The State of Soil Organic Carbon in Vineyards as Affected by Soil Types and Fertilization Strategies (Tri Morave Region, Serbia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Fertilization Strategies
2.4. Laboratory Analysis
2.5. Calculation of SOC Stocks
2.6. Statistical Analyses
3. Results and Discussion
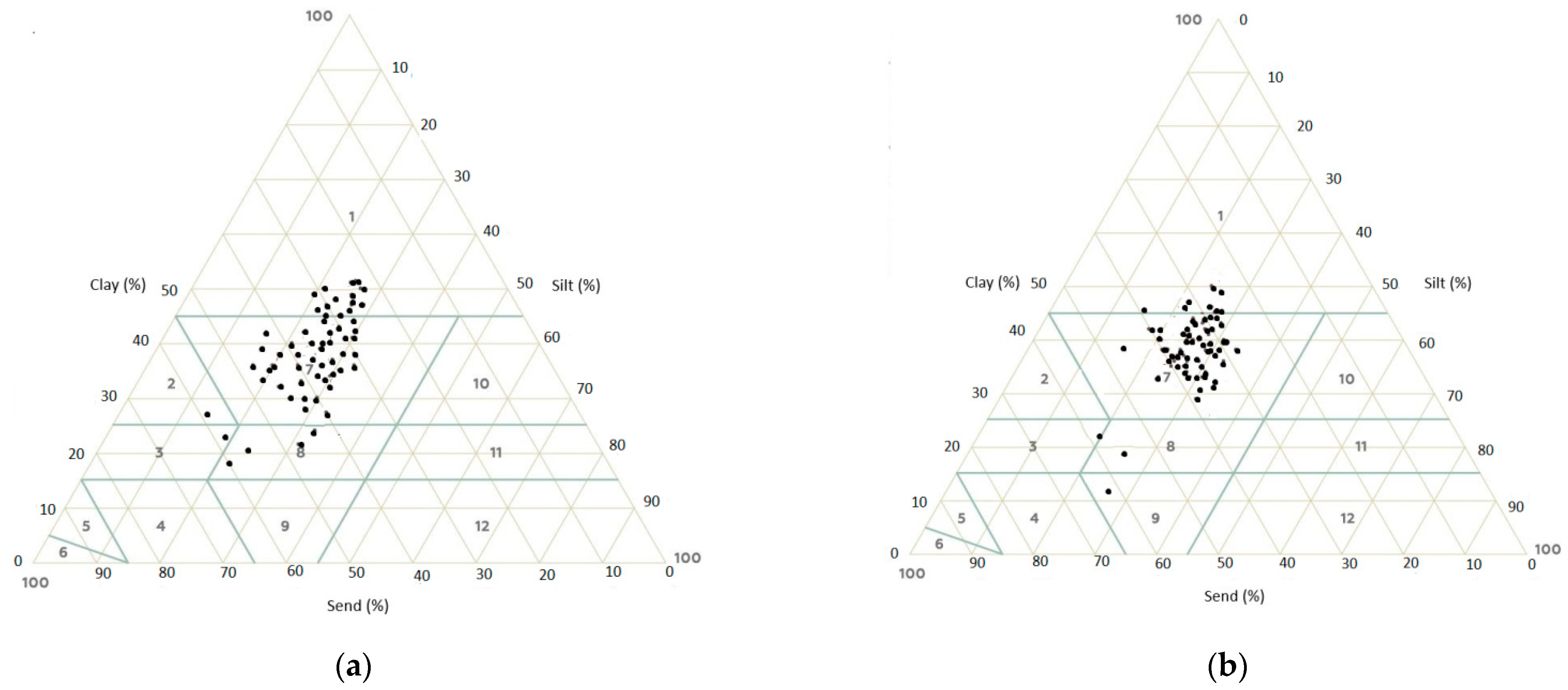
3.1. Characteristics of the Soil
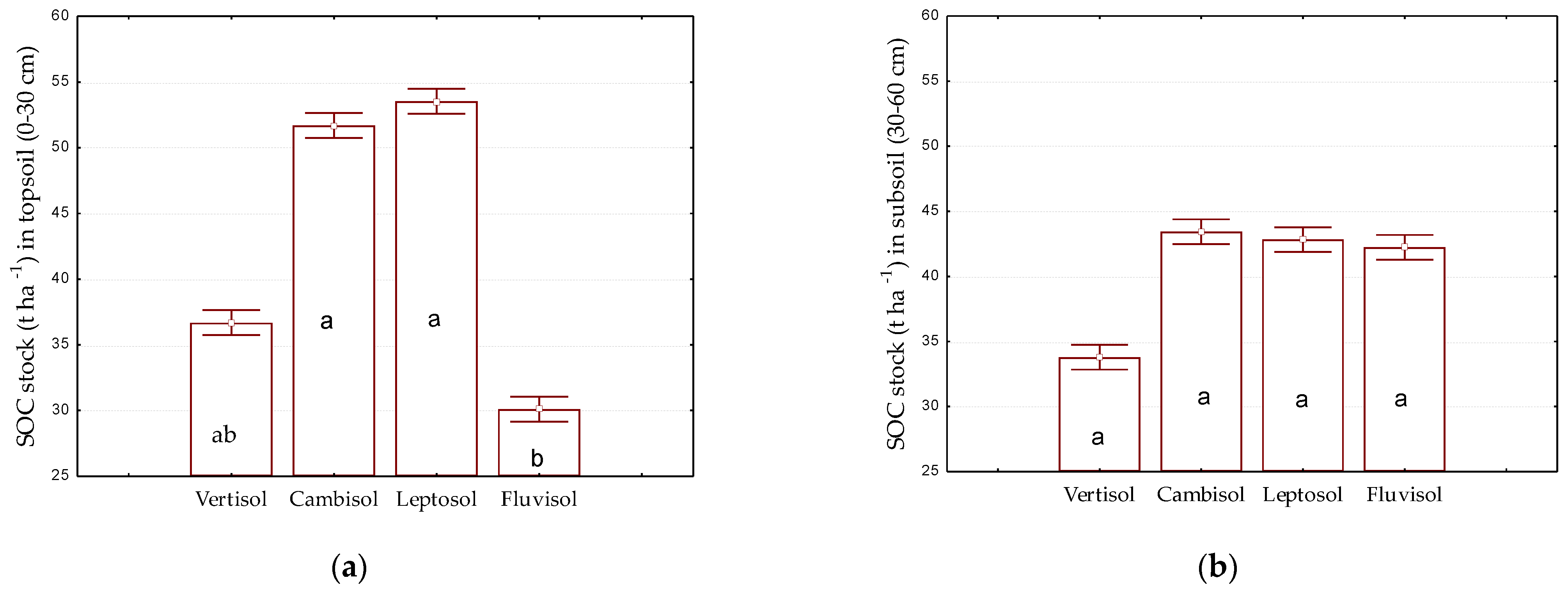
3.2. Soil Organic Carbon Stock
3.3. Organic Carbon Concentrations in Observed Soil Types
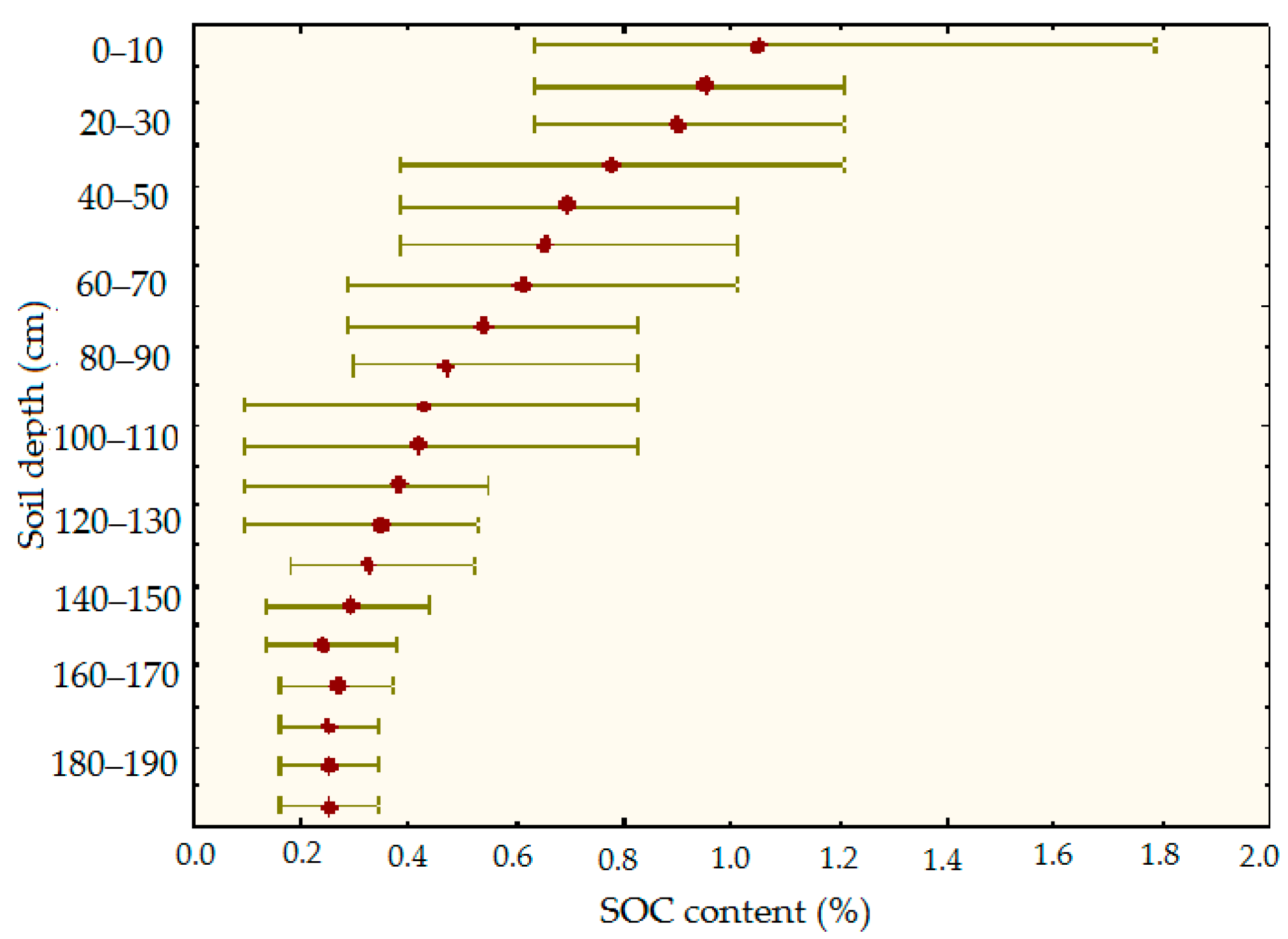
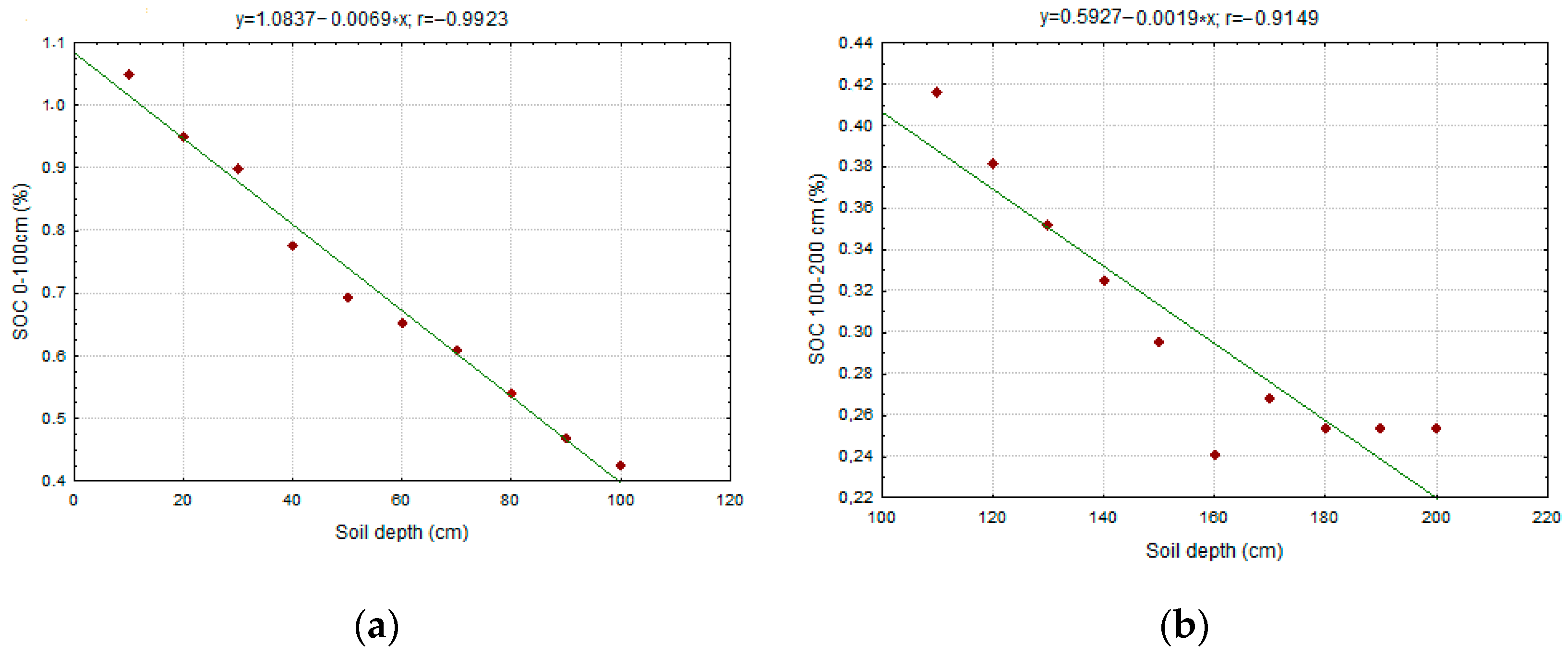
3.4. Distribution of Organic Carbon in the Soil Profile
3.5. Effect of Fertilization Strategies on SOC Concentration
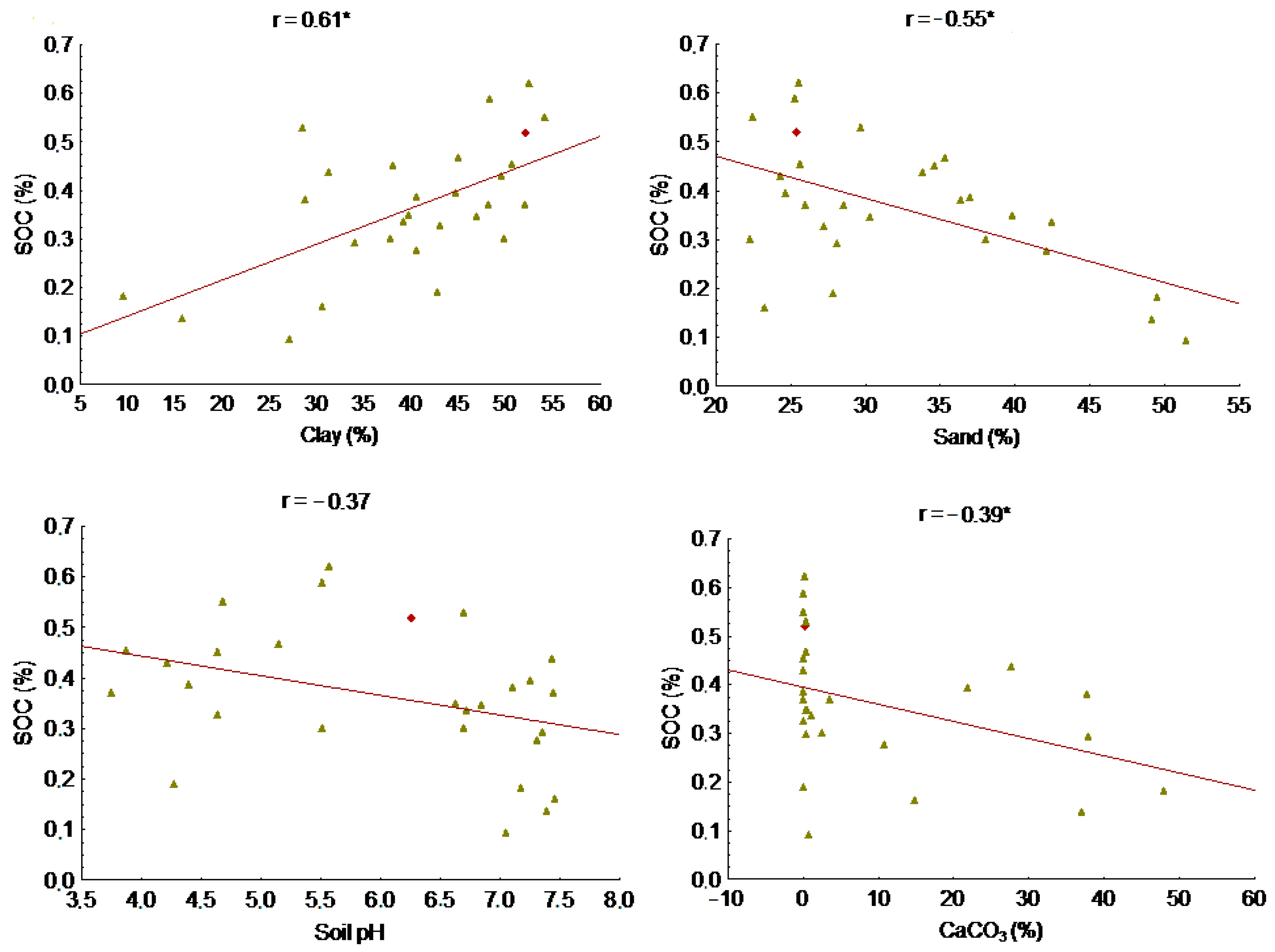
3.6. Correlation of SOC with Soil Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fang, H.J.; Yang, X.M.; Zhang, X.P.; Liang, A.J. Spatial heterogeneity and pattern of black soil organic carbon of sloping field. Bull. Soil Water Conserv. 2005, 25, 20–24. [Google Scholar]
- Zhao, J.; Meng, K.; Sui, Y.Y.; Han, B.J.; Zhang, Y.; Li, H.W. Analysis for spatial heterogeneity of organic carbon and available nutrients in black soil region of Hailun county. Chin. J. Soil Sci. 2005, 36, 487–492. [Google Scholar]
- Bauer, A.; Black, A.L. Quantification of the Effect of Soil Organic Matter Content on Soil Productivity. Soil Sci. Soc. Am. J. 1994, 58, 185–193. [Google Scholar] [CrossRef]
- Loveland, P. Is There a Critical Level of Organic Matter in the Agricultural Soils of Temperate Regions: A Review. Soil Tillage Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Campos, R.; Gabrielle, F.P.; Marcos, H.C. Soil Carbon Sequestration in Rainfed and Irrigated Production Systems in a New Brazilian Agricultural Frontier. Agriculture 2020, 10, 156. [Google Scholar] [CrossRef]
- Arunrat, N.; Praeploy, K.; Sukanya, S.; Ryusuke, H. Soil Organic Carbon in Sandy Paddy Fields of Northeast Thailand: A Review. Agronomy 2020, 10, 1061. [Google Scholar] [CrossRef]
- Kumputa, S.; Vityakon, P.; Saenjan, S.; Lawongsa, P. Carbonaceous Greenhouse Gases and Microbial Abundance in Paddy Soil under Combined Biochar and Rice Straw Amendment. Agronomy 2019, 9, 228. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil Organic Carbon and Total Nitrogen Stocks under Different Land Uses in a Semi-Arid Watershed in Tigray, Northern Ethiopia. Agric. Ecosys. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil Carbon and Nitrogen Stocks in Forests along an Altitudinal Gradient in the Eastern Himalayas and a Meta-Analysis of Global Data. Glob. Chan. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef]
