A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks
Abstract
1. Introduction
2. Materials and Methods
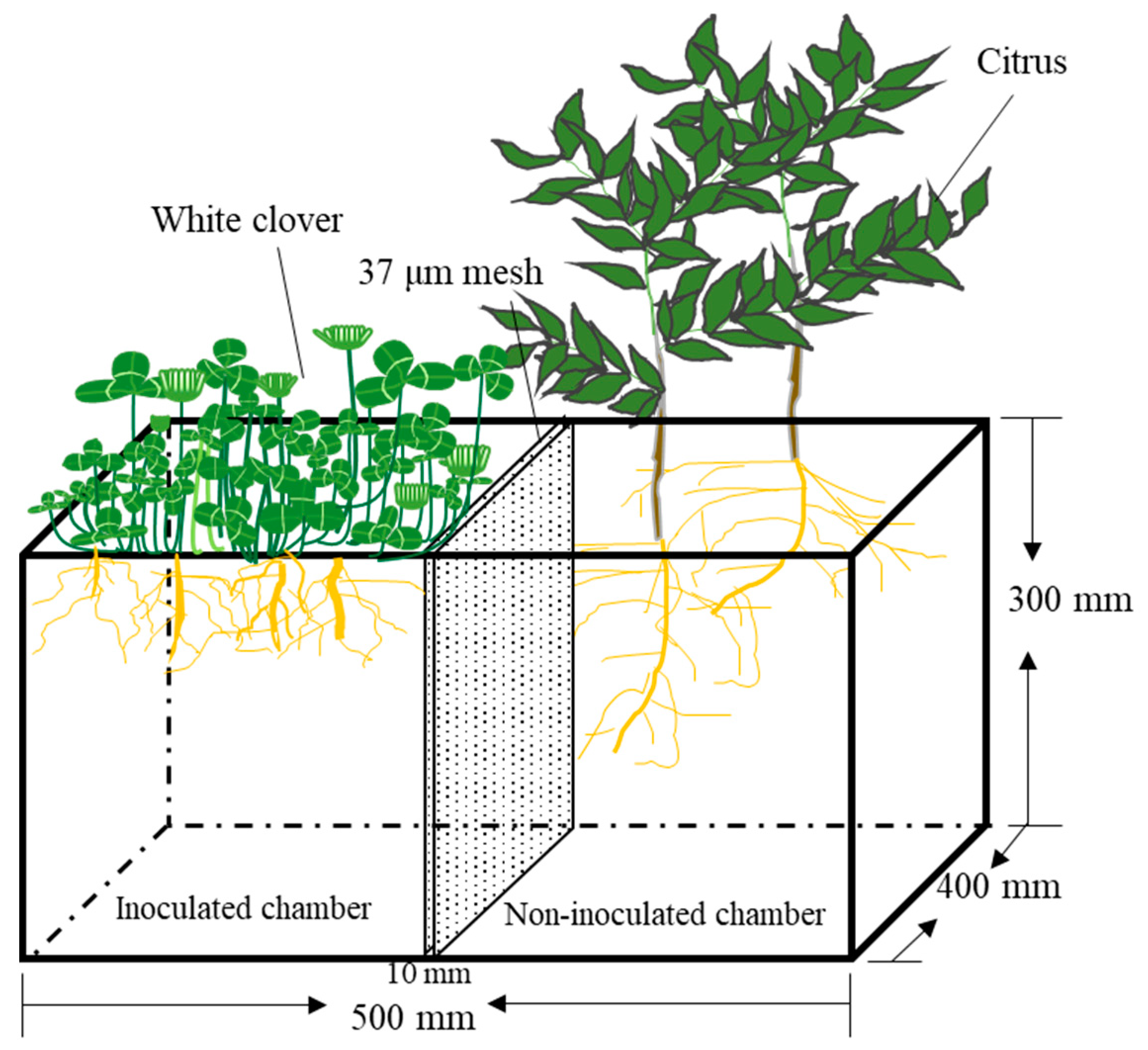
2.1. Experimental Pots and Plant Growth Conditions
2.2. Experimental Design
2.3. 15N Labeling
2.4. Plant Harvest
2.5. Calculation and Statistical Analysis
3. Results
3.1. Biological Nitrogen Fixation in White Clover and Formation of Mycorrhizas
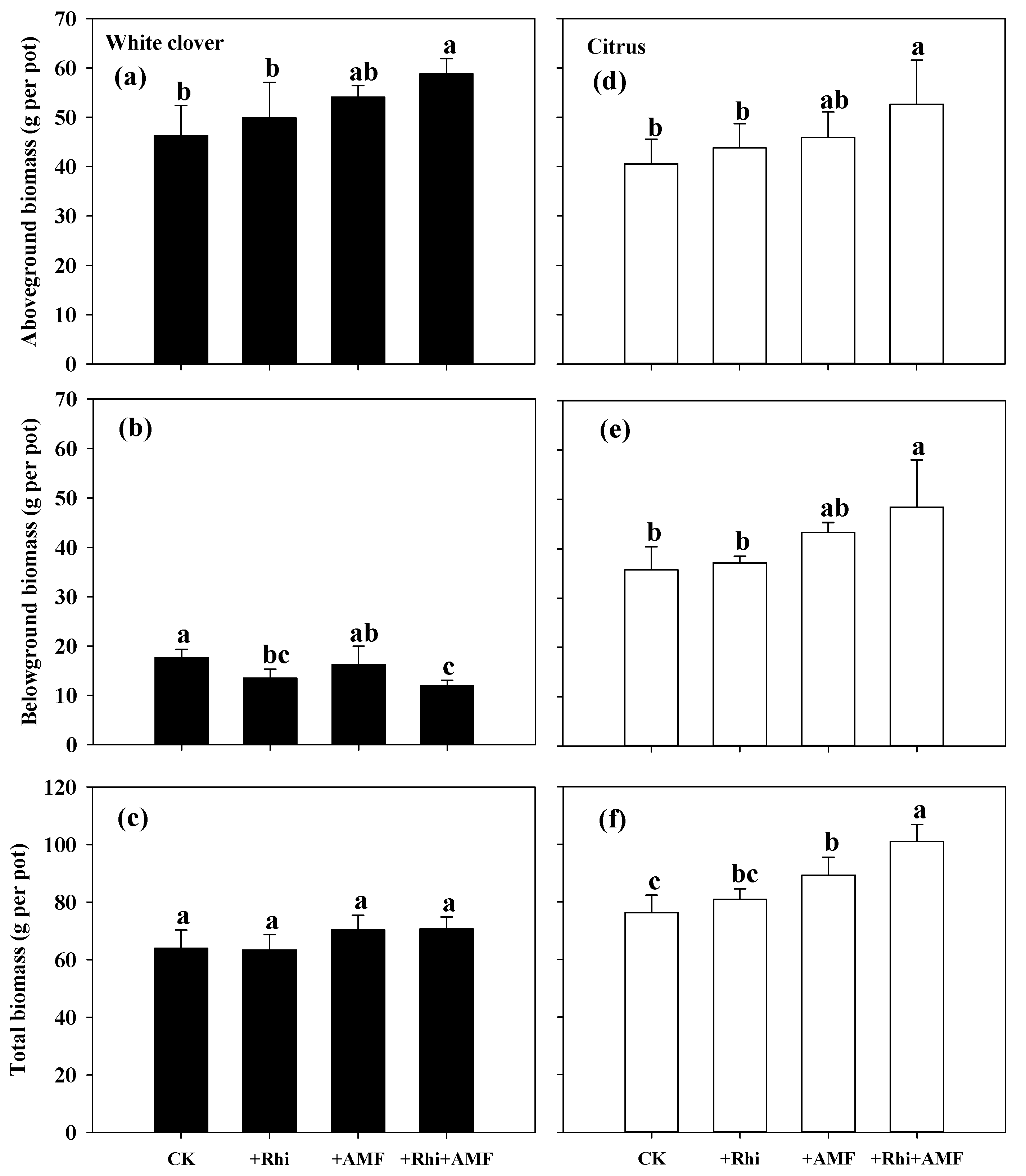
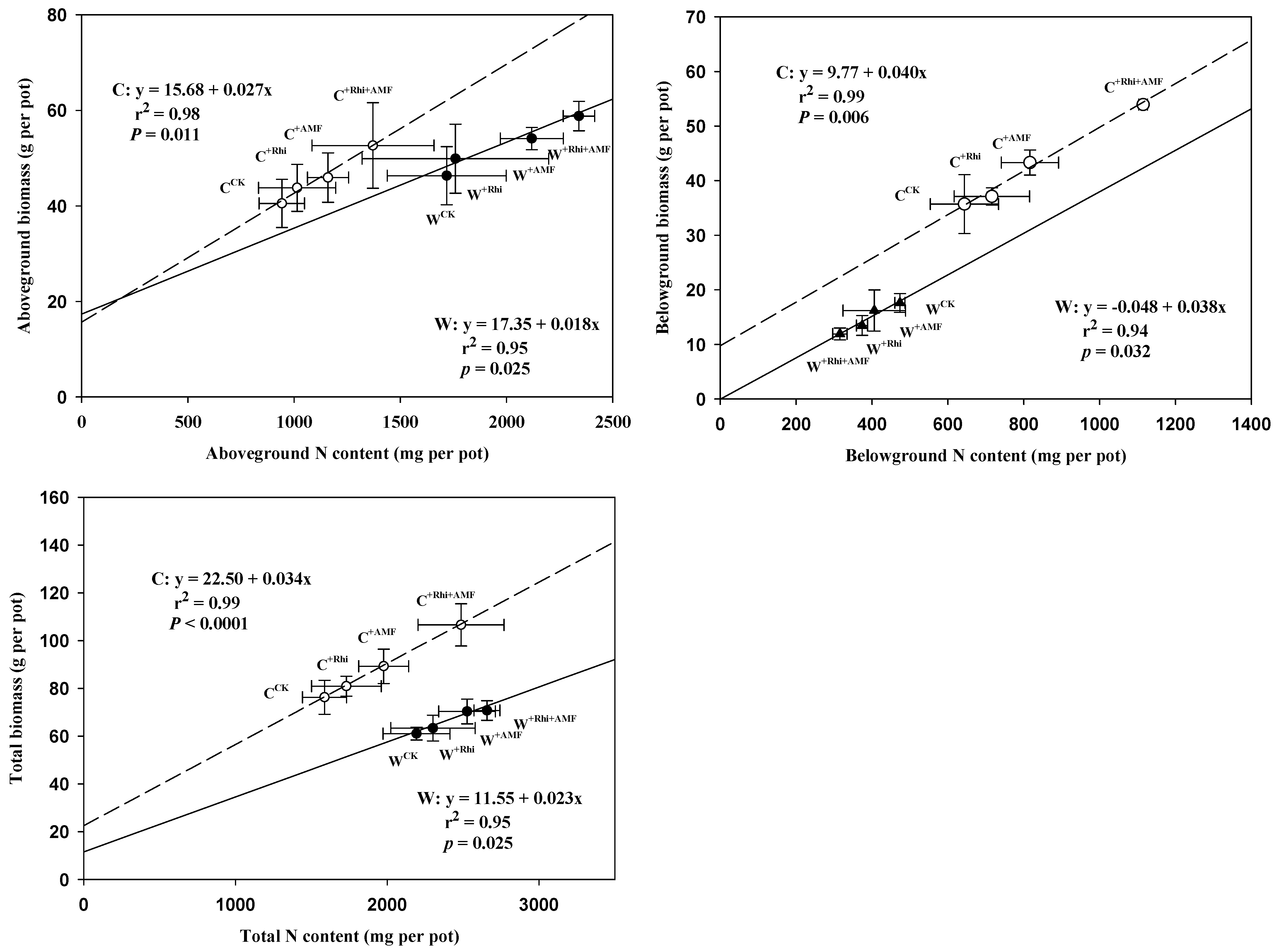
3.2. Plant Biomass
3.3. Plant N Concentration and Content
3.4. 15N Atom Excess and 15N Content
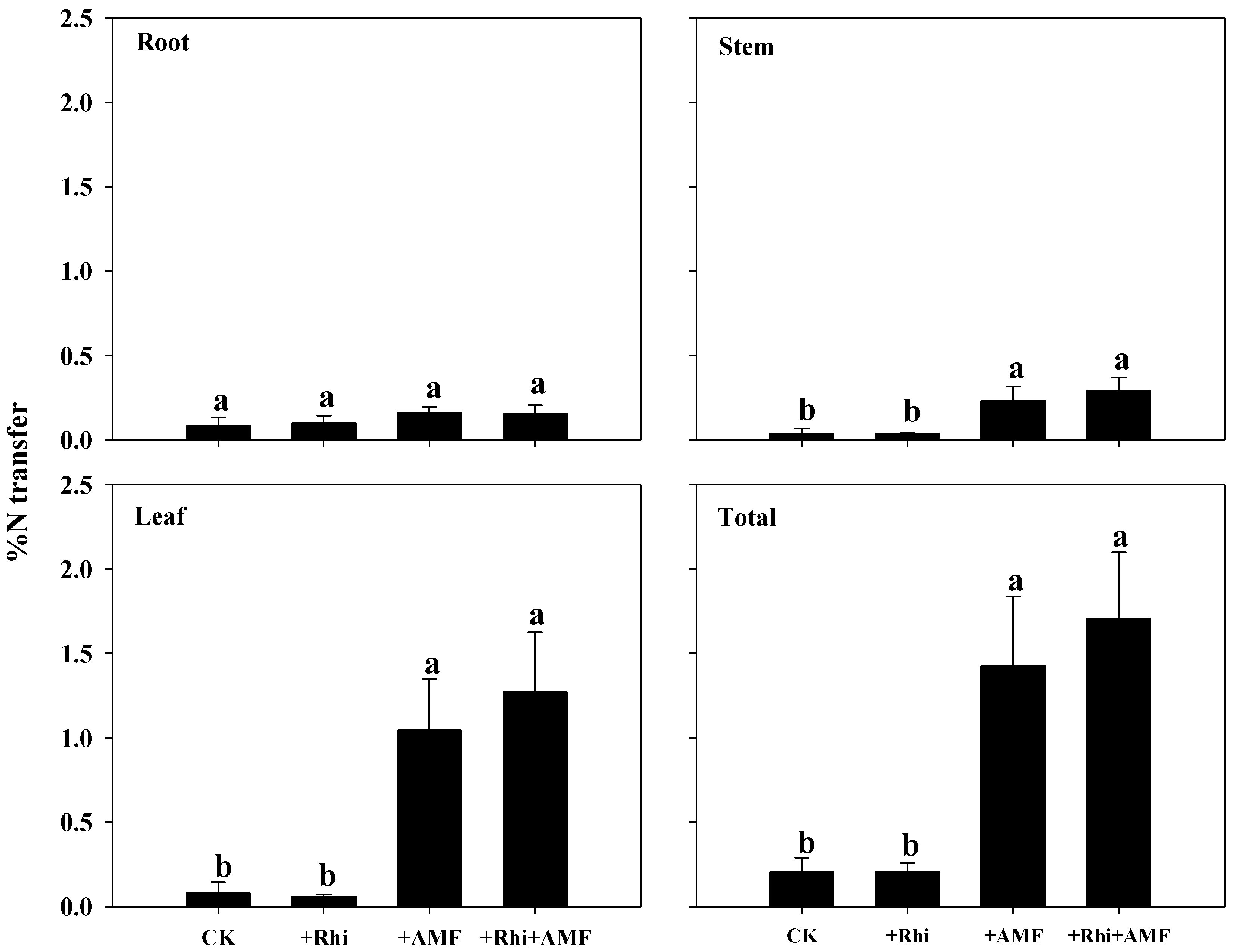
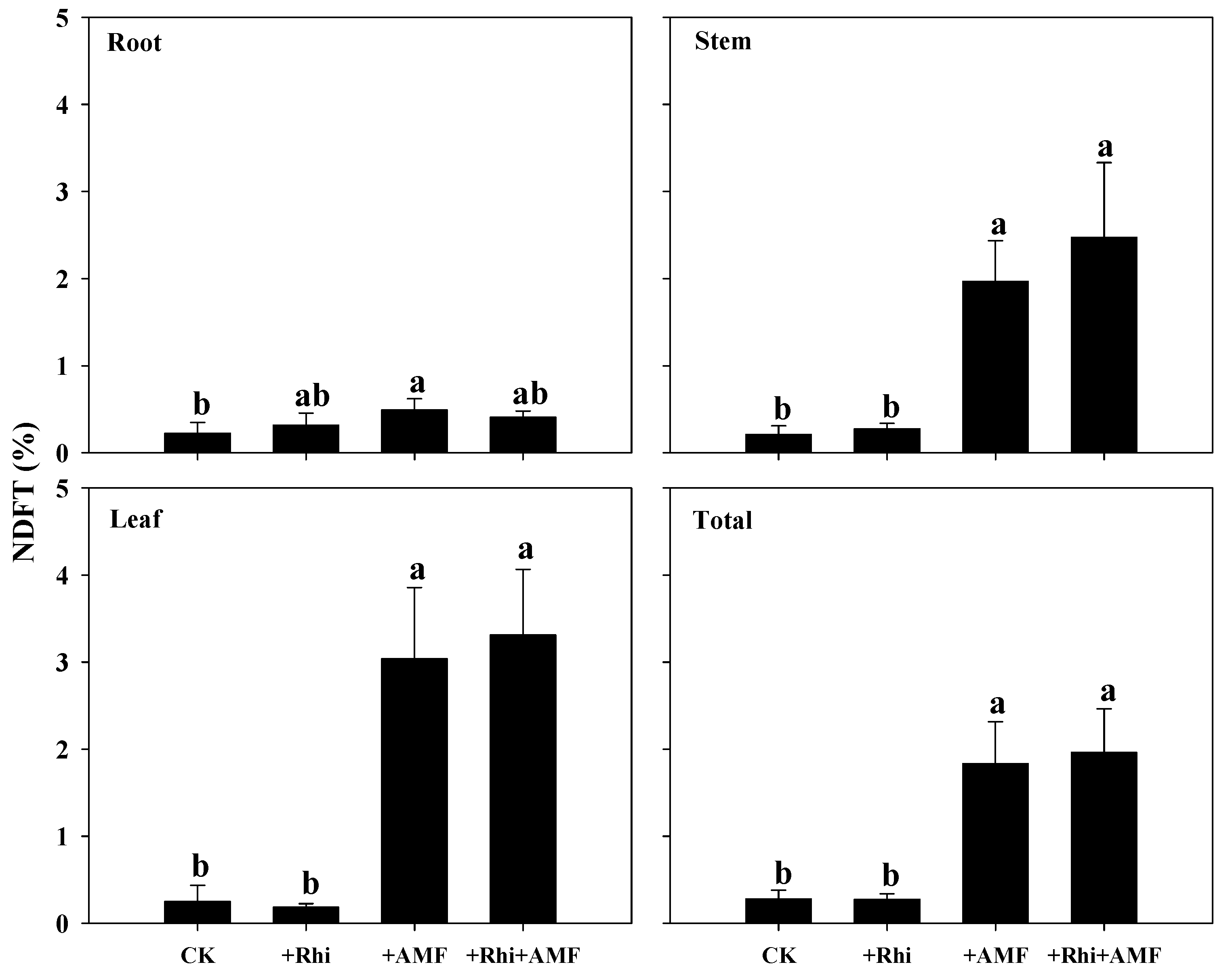
3.5. N Transfer from White Clover to Citrus
4. Discussion
4.1. Biological Nitrogen Fixation and Mycorrhizal Colonization
4.2. Response of Plant Growth Inoculated with Rhizobium and Arbuscular Mycorrhizal Fungus
4.3. N Transfer from White Clover to Citrus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Database-Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Long, Q.; Li, W.; Wang, Z.; He, X.; Wang, J.; Wang, X.; Xiong, H.; Guo, C.; Zhang, G.; et al. Mapping the Environmental Cost of a Typical Citrus-Producing County in China: Hotspot and Optimization. Sustainability 2020, 12, 1827. [Google Scholar] [CrossRef]
