Tree—Open Grassland Structure and Composition Drive Greenhouse Gas Exchange in Holm Oak Meadows of the Iberian Peninsula
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Design
2.2. Greenhouse Gas Exchange (GHG) Measurements
2.3. Vegetation Sampling
2.4. Belowground Biomass Sampling and Soil Water Content Determination
2.5. Data Analysis: Greenhouse Gas Exchange Modeling
- (a)
- Identity model, which includes PFT identity effects, meaning the biomass proportion of each PFT (Equation (2)), where P indicates the proportion of the given PFT and the sub-index F indicates forbs, G grasses, and L legumes, respectively:Identity model
- (b)
- Average interaction model, which includes PFT identity effects, plus evenness [46] as an average interaction term (Equation (3)). Evenness (E) calculated as , where PFT is the number of PFT present in the community, and the relative abundance of the PFTs. Evenness lies between 0 for mono-PFT plots, and 1 when all PFT are equally represented.Average interaction model
- (c)
- Specific interaction model, which includes specific interactions between PFT in addition to the identity effects (Equation (4)):Specific interaction model
3. Results
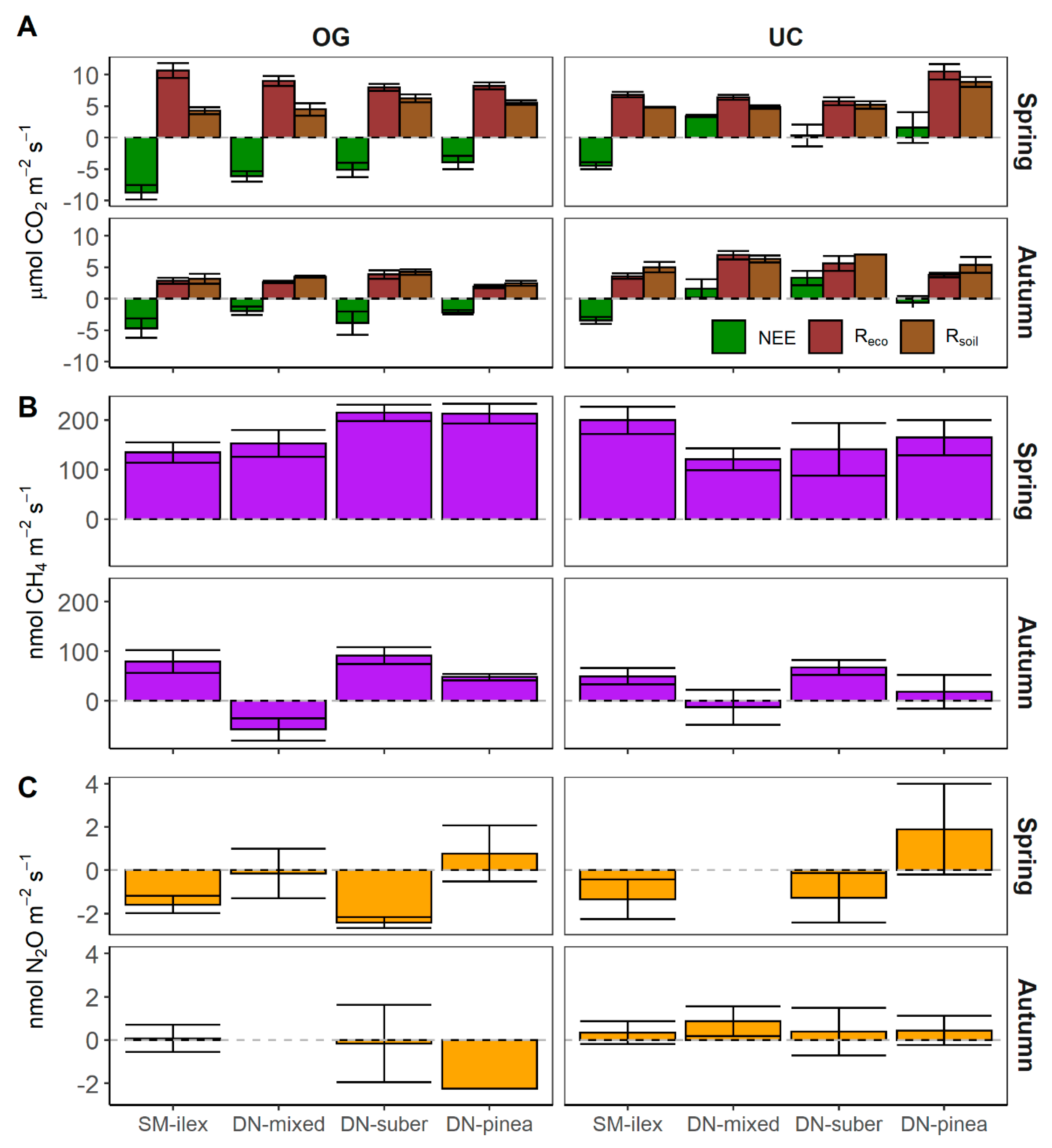
3.1. CO2 Exchange
3.2. CH4 and N2O Exchange
4. Discussion
4.1. CO2 Exchange Drivers
4.2. CH4 and N2O Exchange Drivers
4.3. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eichhorn, M.P.; Paris, P.; Herzog, F.; Incoll, L.D.; Liagre, F.; Mantzanas, K.; Mayus, M.; Moreno, G.; Papanastasis, V.P.; Pilbeam, D.J.; et al. Silvoarable systems in Europe — Past, present and future prospects. Agrofor. Syst. 2006, 67, 29–50. [Google Scholar] [CrossRef]
- Olea, L.; López-Bellido, R.J.; Poblaciones, M. Europe types of silvopastoral systems in the Mediterranean area: Dehesa. In Silvopastoralism and Sustainable Land Management. In Proceedings of the International Congress on Silvopastoralism and Sustainable Management, Lugo, Spain, 18–24 April 2004; Mosquera-Losada, M.R., Rigueiro-Rodríguez, A., McAdam, J., Eds.; CAB International: Wallingford, Oxfordshire, UK, 2005; pp. 30–35. [Google Scholar]
- Huntsinger, L.; Campos, P.; Starrs, P.F.; Oviedo, J.L.; Díaz, M.; Standiford, R.B.; Gregorio, M. Working Landscapes of the Spanish Dehesa and California Oak Woodlands: An Introduction. In Mediterranean Oak Woodland Working Landscapes. Dehesas of Spain and Ranchlands of California; Campos, P., Huntsinger, L., Oviedo, J.L., Starrs, P.F., Diaz, M., Standiford, R.B., Montero, G., Eds.; Springer: New York, NY, USA, 2013; pp. 3–23. ISBN 978-94-007-6706-5. [Google Scholar]