- Lal, R. Sequestering Carbon in Soils of Agro-Ecosystems. Food Policy 2011, 36, S33–S39. [Google Scholar] [CrossRef]
- Calvo de Anta, R.; Luís, E.; Febrero-Bande, M.; Galiñanes, J.; Macías, F.; Ortíz, R.; Casás, F. Soil Organic Carbon in Peninsular Spain: Influence of Environmental Factors and Spatial Distribution. Geoderma 2020, 370, 114365. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den Berg, E.; Reich, P. Organic Carbon in Soils of the World. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Batjes, N.H. Total Carbon and Nitrogen in the Soils of the World. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Scharlemann, J.; Hiederer, R.; Kapos, V. Global Map of Terrestrial Soil Organic Carbon Stocks; UNEP-WCMC&EU-JRC: Cambridge, UK, 2009. [Google Scholar]
- Hiederer, R. Distribution of Organic Carbon in Soil Profile Data. EUR 23980 EN; Office for Official Publications of the European Communities: Luxembourg, 2009; p. 126. [Google Scholar]
- Batjes, N.H. Soil Property Values for Broad-Scale Modelling (WISE30sec) with Estimates of Global Soil Carbon Stocks. Geoderma 2016, 269, 61–68. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Lal, R. Food security impacts of the “4 per Thousand” initiative. Geoderma 2020, 374, 114427. [Google Scholar] [CrossRef]
- Yigini, Y.; Panagos, P. Assessment of Soil Organic Carbon Stocks under Future Climate and Land Cover Changes in Europe. Sci. Total Environ. 2016, 557–558, 838–850. [Google Scholar] [CrossRef]
- IPCC. Summary for policy makers. In Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T., Qin, D., Plattner, G., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Wiesmeier, M.; Barthold, F.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; Angst, G.; von Lützow, M.; Kögel-Knabner, I. Estimation of Total Organic Carbon Storage and Its Driving Factors in Soils of Bavaria (Southeast Germany). Geoderma 2014, 67–78. [Google Scholar] [CrossRef]
- Hobley, E.U.; Baldock, J.; Wilson, B. Environmental and Human Influences on Organic Carbon Fractions down the Soil Profile. Agric. Ecosys. Environ. 2016, 223, 152–166. [Google Scholar] [CrossRef]
- Hobley, E.; Wilson, B.; Wilkie, A.; Gray, J.; Koen, T. Drivers of Soil Organic Carbon Storage and Vertical Distribution in Eastern Australia. Plant Soil 2015, 390, 111–127. [Google Scholar] [CrossRef]