- Islam, M.A.; Adjesiwor, A.T. Nitrogen Fixation and Transfer in Agricultural Production Systems. In Nitrogen in Agriculture—Updates; IntechOpen: London, UK, 2018; pp. 95–110. [Google Scholar]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Ordóñez-Fernández, R.; De Torres, M.A.R.-R.; Márquez-García, J.; Moreno-García, M.; Carbonell-Bojollo, R.M. Legumes used as cover crops to reduce fertilisation problems improving soil nitrate in an organic orchard. Eur. J. Agron. 2018, 95, 1–13. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Dimande, P.; Pereira, E.L.; Ferreira, I.Q.; Freitas, S.; Correia, C.; Moutinho-Pereira, J.M.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N.; Malhotra, S.K. Mycorrhizas in citrus: Beyond soil fertility and plant nutrition. Indian J. Agric. Sci. 2017, 87, 427–443. [Google Scholar]
- Wei, H.; Xiang, Y.; Liu, Y.; Zhang, J. Effects of sod cultivation on soil nutrients in orchards across China: A meta-analysis. Soil Tillage Res. 2017, 169, 16–24. [Google Scholar] [CrossRef]
- Ovalle, C.; del Pozo, A.; Peoples, M.B.; Lavín, A. Estimating the contribution of nitrogen from legume cover crops to the nitrogen nutrition of grapevines using a 15N dilution technique. Plant Soil 2010, 334, 247–259. [Google Scholar] [CrossRef]