- Marañón, T.; Pugnaire, F.I.; Callaway, R.M. Mediterranean-climate oak savannas: The interplay between abiotic environment and species interactions. Web Ecol. 2009, 9, 30–43. [Google Scholar] [CrossRef]
- Gaman, T.; Firman, J. Oaks 2040: The Status and Future of Oaks in California; California Oak Foundation: Oakland, CA, USA, 2006. [Google Scholar]
- Mohan Kumar, B.; Ramachandran Nair, P.K. (Eds.) Carbon Sequestration Potential of Agroforestry Systems; Springer: Berlin, Germany, 2011; ISBN 9789400716308. [Google Scholar]
- Costa Pérez, J.C.; Martín Vicente, Á.; Fernández Alés, R.; Estirado Oliet, M.; Montes, I.D.; Medio, C.D. Dehesas de Andalucía. Caracterización Ambiental; Consejería de Medio Ambiente, Junta de Andalucía, Eds.; Tecnographic, S.L.: Sevilla, Spain, 2006; ISBN 849632981X. [Google Scholar]
- Costa, A.; Madeira, M.; Lima Santos, J.; Oliveira, Â. Change and dynamics in Mediterranean evergreen oak woodlands landscapes of Southwestern Iberian Peninsula. Landsc. Urban Plan. 2011, 102, 164–176. [Google Scholar] [CrossRef]
- Howlett, D.S.; Moreno, G.; Mosquera Losada, M.R.; Nair, P.K.R.; Nair, V.D. Soil carbon storage as influenced by tree cover in the Dehesa cork oak silvopasture of central-western Spain. J. Environ. Monit. 2011, 13, 1897–1904. [Google Scholar] [CrossRef]
- Gómez-Rey, M.X.; Madeira, M.; Gonzalez-Prieto, S.J.; Coutinho, J. Soil C and N dynamics in a Mediterranean oak woodland with shrub encroachment. Plant Soil 2013, 371, 339–354. [Google Scholar] [CrossRef]
- Pulido-Fernández, M.; Schnabel, S.; Lavado-Contador, J.F.; Miralles Mellado, I.; Ortega Pérez, R. Soil organic matter of Iberian open woodland rangelands as influenced by vegetation cover and land management. Catena 2013, 109, 13–24. [Google Scholar] [CrossRef]
- Andivia, E.; Fernández, M.; Alejano, R.; Vázquez-Piqué, J. Tree patch distribution drives spatial heterogeneity of soil traits in cork oak woodlands. Ann. For. Sci. 2015, 549–559. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Xu, L.; Kiang, N. How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak-grass savanna and an annual grassland. Agric. For. Meteorol. 2004, 123, 13–39. [Google Scholar] [CrossRef]
- Ma, S.; Eichelmann, E.; Wolf, S.; Rey-Sanchez, C.; Baldocchi, D.D. Transpiration and evaporation in a Californian oak-grass savanna: Field measurements and partitioning model results. Agric. For. Meteorol. 2020, 295, 1–13. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Montero, G.; Cañellas, I. Changes in limiting resources determine spatio-temporal variability in tree-grass interactions. Agrofor. Syst. 2009, 76, 375–387. [Google Scholar] [CrossRef]