- Novara, A.; Favara, V.; Novara, A.; Francesca, N.; Santangelo, T.; Columba, P.; Chironi, S.; Ingrassia, M.; Gristina, L. Soil Carbon Budget Account for the Sustainability Improvement of a Mediterranean Vineyard Area. Agronomy 2020, 10, 336. [Google Scholar] [CrossRef]
- Napoli, M.; Dalla Marta, A.; Zanchi, C.A.; Orlandini, S. Assessment of Soil and Nutrient Losses by Runoff under Different Soil Management Practices in an Italian Hilly Vineyard. Soil Tillage Res. 2017, 168, 71–80. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J. Five Decades of Soil Erosion Research in ‘Terroir’. The State-of-the-Art. Earth-Sci. Rev. 2018, 179, 436–447. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Blavet, D.; De Noni, G.; Laurent, J.-Y.; Asseline, J.; Chenu, C. Erodibility of Mediterranean Vineyard Soils: Relevant Aggregate Stability Methods and Significant Soil Variables. Eur. J. Soil Sci. 2007, 58, 188–195. [Google Scholar] [CrossRef]
- Martínez-Casasnovas, J.A.; Concepción Ramos, M. Soil Alteration Due to Erosion, Ploughing and Levelling of Vineyards in North East Spain. Soil Use Manag. 2009, 25, 183–192. [Google Scholar] [CrossRef]
- Belmonte, S.A.; Celi, L.; Stahel, R.J.; Bonifacio, E.; Novello, V.; Zanini, E.; Steenwerth, K.L. Effect of Long-Term Soil Management on the Mutual Interaction Among Soil Organic Matter, Microbial Activity and Aggregate Stability in a Vineyard. Pedosphere 2018, 28, 288–298. [Google Scholar] [CrossRef]
- Chiti, T.; Gardin, L.; Perugini, L.; Quaratino, R.; Vaccari, P.; Franco, F.; Valentini, V. Soil organic carbon stock assessment for the different cropland land uses in Italy. Biol. Fertil. Soils 2012, 48, 9–17. [Google Scholar] [CrossRef]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial Distribution of Soil Organic Carbon Stocks in France. Biogeosciences 2011, 5, 1053–1065. [Google Scholar] [CrossRef]
- Rodríguez-Murillo, J.C. Organic Carbon Content under Different Types of Land Use and Soil in Peninsular Spain. Biol. Fertil. Soils 2001, 33, 53–61. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of Agricultural Management on Soil Organic Matter and Carbon Transformation; a Review. Plant Soil Environ. 2011, 52, 531–543. [Google Scholar] [CrossRef]
- Jakšić, S.; Milić, S.; Ninkov, J. Basic chemical properties of the soil. In Pedological and Agrochemical Properties of the Tri Morave Wine Region; Ninkov, J., Ed.; Institute of Field and Vegetable Crops: Novi Sad, Serbia, 2016. [Google Scholar]
- Ivanišević, D.; Jakšić, D.; Korać, N. Vineyard Atlas; Republican Bureau of Statistics: Belgrade, Serbia, 2015.