- Cheng, X.; Baumgartner, K. Arbuscular mycorrhizal fungi-mediated nitrogen transfer from vineyard cover crops to grapevines. Biol. Fertil. Soils 2004, 40, 406–412. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zhang, Y.C.; Zhang, Z.Z.; Srivastava, A.K. Underground communication of root hormones by common mycorrhizal network between trifoliate orange and white clover. Arch. Agron. Soil Sci. 2017, 63, 1187–1197. [Google Scholar] [CrossRef]
- TerAvest, D.; Smith, J.L.; Carpenter-Boggs, L.; Hoagland, L.; Granatstein, D.; Reganold, J.P. Influence of Orchard Floor Management and Compost Application Timing on Nitrogen Partitioning in Apple Trees. HortScience 2010, 45, 637–642. [Google Scholar] [CrossRef]
- Daudin, D.; Sierra, J. Spatial and temporal variation of below-ground N transfer from a leguminous tree to an associated grass in an agroforestry system. Agric. Ecosyst. Environ. 2008, 126, 275–280. [Google Scholar] [CrossRef]
- Sierra, J.; Daudin, D.; Domenach, A.M.; Nygren, P.; Desfontaines, L. Nitrogen transfer from a legume tree to the associated grass estimated by the isotopic signature of tree root exudates: A comparison of the 15N leaf feeding and natural 15N abundance methods. Eur. J. Agron. 2007, 27, 178–186. [Google Scholar] [CrossRef]