- Moreno, G.; Obrador, J.J.; García, A. Impact of evergreen oaks on soil fertility and crop production in intercropped dehesas. Agric. Ecosyst. Environ. 2007, 119, 270–280. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Otieno, D.O.; Mirzae, H.; Li, Y.L.; Schmidt, M.W.T.; Siebke, L.; Foken, T.; Ribeiro, N.A.; Pereira, J.S.; Tenhunen, J.D. CO2 exchange and biomass development of the herbaceous vegetation in the Portuguese montado ecosystem during spring. Agric. Ecosyst. Environ. 2009, 132, 143–152. [Google Scholar] [CrossRef]
- Rossetti, I.; Bagella, S.; Cappai, C.; Caria, M.C.; Lai, R.; Roggero, P.P.; Martins, P.; Sousa, J.P.; Querner, P.; Seddaiu, G. Isolated cork oak trees affect soil properties and biodiversity in a Mediterranean wooded grassland. Agric. Ecosyst. Environ. 2015, 202, 203–216. [Google Scholar] [CrossRef]
- Lopez-Carrasco, C.; Lopez-Sanchez, A.; San Miguel, A.; Roig, S. The effect of tree cover on the biomass and diversity of the herbaceous layer in a Mediterranean dehesa. Grass Forage Sci. 2015, 70, 639–650. [Google Scholar] [CrossRef]
- Uribe, C.; Inclán, R.; Hernando, L.; Román, M.; Clavero, M.A.; Roig, S.; Van Miegroet, H. Grazing, tilling and canopy effects on carbon dioxide fluxes in a Spanish dehesa. Agrofor. Syst. 2015, 89, 305–318. [Google Scholar] [CrossRef]
- Casals, P.; Gimeno, C.; Carrara, A.; Lopez-sangil, L. Soil CO2 efflux and extractable organic carbon fractions under simulated precipitation events in a Mediterranean Dehesa. Soil Biol. Biochem. 2009, 41, 1915–1922. [Google Scholar] [CrossRef]
- Casals, P.; Sangil, L.L.; Carrara, A.; Gimeno, C.; Nogués, S. Autotrophic and heterotrophic contributions to short-term soil CO2 efflux following simulated summer precipitation pulses in a Mediterranean dehesa. Global Biogeochem. Cycles 2011, 25, 1–12. [Google Scholar] [CrossRef]
- Ma, S.; Baldocchi, D.D.; Xu, L.; Hehn, T. Inter-annual variability in carbon dioxide exchange of an oak/grass savanna and open grassland in California. Agric. For. Meteorol. 2007, 147, 157–171. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D. Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Scott, R.L.; Jenerette, G.D.; Huxman, T.E. The relative controls of temperature, soil moisture, and plant functional group on soil CO2 efflux at diel, seasonal, and annual scales. J. Geophys. Res. 2011, 116, 1–16. [Google Scholar] [CrossRef]
- Li, Y.L.; Tenhunen, J.; Mirzaei, H.; Hussain, M.Z.; Siebicke, L.; Foken, T.; Otieno, D.; Schmidt, M.; Ribeiro, N.; Aires, L.; et al. Assessment and up-scaling of CO2 exchange by patches of the herbaceous vegetation mosaic in a Portuguese cork oak woodland. Agric. For. Meteorol. 2008, 148, 1318–1331. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Martens, D.A. Moisture Controls on Trace Gas Fluxes in Semiarid Riparian Soils. Soil Sci. Soc. Am. J. 2006, 70, 367–377. [Google Scholar] [CrossRef]