- Tomic, N.; Kokovic, J.; Jaksic, D.; Ninkov, J.; Vasin, J.; Malicanin, M.; Markovic, S. Terroir of the Tri Morave Wine Region (Serbia) as a Basis for Producing Wines with Geographical Indication. Geogr. Pannon. 2017, 21, 166–178. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017. General Requirements for the Competence of Testing and Calibration Laboratories; Institute for Standardization of Serbia: Belgrade, Serbia, 2017. [Google Scholar]
- ISO 11464:2006. Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis; Institute for Standardization of Serbia: Belgrade, Serbia, 2006. [Google Scholar]
- ISO 10390:2005. Soil Quality—Determination of pH; Institute for Standardization of Serbia: Belgrade, Serbia, 2005. [Google Scholar]
- ISO 10693:1995. Soil Quality—Determination of Carbonate Content—Volumetric Method; Institute for Standardization of Serbia: Belgrade, Serbia, 1995. [Google Scholar]
- ISO 106941995. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis); Institute for Standardization of Serbia: Belgrade, Serbia, 1995. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; ISRIC FAO Technical Paper Vol. 9; International Soil Reference and Information Centre Wageningen: Wageningen, The Netherlands, 2002. [Google Scholar]
- Ninkov, J. Karakterizacija Zemljišta Niškog Vinogradarskog Rejona; Institute of Field and Vegetable Crops: Novi Sad, Serbia, 2014. [Google Scholar]
- Leake, S. Vineyard Soil Management; Sydney Environmental & Soil Laboratory Pty Ltd.: Sydney, Australia, 1999. [Google Scholar]
- White, R.E. Soils for Fine Wines; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Gücüyen, A. Manisa ili ve Çevresinde Bağcılıkta Mekanizasyon Durumu, Sorunları ve iyi Tarım Uygulamalarına Yönelik Çözüm Önerileri; Ege Üniversitesi Fen Bilimleri Enstitüsü: İzmir, Turkey, 2007; p. 146s. (In Turkish) [Google Scholar]
- Kaĉinskij, N.A. Mehaničeskij i Mikroagregatnij Sostav Počvi, Metodi Ego Izučenija; Izdateljstvo Akademi nauk SSSR: Moskva, Russia, 1958. [Google Scholar]
- Vučić, N. Water, Air and Soil Temperature Regime; Matica srpska Novi Sad, Faculty of Agriculture University of Novi Sad: Novi sad, Serbia, 1987. [Google Scholar]
- Doğan, B.; Gülser, C. Assessment of Soil Quality for Vineyard Fields: A Case Study in Menderes District of Izmir, Turkey. Eurasian J. Soil Sci. 2019, 8, 176–183. [Google Scholar] [CrossRef]
- Vidojević, D. Estimation of Soil Organic Matter in the Soils of Serbia; Faculty of Agriculture: Novi Sad, Serbia, 2015. [Google Scholar]
- Smith, W.N.; Desjardins, R.L.; Pattey, E. The Net Flux of Carbon from Agricultural Soils in Canada 1970–2010. Glob. Chang. Biol. 2000, 6, 557–568. [Google Scholar] [CrossRef]
- Arrouays, D.; Deslais, W.; Badeau, V. The carbon content of topsoil and its geographical distribution in France. Soil Use Manag. 2001, 17, 7–11. [Google Scholar] [CrossRef]
- Gardi, C.; Sconosciuto, F. Evaluation of carbon stock variation in Northern Italian soils over the last 70 years. Sustain. Sci. 2007, 2, 237–243. [Google Scholar] [CrossRef]
- Morari, F.; Lugato, E.; Berti, A.; Giardini, L. Long-Term Effects of Recommended Management Practices on Soil Carbon Changes and Sequestration in North-Eastern Italy. Soil Use Manag. 2006, 22, 71–81. [Google Scholar] [CrossRef]
