316 | IC488526 |
| IC507376 | IC590082 | IC76422 | IC325853 | EC396413 | IC325853 |
| IC398746 | IC623704 | IC488966 | IC553566 | IC315045 | IC507296 |
| IC282110 | IC623821 | EC398949 | IC507296 | IC623821 | IC590082 |
| IC488966 | IC584699 | IC314512 | IC590082 | IC573083 | IC623704 |
| EC398949 | IC415097 | IC121316 | IC623821 | IC623705 | IC415097 |
| IC305249 | HUM-1 | IC507319 | IC393792 | IC512343 | IC393792 |
| Low P | Drought | Combined | Mean | ||||
|---|---|---|---|---|---|---|---|
| AGDM | Seed Yield | AGDM | Seed Yield | AGDM | Seed Yield | ||
| IC333090 | 1 | 1 | 1 | 1 | 1 | 2 | 1.2 |
| IC507340 | 6 | 2 | 2 | 2 | 2 | 1 | 2.5 |
| IC73401 | 3 | 4 | 3 | 4 | 7 | 4 | 4.2 |
| IC121316 | 5 | 3 | 4 | 5 | 6 | 7 | 5.0 |
| IC76415 | 2 | 7 | 7 | 7 | 4 | 5 | 5.3 |
| IC280489 | 4 | 8 | 8 | 6 | 5 | 3 | 5.7 |
| EC397142 | 7 | 5 | 9 | 3 | 8 | 6 | 6.3 |
| IC119005 | 10 | 10 | 6 | 8 | 3 | 8 | 7.5 |
| IC325853 | 8 | 6 | 5 | 9 | 9 | 9 | 7.7 |
| IC488526 | 9 | 9 | 10 | 10 | 10 | 10 | 9.7 |
| Accession | Oxalate (µM g−1 Root FW) | Citrate (µM g−1 Root FW) | Maleate (µM g−1 Root FW) | α-Ketoglutarate (µM g−1 FW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | |
| IC333090 | nd | 6.11 | nd | nd | nd | 17.5 | nd | 1.69 | nd | 13.8 | 5.0 | 25.3 | 1.94 | 117.2 | 2.09 | 36.2 |
| IC507340 | nd | 2.71 | nd | 4.64 | nd | 0.92 | nd | 2.77 | 0.27 | 171.1 | 27.4 | 2.88 | 2.07 | 73.8 | 5.75 | 9.89 |
| IC73401 | nd | nd | nd | nd | nd | 0.91 | 48.0 | 4.01 | 0.40 | 137 | nd | 8.19 | 14.0 | 54.2 | 5.13 | 3.75 |
| IC121316 | nd | 11.1 | nd | nd | nd | 25.0 | nd | 0.42 | nd | 58.2 | 14.0 | 1.85 | nd | 36.9 | 14.9 | 2.56 |
| IC76415 | nd | 6.43 | nd | 9.85 | nd | 14.2 | 47.5 | 0.54 | nd | 20.4 | 245 | 0.37 | 8.65 | 33.4 | 0.13 | 0.65 |
| Mean (Tolerant) | -- | 6.59 | -- | 7.25 | -- | 11.7 | 47.8 | 1.89 | 0.34 | 80.1 | 72.9 | 7.72 | 6.67 | 63.1 | 5.6 | 10.6 |
| IC280489 | nd | nd | nd | nd | nd | 7.68 | nd | 1.07 | 4.14 | 40.3 | 3.50 | 4.34 | 8.91 | 16.3 | 12.8 | 2.47 |
| EC397142 | nd | 16.5 | nd | 20.2 | nd | 1.33 | 54.1 | 2.56 | 0.02 | 15.1 | 31.4 | 3.75 | 8.83 | 12.4 | 22.9 | nd |
| IC119005 | nd | 8.57 | nd | nd | nd | 5.60 | nd | 0.22 | nd | 18.2 | 8.43 | 0.26 | 28.2 | 26.3 | 15.7 | 0.64 |
| IC325853 | nd | 4.29 | nd | nd | nd | 7.85 | nd | 7.89 | 7.93 | 8.12 | 5.56 | 23.9 | 6.60 | 17.4 | 6.96 | 0.22 |
| IC488526 | nd | 5.42 | nd | nd | nd | 2.24 | 34.4 | 0.74 | nd | 1.81 | 7.56 | 6.67 | 17.3 | 37.5 | 6.45 | 5.31 |