- Fan, Z.; Neff, J.C.; Hanan, N.P. Modeling pulsed soil respiration in an African savanna ecosystem. Agric. For. Meteorol. 2015, 200, 282–292. [Google Scholar] [CrossRef]
- Shvaleva, A.; Costa, F.; Costa, J.M.; Correia, A.; Anderson, M.; Lobo-do-vale, R.; Fangueiro, D.; Bicho, C. Comparison of methane, nitrous oxide fluxes and CO2 respiration rates from a Mediterranean cork oak ecosystem and improved pasture. Plant Soil 2014, 374, 883–898. [Google Scholar] [CrossRef]
- Shvaleva, A.; Siljanen, H.M.P.; Correia, A.; Silva, F.C.; Lamprecht, R.E.; Lobo-do-Vale, R.; Bicho, C.; Fangueiro, D.; Anderson, M.; Pereira, J.S.; et al. Environmental and microbial factors influencing methane and nitrous oxide fluxes in Mediterranean cork oak woodlands: Trees make a difference. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Herman, D.; Halverson, L.; Firestone, M. Nitrogen dynamics in an annual grassland: Oak canopy, climate, and microbial population effects. Ecol. Appl. 2003, 13, 593–604. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Marañon, A. Diversidad floristica y heterogenidad ambiental en una dehesa de Sierra Morena. Edafol. Agro-Biol. 1985, 44, 1183–1197. [Google Scholar]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Quirk, H.; Yi, Z.; Oakley, S.; Ostle, N.J.; Bardgett, R.D. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J. Ecol. 2009, 97, 864–875. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Wardle, D.A.; Tate, K.R. Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil 2006, 83–98. [Google Scholar] [CrossRef]
- Ribas, A.; Llurba, R.; Gouriveau, F.; Altimir, N.; Connolly, J.; Sebastià, M.T. Plant identity and evenness affect yield and trace gas exchanges in forage mixtures. Plant Soil 2015, 391, 93–108. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Porta Casanellas, J.; López-Acevedo Reguerín, M.; Rodríguez Ochoa, R. Determinación de pH y conductividad eléctrica en extractos acuosos 1/5 (p/v). In Técnicas y Experimentos en Edafología; Col·legi Oficial d’Enginyers Agrònoms de Catalunya, Ed.; Col·legi d’Enginyers Agrònoms de Catalunya, Universidad Politécnica de Cataluña, Escola Tècnica Superior d’Enginyers Agrònoms de Lleida: Lleida, Spain, 1986. [Google Scholar]
- Walkley, A.; Black, C.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis. Part 1; Klute, A., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; pp. 383–409. [Google Scholar]
- LumaSense Technologies Gas Detection Limits. Available online: https://www.advancedenergy.com/globalassets/resources-root/diagrams--charts/en-gs-innova-detection-limit-tables.pdf (accessed on 10 December 2020).