- Novara, A.; Minacapilli, M.; Santoro, A.; Rodrigo-Comino, J.; Carrubba, A.; Sarno, M.; Venezia, G.; Gristina, L. Real Cover Crops Contribution to Soil Organic Carbon Sequestration in Sloping Vineyard. Sci. Total Environ. 2019, 652, 300–306. [Google Scholar] [CrossRef]
- Robinson, C.A.; Cruse, R.M.; Kohler, K.A. Soil management. In Sustainable Agricultural Systems; Hatfield, J.L., Karlen, D.L., Eds.; Lewis Publ.: Boca Raton, FL, USA, 1994; pp. 109–134. [Google Scholar]
- Kladivko, E.J. Tillage Systems and Soil Ecology. Soil Tillage Res. 2011, 61, 61–76. [Google Scholar] [CrossRef]
- Beare, M.H.; Hendrix, P.F.; Cabrera, M.L.; Coleman, D.C. Aggregate-Protected and Unprotected Organic Matter Pools in Conventional- and No-Tillage Soils. Soil Sci. Soc. Am. J. 1994, 58, 787–795. [Google Scholar] [CrossRef]
- Buckman, H.O.; Brady, N.C. The Nature and Properties of Soil, 6th ed.; MacMillan: New York, NY, USA, 1960. [Google Scholar]
- Belić, M.; Manojlović, M.; Nešić, L.J.; Ćirić, V.; Vasin, J.; Benka, P.; Šeremešić, S. Significance of Soil Organic Carbon Stock in Southe-Eastern Panonnian Basin. Carpathian J. Earth Environ. Sci. 2013, 1, 171–178. [Google Scholar]
- Schöning, I.; Totsche, K.U.; Kögel-Knabner, I. Small Scale Spatial Variability of Organic Carbon Stocks in Litter and Solum of a Forested Luvisol. Geoderma 2006, 136, 631–642. [Google Scholar] [CrossRef]
- Grüneberg, E.; Ziche, D.E.; Wellbrock, N. Organic carbon stocks and sequestration rates of forest soils in Germany. Glob. Chang. Biol. 2014, 20, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, V. Kvalitativne i kvantitativne karakteristike organske materije različitih tipova zemljišta. In Doktorska Disertacija; Poljoprivredni Fakultet: Novi Sad, Serbia, 2013. [Google Scholar]
- Vasques, G.M.; Grunwald, S.; Comerford, N.B.; Sickman, J.O. Regional Modelling of Soil Carbon at Multiple Depths within a Subtropical Watershed. Geoderma 2010, 156, 326–336. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zha, T.; Zhang, X.; Ma, L. Vertical Distribution and Influencing Factors of Soil Organic Carbon in the Loess Plateau, China. Sci. Total Environ. 2019, 693, 133632. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Tong-Gang, Z.H.A.; Xiao-Xia, Z.; Zhi-Qiang, Z.; Yu-Shen, Z.H.U.; Ya, Z.; Yi-Han, L.I.U.; Zhu, L.I.N. Effects of Vegetation Type and Terrain on Vertical Distribution of Soil Organic Carbon on abandoned Farmlands in the Loess Plateau. Chin. J. Ecol. 2017, 36, 2447. [Google Scholar]
- Van den Bygaart, A.J.; Gregorich, E.G.; Angers, D.A.; McConkey, B.G. Assessment of the Lateral and Vertical Variability of Soil Organic Carbon. Can. J. Soil Sci. 2007, 87, 433–444. [Google Scholar] [CrossRef]
- Popović, T.; Mijović, S.; Pajović Šćepanović, R.; Raičević, D. Analysis of possibilities of reducing the quantity of mineral fertilizer application using different types of organic fertilizers in Cardinal grape variety. Agric. For. 2020, 66, 261–268. [Google Scholar] [CrossRef]
- Ozdemir, G. Determination of the effect of some organic and organo-mineral fertilizers on total phenolic, flavonoid and anthocyanin content of Bogazkere (Vitis vinifera L.) grapes. Fresenius Environ. Bull. 2018, 27, 3199–3205. [Google Scholar]
- Malusà, E.; Laurenti, E.; Ghibaudi, E.; Rolle, L. Influence of organic and conventional management on yield and composition of grape cv. “Grignolino”. Acta Hortic. 2004, 640, 135–141. [Google Scholar] [CrossRef]