- Bhuvaneswari, T.V.; Solheim, B. Root hair deformation in the white clover/Rhizobium trifolii symbiosis. Physiol. Plant. 1985, 63, 25–34. [Google Scholar] [CrossRef]
- Frank, D.A.; Evans, R.D.; Tracy, B.F. The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland. Biogeochemistry 2004, 68, 169–178. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Laidlaw, A.; Christie, P.; Hammond, M. The specificity of arbuscular mycorrhizal fungi in perennial ryegrass-white clover pasture. Agric. Ecosyst. Environ. 2000, 77, 211–218. [Google Scholar] [CrossRef]
- Rogers, J.B.; Laidlaw, A.S.; Christie, P. The role of arbuscular mycorrhizal fungi in the transfer of nutrients between white clover and perennial ryegrass. Chemosphere 2001, 42, 153–159. [Google Scholar] [CrossRef]
- He, X.; Critchley, C.; Ng, H.; Bledsoe, C. Reciprocal N (15NH4+ or 15NO3−) transfer between nonN2-fixing Eucalyptus macu-lata and N2-fixing Casuarina cunninghamiana linked by the ectomycorrhizal fungus Pisolithus sp. New Phytol. 2003, 163, 629–640. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Richard, F.; He, X.; Simard, S.W. Mycorrhizal networks: Des liaisons dangereuses? Trends Ecol. Evol. 2006, 21, 621–628. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Y.; Dong, T.; Liao, Y.; Li, D.; He, X.; Xu, X. Additional AM fungi inoculation increase Populus cathayana intersexual competition. Front. Plant Sci. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 11–32. [Google Scholar]
- He, X.; Critchley, C.; Ng, H.; Bledsoe, C. Nodulated N2-fixing Casuarina cunninghamiana is the sink for net N transfer from non-N2-fixing Eucalyptus maculata via an ectomycorrhizal fungus Pisolithus sp. using 15NH4+ or 15NO3− supplied as ammonium nitrate. New Phytol. 2005, 167, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Lou, Y.G.; Deng, D.J.; Rahman, M.M.; Wu, Q.S. Effects of common mycorrhizal network on plant carbohydrates and soil properties in trifoliate orange-white clover association. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kurppa, M.; Leblanc, H.A.; Nygren, P. Detection of nitrogen transfer from N2-fixing shade trees to cacao saplings in 15N labelled soil: Ecological and experimental considerations. Agrofor. Syst. 2010, 80, 223–239. [Google Scholar] [CrossRef]
- Li, Y.; Ran, W.; Zhang, R.; Sun, S.; Xu, G. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil. 2009, 315, 285–296. [Google Scholar] [CrossRef]
- Lu, J.K.; Kang, L.H.; Sprent, J.I.; Xu, D.P.; He, X.H. Research paper Two-way transfer of nitrogen between Dalbergia odorifera and its hemiparasite Santalum album is enhanced when the host is effectively nodulated and fixing nitrogen. Tree Physiol. 2013, 33, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Alva, A.K.; Paramasivam, S.; Obreza, T.A.; Schumann, A.W. Nitrogen best management practice for citrus trees: I. Fruit yield, quality, and leaf nutritional status. Sci. Hortic. 2006, 107, 233–244. [Google Scholar] [CrossRef]
- Group, S. World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. Update 2015. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; pp. 172–173. [Google Scholar]
- Meng, L.; Zhang, A.; Wang, F.; Han, X.; Wang, D.; Li, S. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 2015, 6, 6. [Google Scholar] [CrossRef]
- He, X.; Bledsoe, C.S.; Zasoski, R.J.; Southworth, D.; Horwath, W.R. Rapid nitrogen transfer from ectomycorrhizal pines to adjacent ectomycorrhizal and arbuscular mycorrhizal plants in a California oak woodland. New Phytol. 2006, 170, 143–151. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Qiu, G.Y.; Zhou, J. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2009, 2, 107–118. [Google Scholar] [CrossRef]