- Moody, L.B.; Li, H.; Burns, R.T.; Xin, H.; Gates, R.S.; Hoff, S.J.; Overhults, D.G. A quality assurance project plan for monitoring gaseous and particulate matter emissions from broiler housing. Am. Soc. Agric. Biol. Eng. 2008, 1–27. [Google Scholar] [CrossRef]
- Debouk, H.; Altimir, N.; Sebastià, M.T. Maximizing the information obtained from chamber-based greenhouse gas exchange measurements in remote areas. MethodsX 2018, 5, 973–983. [Google Scholar] [CrossRef]
- Eddy Covariance. A Practical Guide to Measurement and Data Analysis; Aubinet, M., Vesala, T., Papale, D., Eds.; Springer: Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2012; ISBN 9789400723504. [Google Scholar]
- Kirwan, L.; Lüscher, A.; Sebastià, M.T.; Finn, J.A.; Collins, R.P.; Porqueddu, C.; Helgadottir, A.; Baadshaug, O.H.; Brophy, C.; Coran, C.; et al. Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. J. Ecol. 2007, 95, 530–539. [Google Scholar] [CrossRef]
- Kirwan, L.; Connolly, J.; Finn, J.A.; Brophy, C.; Lüscher, A.; Nyfeler, D.; Sebastia, M.-T. Diversity–interaction modeling: Estimating contributions of species identities and interactions to ecosystem function. Ecology 2009, 90, 2032–2038. [Google Scholar] [CrossRef]
- Fabozzi, F.J.; Focardi, S.M.; Rachev, S.T.; Arshanapalli, B.G. Appendix E: Model Selection Criterion: AIC and BIC. In The Basics of Financial Econometrics: Tools, Concepts, and Asset Management Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 399–403. [Google Scholar]
- Wohlfahrt, G.; Hammerle, A.; Haslwanter, A.; Bahn, M.; Tappeiner, U.; Cernusca, A. Disentangling leaf area and environmental effects on the response of the net ecosystem CO2 exchange to diffuse radiation. Geophys. Res. Lett. 2008, 35, 1–5. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Scott, R.L.; Jenerette, G.D.; Hamerlynck, E.P.; Huxman, T.E. Temperature and precipitation controls over leaf- and ecosystem-level CO2 flux along a woody plant encroachment gradient. Glob. Chang. Biol. 2012, 18, 1389–1400. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Martens, D.A.; McClaran, M.P. Soil cycling of trace gases in response to mesquite management in a semiarid grassland. J. Arid Environ. 2008, 72, 1654–1665. [Google Scholar] [CrossRef]
- Ibañez, M. Vegetation Drives Greenhouse Gas Exchange, and Carbon and Nitrogen Cycling in Grassland Ecosystems. Ph.D. Thesis, University of Lleida, Lleida, Spain, 2019. [Google Scholar]
- Busch, F.A.; Sage, R.F.; Farquhar, G.D. Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nat. Plants 2018, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Reich, P.B.; Tjoelker, M.G. Legume presence increases photosynthesis and N concentrations of co-occurring non-fixers but does not modulate their responsiveness to carbon dioxide enrichment. Oecologia 2003, 137, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Ecology 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. Leaf Structure (Specific Leaf Area) Modulates Photosynthesis-Nitrogen relations: Evidence from within Across Species and Functional Groups. Funct. Ecol. 1998, 12, 948–958. [Google Scholar] [CrossRef]