- Blidariu, C.; Sala, F. Influence of organic and mineral fertilization on sugar content in Italian Riesling grape variety. J. Hortic. For. Biotechnol. 2012, 16, 251–254. [Google Scholar]
- Yang, X.M.; Zhang, X.P.; Fang, H.J.; Zhu, P.; Ren, J.; Wang, L.C. Effects of fertilization under continuous on organic carbon in black soil: Simulation by RothC-26.3 model. Agric. Sci. China 2003, 36, 1318–1324. [Google Scholar]
- Liu, X.; Liu, J.; Xing, B.; Herbert, S.J.; Meng, K.; Han, X.; Zhang, X. Effects of Long-Term Continuous Cropping, Tillage, and Fertilization on Soil Organic Carbon and Nitrogen of Black Soils in China. Commun. Soil Sci. Plant Anal. 2005, 36, 1229–1239. [Google Scholar] [CrossRef]
- Hao, Y.; Lal, R.; Owens, L.B.; Izaurralde, R.C.; Post, W.M.; Hothem, D.L. Effect of Cropland Management and Slope Position on Soil Organic Carbon Pool at the North Appalachian Experimental Watersheds. Soil Tillage Res. 2002, 68, 133–142. [Google Scholar] [CrossRef]
- Biddoccu, M.; Zecca, O.; Audisio, C.; Godone, F.; Barmaz, A.; Cavallo, E. Assessment of long-term soil erosion in a mountain vineyard, Aosta Valley (NW Italy). Land Degrad. Dev. 2018, 29, 617–629. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon Sequestration in the Agricultural Soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Triberti, L.; Nastri, A.; Giordani, G.; Comellini, F.; Baldoni, G.; Toderi, G. Can Mineral and Organic Fertilization Help Sequestrate Carbon Dioxide in Cropland? Eur. J. Agron. 2008, 29, 13–20. [Google Scholar] [CrossRef]
- Garcia Diaz, A.; Sastre, B.; Aton, O. The influence of the soil management strategy on the soil organic carbon concentration in meditarrean vineyards. In Proceedings of the 1st World Conference on Soil and Water Conservation under Global Change-CONSOWA, Lleida, Spain, 12–16 June 2017. [Google Scholar]
- Cervantes, V.A.; Don, A.; Well, R.; Schneider, F.; Nieder, R. Deep ploughing mineral soils for SOC sequestration. In Global Symposium on Soil Organic Carbon; FAO: Rome, Italy, 2017. [Google Scholar]
- Arshad, M.A.; Schnitzer, M.; Angers, D.A.; Ripmeester, J.A. Effects of till vs. no-till on the quality of soil organic matter. Soil Biol. Biochem. 1990, 22, 595–599. [Google Scholar] [CrossRef]
- Dalal, R.C.; Henderson, P.A.; Glasby, J.M. Organic matter and microbial biomass in a Vertisol after 20 yr. of zero-tillage. Soil Biol. Biochem. 1991, 23, 435–441. [Google Scholar] [CrossRef]
- Ding, G.; Novak, J.M.; Amarasiriwardena, D.; Hunt, P.G.; Xing, B. Soil organic matter characteristics as affected by tillage management. Soil Sci. Soc. Am. J. 2002, 66, 421–429. [Google Scholar] [CrossRef]
- Campbell, C.A.; Selles, F.; Lafond, G.P.; Biederbeck, V.O.; Zentner, R.P. Tillage-fertilizer changes: Effect on some soil quality attributes under long-term crop rotation in a thin Black Chernozem. Can. J. Soil Sci. 2001, 81, 157–165. [Google Scholar] [CrossRef]
- Liebig, M.A.; Tanaka, D.L.; Wienhold, B.J. Tillage and cropping effects on soil quality indicators in the northern Great Plains. Soil Tillage Res. 2004, 78, 131–141. [Google Scholar] [CrossRef]
- Vos, C.; Don, A.; Hobley, E.U.; Prietz, R.; Heidkamp, A.; Freibauer, A. Factors Controlling the Variation in Organic Carbon Stocks in Agricultural Soils of Germany. Eur. J. Soil Sci. 2019, 70, 550–564. [Google Scholar] [CrossRef]
- Meersmans, J.; De Ridder, F.; Canters, F.; De Baets, S.; Van Molle, M. A Multiple Regression Approach to Assess the Spatial Distribution of Soil Organic Carbon (SOC) at the Regional Scale (Flanders, Belgium). Geoderma 2008, 143, 1–13. [Google Scholar] [CrossRef]