| Mean (Sensitive) | -- | 8.70 | -- | 20.2 | -- | 4.94 | 44.3 | 2.50 | 4.03 | 16.7 | 11.3 | 7.78 | 14.0 | 22.0 | 13.0 | 2.16 |
| Accession | Pyruvate (µM g−1 Root FW) | Malonate (µM g−1 Root FW) | Malate (µM g−1 Root FW) | Succinate (µM g−1 Root FW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | |
| IC333090 | 199.6 | 21.4 | 6.11 | 60.8 | 3.98 | 10.7 | 0.46 | 6.49 | 1.71 | 22.5 | 2.94 | 3.22 | 4.55 | 14.9 | 0.33 | 12.2 |
| IC507340 | 25.9 | 3.5 | 7.24 | 2.49 | 2.70 | 5.76 | 1.56 | 3.54 | 1.52 | 28.5 | 0.52 | 3.58 | 0.19 | 13.9 | 0.26 | 8.59 |
| IC73401 | 10.3 | 26.3 | 8.53 | 60.8 | 3.03 | 16.0 | 2.44 | 0.29 | 4.13 | 1.03 | 8.60 | 7.88 | 1.13 | 21.0 | 9.89 | 12.3 |
| IC121316 | 7.27 | 3.08 | 17.3 | 53.3 | 0.18 | 5.12 | 0.90 | 11.9 | 0.70 | 0.60 | 7.33 | 5.15 | 0.68 | 19.5 | 19.2 | 4.95 |
| IC76415 | 212 | 3.16 | 40.2 | 30.2 | 7.26 | 8.47 | 14.5 | 4.23 | 6.01 | 23.7 | 3.62 | 4.60 | 2.07 | 45.3 | 1.42 | 17.3 |
| Mean (Tolerant) | 91.0 | 11.5 | 15.9 | 41.5 | 3.43 | 9.21 | 3.98 | 5.29 | 2.81 | 15.3 | 4.60 | 4.89 | 1.72 | 22.9 | 6.22 | 11.1 |
| IC280489 | 125.7 | 2.40 | 8.92 | 1.33 | 11.9 | 2.36 | 0.89 | 5.29 | 2.03 | 17.2 | 2.51 | 4.75 | 1.82 | 8.40 | 0.01 | 1.61 |
| EC397142 | 230.6 | 41.5 | 8.47 | 46.9 | 6.33 | 21.7 | 6.19 | 2.85 | 1.49 | 1.11 | 0.51 | 4.20 | 0.17 | 7.16 | 0.42 | 16.3 |
| IC119005 | 152.0 | 59.5 | 21.1 | 3.80 | 5.18 | 4.28 | 3.76 | 1.73 | 3.15 | 51.0 | 4.32 | 2.76 | 0.54 | 7.71 | 0.52 | 2.41 |
| IC325853 | 212.1 | 69.6 | 55.8 | 73.5 | 16.5 | 12.5 | 4.23 | 24.9 | 2.69 | 0.73 | 1.95 | 11.1 | 1.39 | 26.9 | 2.75 | 22.4 |
| IC488526 | 4.13 | 27.9 | 4.00 | 46.7 | 1.44 | 11.4 | 1.65 | 15.0 | 5.66 | 0.11 | 1.06 | 9.09 | 5.09 | 35.3 | 7.62 | 18.7 |
| Mean (Sensitive) | 144.9 | 40.2 | 19.7 | 34.5 | 8.3 | 10.4 | 3.34 | 10.0 | 3.00 | 14.0 | 2.07 | 6.38 | 1.80 | 17.1 | 2.26 | 12.3 |
| Accession | Lactate (µM g−1 Root FW) | Acetate (µM g−1 Root FW) | Fumarate (µM g−1 Root FW) | Total Organic Acid (µM g−1 Root FW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | Con | LP | Dt | CS | |
| IC333090 | 1.18 | 4.94 | 23.0 | 2.30 | 14.5 | 81.5 | 7.04 | 17.4 | 0.40 | 22.5 | 75.2 | 35.4 | 227.9 | 333.0 | 122.2 | 201.1 |
| IC507340 | 3.71 | 197.3 | 18.5 | 3.93 | 6.88 | 25.3 | 228.5 | 8.51 | 2.73 | 66.8 | 7.81 | 1.43 | 46.0 | 589.5 | 297.4 | 52.2 |
| IC73401 | 3.39 | 252.2 | 81.7 | 0.53 | 3.00 | 50.9 | 110.2 | 4.66 | 3.74 | 78.3 | 78.6 | 0.55 | 43.1 | 637.8 | 353.0 | 102.9 |