- Ibañez, M.; Altimir, N.; Ribas, A.; Eugster, W.; Sebastià, M.T. Phenology and plant functional type dominance drive CO2 exchange in seminatural grasslands in the Pyrenees. J. Agric. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Mulder, C.P.H.; Jumpponen, A.; Högberg, P. How plant diversity and legumes affect nitrogen dynamics in experimental grassland communities. Community Ecol. 2002, 133, 412–421. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Rasmussen, J.; Høgh-Jensen, H.; Eriksen, J.; Søegaard, K.; Rasmussen, J. Nitrogen transfer from forage legumes to nine neighbouring plants in a multi-species grassland. Plant Soil 2012, 350, 71–84. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Debouk, H.; Emeterio, L.S.; Marí, T.; Canals, R.M.; Sebastià, M.T. Plant functional diversity, climate and grazer type regulate soil activity in natural grasslands. Agronomy 2020, 10, 1291. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Aljazairi, S.; Arias, C.; Nogués, S. Carbon and nitrogen allocation and partitioning in traditional and modern wheat genotypes under pre-industrial and future CO2 conditions. Plant Biol. 2015, 17, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.S.; Ibrom, A.; Beier, C.; Jonasson, S.; Michelsen, A. Ecosystem respiration depends strongly on photosynthesis in a temperate heath. Biogeochemistry 2007, 85, 201–213. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Y.; Chen, D. Stable isotopes of carbon and nitrogen help to predict the belowground communities at a regional scale. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, Y.; Wu, H.; Sun, T.; La Pierre, K.J.; Sun, Z.; Yu, Q. Divergent effects of nitrogen addition on soil respiration in a semiarid grassland. Sci. Rep. 2016, 1–8. [Google Scholar] [CrossRef]
- Corrêa Dias, A.T.; van Ruijven, J.; Berendse, F. Plant species richness regulates soil respiration through changes in productivity. Oecologia 2010, 163, 805–813. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M. Map-Making of Plant Biomass and Leaf Area Index for Management of Protected Areas. Aliso A J. Syst. Evol. Bot. 2000, 19, 1–12. [Google Scholar] [CrossRef]
- Sheffer, E.; Canham, C.D.; Kigel, J.; Perevolotsky, A. Countervailing effects on pine and oak leaf litter decomposition in human-altered Mediterranean ecosystems. Oecologia 2015, 177, 1039–1051. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Pellegrino, A.; Fuggi, A. Leaf litter decomposition dynamics in Mediteranean area. In Soil Ecology Research Developements; Tian-Xiao, L., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Liu, X.P.; Zhang, W.J.; Hu, C.S.; Tang, X.G. Soil greenhouse gas fluxes from different tree species on Taihang Mountain, North China. Biogeosciences 2014, 11, 1649–1666. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Jugold, A.; Althoff, F.; Hurkuck, M.; Greule, M.; Lenhart, K.; Lelieveld, J.; Keppler, F. Non-microbial methane formation in oxic soils. Biogeosciences 2012, 9, 5291–5301. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Global Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- Lin, S.; Iqbal, J.; Hu, R.; Cai, J.; Shaaban, M.; Chen, X. Nitrous Oxide Emissions from Yellow Brown Soil as Affected by Incorporation of Crop Residues With Different Carbon-to-Nitrogen Ratios: A Case Study in Central China. Arch. Environ. Contam. Toxicol. 2013, 65, 183–192. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.; Hu, R.; Lin, S.; Zhao, J. Soil Nitrous oxide and Carbon dioxide emissions following incorporation of above- and below-ground biomass of green bean. Int. J. Environ. Sci. Technol. 2016, 13, 179–186. [Google Scholar] [CrossRef]


| Plot | SM-ilex | DN-mixed | DN-suber | DN-pinea | ||||
|---|---|---|---|---|---|---|---|---|
| Depth (cm) | 0–40 | 40–80 | 0–30 | 30–60 | 0–30 | 30–60 | 0–30 | 30–60 |
| pH | 6.7 | 7.2 | 7.9 | 7.8 | 7.5 | 7.8 | 7.0 | 7.4 |
| Organic C (%) | 0.80 | 0.30 | 0.32 | 0.34 | 0.60 | 0.02 | 1.52 | 0.51 |
| Total N (%) | 0.85 | 0.60 | 0.15 | 0.04 | 0.06 | 0.05 | 0.20 | 0.11 |
| Clay (%) | 18 | 30 | 10 | 11 | 13 | 4 | 16 | 16 |