- Meersmans, J.; Martin, M.P.; Lacarce, E.; De Baets, S.; Jolivet, C.; Boulonne, L.; Lehmann, S.; Philippe, N.; Saby, A.; Bispo, A.; et al. A High Resolution Map of French Soil Organic Carbon. Agron. Sustain. Dev. 2012, 32, 841–851. [Google Scholar] [CrossRef]
- Travnikova, L.S.; Titova, N.A.; Kogut, B.M.; Schulz, E.; Körschens, M. Evaluation of the Different Soil Organic Matter (SOM) Pools Stability in Long-Term Field Experiments of Germany by Physical Fractionation. Arch. Agron. Soil Sci. 2002, 48, 565–576. [Google Scholar] [CrossRef]
- Rice, C.W. Organic matter and nutrient dynamics. In Encyclopedia of Soil Science; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 925–928. [Google Scholar]
- Prasad, R.; Power, J.F. Soil Fertility Management for Sustainable Agriculture; Lewis Publishers in an Imprint of CRC Press: Boca Raton, FL, USA, 1997; p. 243. [Google Scholar]
- Li, Y.; Wang, X.; Niu, Y.; Lian, J.; Luo, Y.; Chen, Y.; Gong, X.; Yang, H.; Yu, P. Spatial Distribution of Soil Organic Carbon in the Ecologically Fragile Horqin Grassland of Northeastern China. Geoderma 2018, 325, 102–109. [Google Scholar] [CrossRef]
- Islam, K.K.; Anusontpornperm, S.; Kheoruenromne, I.; Thanachit, S. Relationship between Carbon Sequestration and Physico-Chemical Properties of Soils in Salt-Affected Areas, Northeast Thailand. Kasetsart J. Nat. Sci. 2014, 48, 560–576. [Google Scholar]
- Ayaz, M.; Akhtar, M.; Rukh, S.; Imran, M.; Hassan, A.; Abbasi, K.; Qayyum, A. Soil Organic Carbon Stock Variation with Climate and Land Use in Shale Derived Soils. J. Serbian Chem. Soc. 2018, 83, 785–793. [Google Scholar]
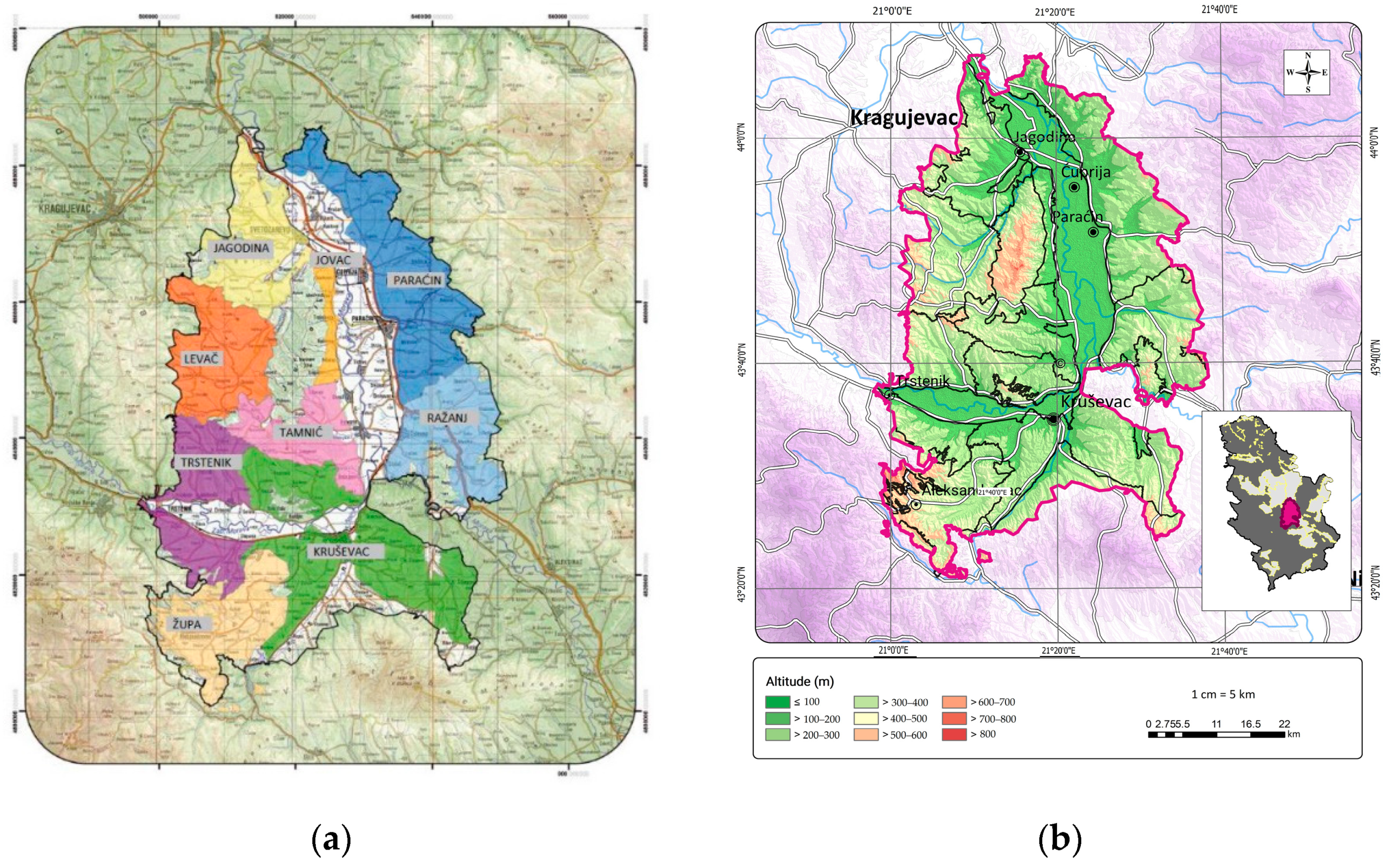
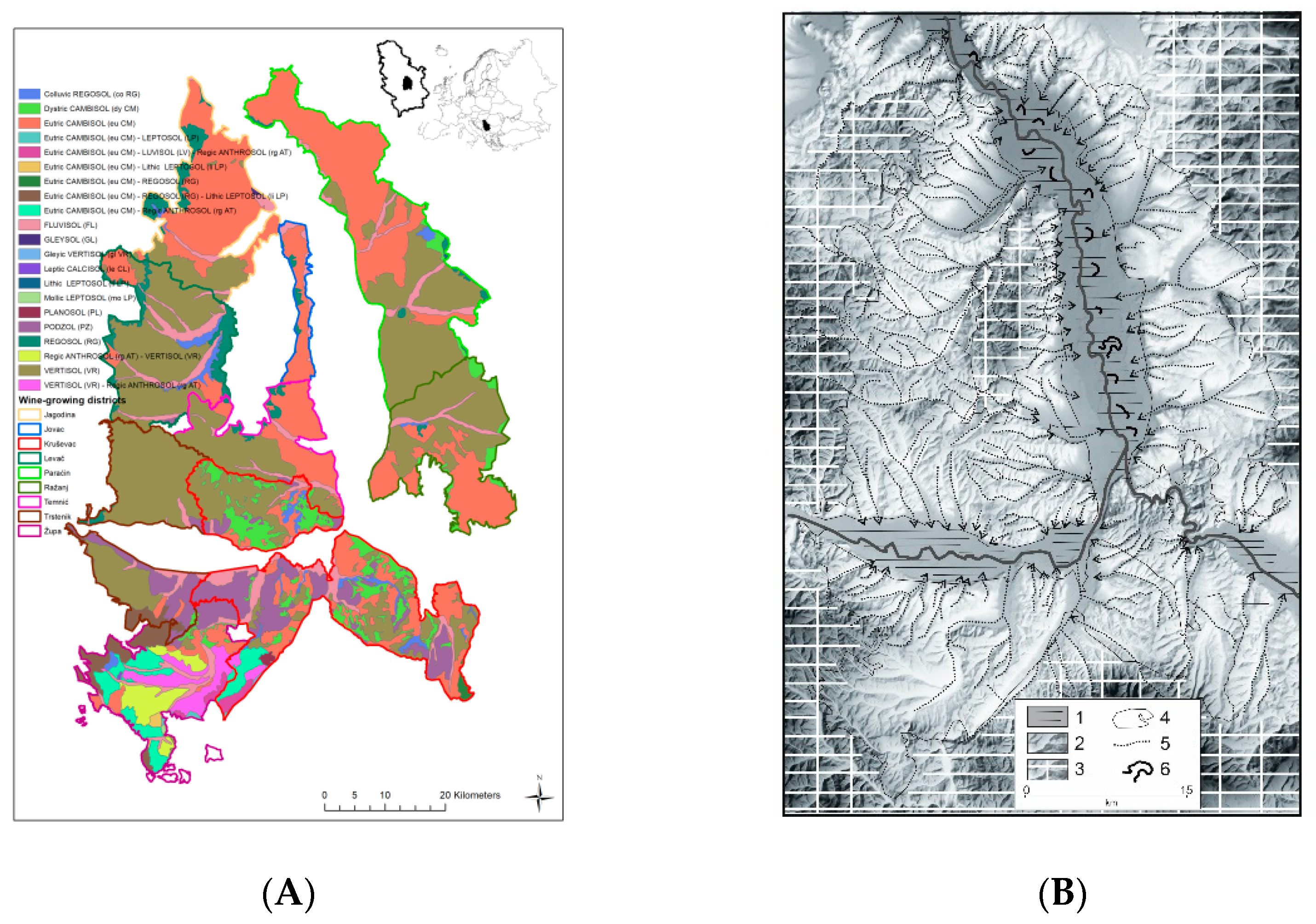

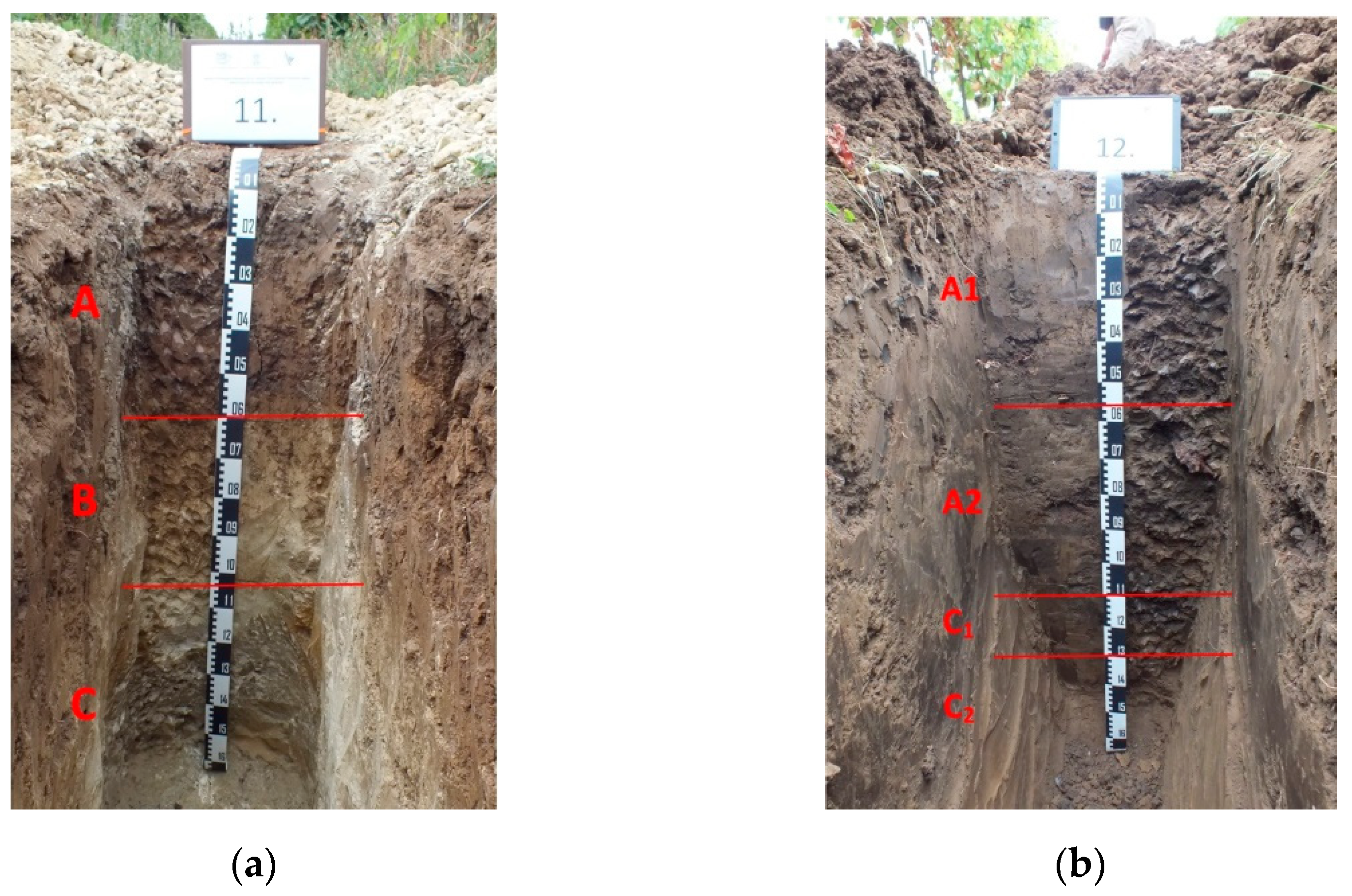
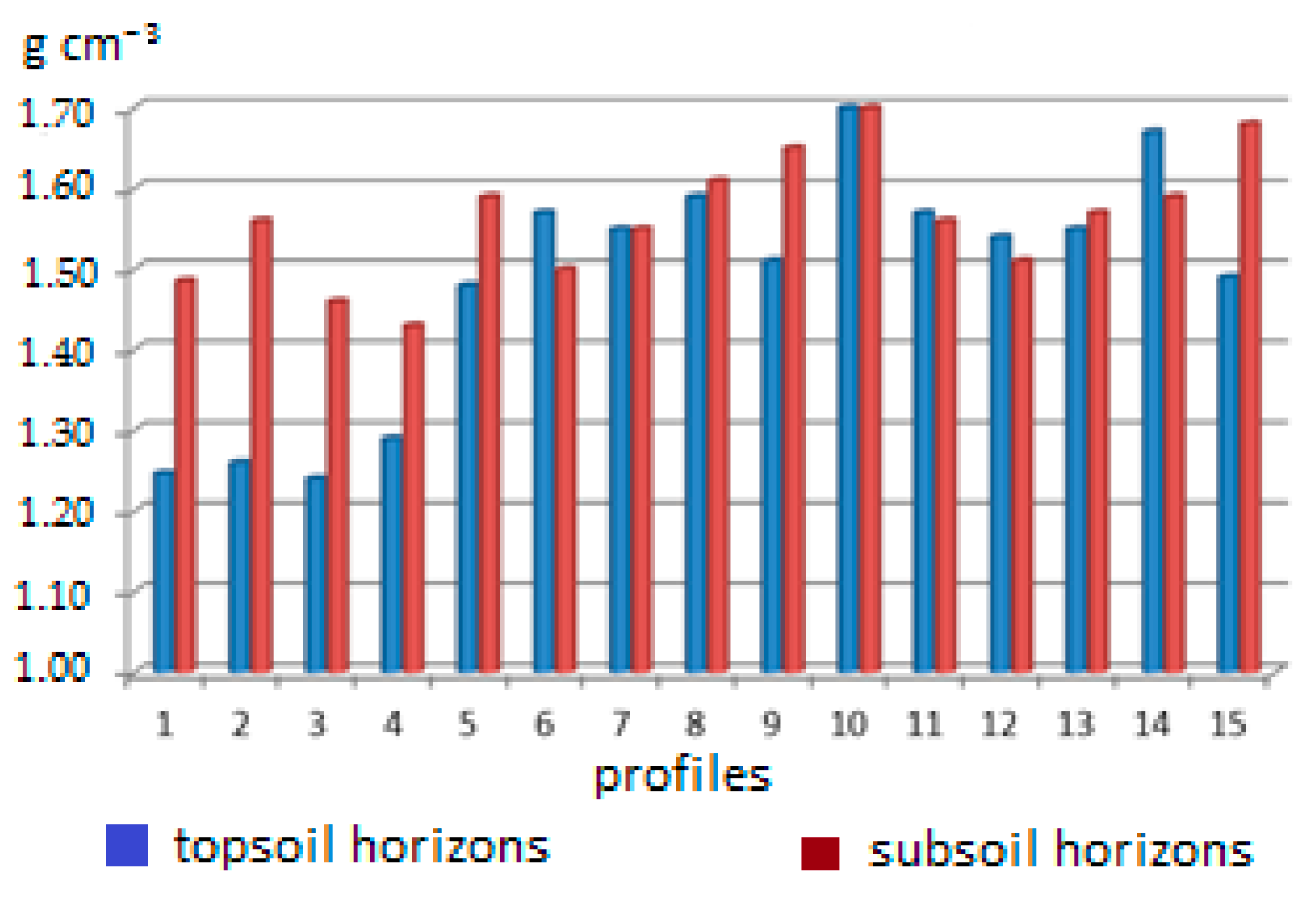
| No | Locality | Vine Growing District | Soil Type (FAO–WRB) |
|---|---|---|---|
| 1 | Levač | Levač | Haplic Vertisol (Clayic) |
| 2 | Dobričevo | Paraćin | Eutric Cambisol |
| 3 | Glavica | Levač | Haplic Vertisol (Clayic) |
| 4 | Oparić | Paraćin | Stagnic, Eutric Cambisol |
| 5 | Lozovik | Jagodina | Eutric Cambisol (Clayic) |
| 6 | Lučina | Kruševac | Eutric Cambisol (Clayic) |
| 7 | Ravnjak | Kruševac | Eutric Cambisol (Clayic) |
| 8 | Bučje | Trstenik | Skeletic Leptosol (Clayic) |
| 9 | Trstenik | Trstenik | Eutric Cambisol (Clayic) |
| 10 | Bačina | Temnić | Eutric Cambisol (Clayic) |
| 11 | Lipovac | Ražanj | Eutric Cambisol (Clayic) |
| 12 | Gornje Zleginje | Župa | Haplic Vertisol |
| 13 | Donje Zleginje | Župa | Haplic Vertisol |
| 14 | Tržac | Župa | Eutric Cambisol (Clayic) |
| 15 | Aleksandrovac | Župa | Haplic Vertisol |
| 16 | Aleksandrovac | Župa | Gleyic, Skeletic Fluvisol (Clayic) |
| AF + FM + NPK | Ameliorative fertilization initially + farmyard manure initially + continuous application of NPK 1 inorganic fertilizers |
| AF + NPK | Ameliorative fertilization initially + continuous application of NPK 1 inorganic fertilizers |
| AF + FM | Ameliorative fertilization initially + farmyard manure initially |
| FM + GM | Farmyard manure initially + green manure continuously |
| F | Foliar microbial fertilizer continuously |
| NF | No fertilizers |
| Soil Properties | Min. | Max. | Mean | Std. Dev. |
|---|---|---|---|---|
| 0–30 cm | ||||
| pH (in 1M KCl) | 3.94 | 7.60 | 5.53 | 1.14 |