| IC121316 | 17.3 | 79.5 | 0.32 | 34.5 | 1.36 | 5.41 | 62.3 | 13.6 | 1.64 | 75.5 | 58.7 | 2.75 | 29.1 | 319.9 | 195.0 | 130.9 |
| IC76415 | 1.46 | 66.0 | 35.6 | 1.44 | 20.1 | 2.80 | 90.6 | 11.5 | 2.95 | 43.3 | 54.9 | 3.18 | 260.5 | 267.2 | 533.7 | 83.9 |
| Mean (Tolerant) | 5.41 | 120 | 31.8 | 8.54 | 9.16 | 33.2 | 99.7 | 11.1 | 2.29 | 57.3 | 55.0 | 8.66 | 121.3 | 429.5 | 300.3 | 114.2 |
| IC280489 | 0.47 | 6.12 | 23.7 | 22.0 | 94.2 | 50.5 | 53.2 | 9.09 | 0.21 | 19.4 | 30.2 | 1.93 | 249.3 | 170.7 | 135.8 | 53.9 |
| EC397142 | 4.41 | 116.4 | 23.5 | 0.52 | 5.43 | 63.0 | 32.3 | 0.75 | 1.62 | 41.4 | 0.09 | 2.41 | 258.9 | 337.6 | 179.9 | 100.5 |
| IC119005 | 3.04 | 13.2 | 54.1 | 15.2 | 1.35 | 22.6 | 162.1 | 4.56 | 19.2 | 16.5 | 34.0 | 1.60 | 212.5 | 233.4 | 304.0 | 33.1 |
| IC325853 | 7.26 | 37.1 | 31.8 | 12.7 | 12.3 | 92.8 | 111.0 | 3.91 | 4.29 | 35.3 | 70.4 | 2.10 | 271.2 | 312.6 | 290.5 | 182.6 |
| IC488526 | 1.65 | 0.71 | 60.6 | 4.21 | 9.77 | 7.61 | 165.0 | 24.6 | 1.94 | 39.8 | 61.6 | 2.20 | 47.0 | 169.7 | 349.9 | 133.3 |
| Mean (Sensitive) | 3.37 | 34.7 | 38.7 | 10.9 | 24.6 | 47.3 | 104.7 | 8.58 | 5.44 | 30.5 | 39.3 | 2.05 | 207.8 | 244.8 | 252.0 | 100.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meena, S.K.; Pandey, R.; Sharma, S.; Gayacharan; Kumar, T.; Singh, M.P.; Dikshit, H.K. Physiological Basis of Combined Stress Tolerance to Low Phosphorus and Drought in a Diverse Set of Mungbean Germplasm. Agronomy 2021, 11, 99. https://doi.org/10.3390/agronomy11010099
Meena SK, Pandey R, Sharma S, Gayacharan, Kumar T, Singh MP, Dikshit HK. Physiological Basis of Combined Stress Tolerance to Low Phosphorus and Drought in a Diverse Set of Mungbean Germplasm. Agronomy. 2021; 11(1):99. https://doi.org/10.3390/agronomy11010099
Chicago/Turabian StyleMeena, Surendra Kumar, Renu Pandey, Sandeep Sharma, Gayacharan, Tarun Kumar, Madan Pal Singh, and Harsh Kumar Dikshit. 2021. "Physiological Basis of Combined Stress Tolerance to Low Phosphorus and Drought in a Diverse Set of Mungbean Germplasm" Agronomy 11, no. 1: 99. https://doi.org/10.3390/agronomy11010099
APA StyleMeena, S. K., Pandey, R., Sharma, S., Gayacharan, Kumar, T., Singh, M. P., & Dikshit, H. K. (2021). Physiological Basis of Combined Stress Tolerance to Low Phosphorus and Drought in a Diverse Set of Mungbean Germplasm. Agronomy, 11(1), 99. https://doi.org/10.3390/agronomy11010099