| Silt (%) | 29 | 28 | 25 | 15 | 18 | 9 | 22 | 21 |
| Sand (%) | 54 | 42 | 65 | 74 | 69 | 87 | 62 | 63 |
| CO2 Flux (µmol m−2 s−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEE | Reco | Rsoil | ||||||||||
| Specific Interaction Model | Null Model | Null Model | ||||||||||
| Par. | SE | t | p-Value | Par. | SE | t | p-Value | Par. | SE | t | p-Value | |
| Plot SM-ilex | −6 | 3 | −1.81 | 0.07 | 8.9 | 0.6 | 14.60 | <0.001 | 0 | 1 | 0.35 | 0.7 |
| Plot DN-mixed | −2 | 3 | −0.54 | 0.6 | 9.0 | 0.5 | 18.82 | <0.001 | 1 | 1 | 0.55 | 0.6 |
| Plot DN-suber | −2 | 3 | −0.57 | 0.6 | 8.6 | 0.6 | 13.74 | <0.001 | 2 | 1 | 1.33 | 0.2 |
| Plot DN-pinea | −1 | 3 | −0.38 | 0.7 | 9.2 | 0.6 | 14.69 | <0.001 | 1 | 1 | 0.65 | 0.5 |
| Season | −6.2 | 0.6 | −9.88 | <0.001 | −1.3 | 0.5 | −2.56 | 0.01 | ||||
| Canopy | 4 | 0.7 | 5.58 | <0.001 | −1.8 | 0.6 | −2.98 | 0.004 | 1.1 | 0.5 | 2.10 | 0.04 |
| Season x canopy | 4.3 | 0.9 | 4.73 | <0.001 | 1.8 | 0.7 | 2.40 | 0.02 | ||||
| PAR (μmol photons m−2 s−1) | −0.003 | 0.001 | −4.58 | <0.001 | ||||||||
| Ta (°C) | 0.15 | 0.05 | 3.06 | 0.003 | ||||||||
| BGB (g DW m−2) | 0.0015 | 0.0007 | 2.21 | 0.03 | ||||||||
| Forbs (fraction) | 0 | 3 | 0.15 | 0.9 | ||||||||
| Grasses (fraction) | −1 | 3 | −0.22 | <0.001 | ||||||||
| Legumes (fraction) | −67 | 17 | −3.97 | <0.001 | ||||||||
| Forbs × legumes (fraction) | 97 | 25 | 3.84 | <0.001 | ||||||||
| Grasses × legumes (fraction) | 96 | 24 | 4.07 | <0.001 | ||||||||
| CH4 (nmol m−2 s−1) | N2O (nmol m−2 s−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Null Model | Identity Model | |||||||
| Par. | SE | t | p-Value | Par. | SE | t | p-Value | |
| Plot SM-ilex | −24 | 81 | −0.30 | 0.8 | ||||
| Plot DN-mixed | −83 | 81 | −1.03 | 0.3 | ||||
| Plot DN-suber | −22 | 85 | −0.26 | 0.8 | ||||
| Plot DN-pinea | −28 | 85 | −0.33 | 0.7 | ||||
| Season spring | −13 | 5 | −2.73 | 0.008 | ||||
| Season autumn | −150 | 15 | −9.68 | <0.001 | −11 | 5 | −2.30 | 0.03 |
| Canopy | 2.1 | 0.8 | 2.68 | 0.010 | ||||
| Ts (°C) | 13 | 5 | 2.72 | 0.008 | 0.4 | 0.2 | 2.44 | 0.02 |
| SWC (fraction) | 1.4 | 0.8 | 1.83 | 0.07 | −0.05 | 0.03 | −2.00 | 0.05 |
| Litter (g DW m−2) | −0.1 | 0.1 | −1.89 | 0.06 | 0.007 | 0.003 | 2.84 | 0.006 |
| BGB (g DW m−2) | 0.002 | 0.001 | 1.75 | 0.09 | ||||
| Forbs (fraction) | 3 | 3 | 1.02 | 0.3 | ||||
| Grasses (fraction) | 2 | 3 | 0.47 | 0.6 | ||||
| Legumes (fraction) | 10 | 4 | 2.49 | 0.02 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibañez, M.; Leiva, M.J.; Chocarro, C.; Aljazairi, S.; Ribas, À.; Sebastià, M.-T. Tree—Open Grassland Structure and Composition Drive Greenhouse Gas Exchange in Holm Oak Meadows of the Iberian Peninsula. Agronomy 2021, 11, 50. https://doi.org/10.3390/agronomy11010050
Ibañez M, Leiva MJ, Chocarro C, Aljazairi S, Ribas À, Sebastià M-T. Tree—Open Grassland Structure and Composition Drive Greenhouse Gas Exchange in Holm Oak Meadows of the Iberian Peninsula. Agronomy. 2021; 11(1):50. https://doi.org/10.3390/agronomy11010050
Chicago/Turabian StyleIbañez, Mercedes, María José Leiva, Cristina Chocarro, Salvador Aljazairi, Àngela Ribas, and Maria-Teresa Sebastià. 2021. "Tree—Open Grassland Structure and Composition Drive Greenhouse Gas Exchange in Holm Oak Meadows of the Iberian Peninsula" Agronomy 11, no. 1: 50. https://doi.org/10.3390/agronomy11010050
APA StyleIbañez, M., Leiva, M. J., Chocarro, C., Aljazairi, S., Ribas, À., & Sebastià, M.-T. (2021). Tree—Open Grassland Structure and Composition Drive Greenhouse Gas Exchange in Holm Oak Meadows of the Iberian Peninsula. Agronomy, 11(1), 50. https://doi.org/10.3390/agronomy11010050