| CaCO3 (%) | 0.00 | 18.45 | 1.29 | 2.87 |
| Clay (%) | 17.76 | 50.48 | 38.81 | 6.33 |
| Silt (%) | 16.36 | 32.36 | 25.48 | 3.70 |
| Fine sand (%) | 19.83 | 46.32 | 29.55 | 5.36 |
| Coarse sand (%) | 1.17 | 19.86 | 6.12 | 4.39 |
| 30–60 cm | ||||
| pH (in 1M KCl) | 3.77 | 7.56 | 5.35 | 1.13 |
| CaCO3 (%) | 0.00 | 10.90 | 1.38 | 2.72 |
| Clay (%) | 13.96 | 49.96 | 39.13 | 6.65 |
| Silt (%) | 15.76 | 33.60 | 25.33 | 3.87 |
| Fine sand (%) | 20.34 | 47.62 | 29.49 | 5.46 |
| Coarse sand (%) | 1.16 | 17.66 | 6.05 | 4.40 |
| profile horizons, 0–200 cm | ||||
| pH (in 1M KCl) | 3.75 | 7.47 | 5.61 | 1.25 |
| CaCO3 (%) | 0.00 | 37.90 | 3.08 | 8.42 |
| Clay (%) | 15.84 | 54.16 | 40.96 | 8.24 |
| Silt (%) | 17.20 | 46.16 | 26.34 | 5.22 |
| Fine sand (%) | 18.91 | 40.71 | 27.12 | 5.50 |
| Coarse sand (%) | 1.09 | 20.47 | 5.59 | 4.67 |
| Bulk density (g cm−3) | 1.24 | 1.70 | 1.52 | 0.13 |
| Soil Type | Min. | Max. | Mean | Std. Dev. |
|---|---|---|---|---|
| 0–30 cm | ||||
| Eutric Cambisol | 0.64 | 2.02 | 1.12 | 0.32 |
| Haplic Vertisol | 0.38 | 1.19 | 0.85 | 0.32 |
| Skeletic Leptosol | 0.94 | 1.25 | 1.12 | 0.16 |
| Gleyic, Skeletic Fluvisol | 0.62 | 0.73 | 0.67 | 0.07 |
| 30–60 cm | ||||
| Eutric Cambisol | 0.31 | 1.15 | 0.93 | 0.32 |
| Haplic Vertisol | 0.38 | 1.03 | 0.74 | 0.32 |
| Skeletic Leptosol | 0.74 | 1.00 | 0.89 | 0.13 |
| Gleyic, Skeletic Fluvisol | 0.52 | 1.14 | 0.83 | 0.43 |
| Fertilization Strategies 1 | SOC (%) | Mean SOC (%) | ||
|---|---|---|---|---|
| 0–30 cm | 30–60 cm | |||
| 1 | AF + FM + NPK | 1.15 | 1.01 | 1.08 a |
| 2 | AF + NPK | 1.14 | 0.97 | 1.05 ab |
| 3 | AF + FM | 0.94 | 0.81 | 0.88 abc |
| 4 | FM + GM | 1.12 | 0.89 | 1.00 ab |
| 5 | F | 0.87 | 0.75 | 0.81 bc |
| 6 | NF | 0.85 | 0.58 | 0.71 c |
| Fertilization Strategies 1 | Years of Cultivation | SOC Concentration Change (g kg−1 y−1) | SOC Stock Change (t ha−1 y−1) | Rate of SOC Stock Change (% y−1) | |||
|---|---|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | ||
| NF | ≤5 | −0.77 | −1.15 | −3.61 | −5.21 | −8.42 | −10.21 |
| IF + FM | ≤5 | −1.66 | 0.47 | −6.75 | 2.01 | −13.29 | 6.00 |
| 6–40 | −0.10 | 0.10 | −0.42 | 0.43 | −0.64 | 1.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakšić, S.; Ninkov, J.; Milić, S.; Vasin, J.; Banjac, D.; Jakšić, D.; Živanov, M. The State of Soil Organic Carbon in Vineyards as Affected by Soil Types and Fertilization Strategies (Tri Morave Region, Serbia). Agronomy 2021, 11, 9. https://doi.org/10.3390/agronomy11010009
Jakšić S, Ninkov J, Milić S, Vasin J, Banjac D, Jakšić D, Živanov M. The State of Soil Organic Carbon in Vineyards as Affected by Soil Types and Fertilization Strategies (Tri Morave Region, Serbia). Agronomy. 2021; 11(1):9. https://doi.org/10.3390/agronomy11010009
Chicago/Turabian StyleJakšić, Snežana, Jordana Ninkov, Stanko Milić, Jovica Vasin, Dušana Banjac, Darko Jakšić, and Milorad Živanov. 2021. "The State of Soil Organic Carbon in Vineyards as Affected by Soil Types and Fertilization Strategies (Tri Morave Region, Serbia)" Agronomy 11, no. 1: 9. https://doi.org/10.3390/agronomy11010009
APA StyleJakšić, S., Ninkov, J., Milić, S., Vasin, J., Banjac, D., Jakšić, D., & Živanov, M. (2021). The State of Soil Organic Carbon in Vineyards as Affected by Soil Types and Fertilization Strategies (Tri Morave Region, Serbia). Agronomy, 11(1), 9. https://doi.org/10.3390/agronomy11010009