- Walzl, K.P.; Rasmussen, J.; Høgh-Jensen, H.; Eriksen, J.; Søegaard, K.; Rasmussen, J. Nitrogen transfer from forage legumes to nine neighbouring plants in a multi-species grassland. Plant Soil 2011, 350, 71–84. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Unkovich, M.; Pate, J.; McNeill, A.; Gibbs, D.J. Stable Isotope Techniques in the Study of Biological Processes and Functioning of Ecosystems; Kluwer Academic: Dordrecht, the Netherlands, 2001; p. 293. [Google Scholar]
- Boddey, R.M.; Peoples, M.B.; Palmer, B.; Dart, P.J. Use of the 15N natural abundance technique to quantify biological Nitrogen fixation by woody perennials. Nutr. Cycl. Agroecosyst. 2000, 57, 235–270. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Rodd, A.V.; Grimmett, M.; Fillmore, S.A.E.; Crouse, M.; Prithiviraj, B.; Papadopoulos, Y.A. Nitrogen fixation and transfer of red clover genotypes under legume-grass forage based production systems. Nutr. Cycl. Agroecosyst. 2016, 106, 233–247. [Google Scholar] [CrossRef]
- Burchill, W.; James, E.K.; Li, D.; Lanigan, G.; Williams, M.; Iannetta, P.P.M.; Humphreys, J. Comparisons of biological nitrogen fixation in association with white clover (Trifolium repens L.) under four fertiliser nitrogen inputs as measured using two 15N techniques. Plant Soil 2014, 385, 287–302. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2008; pp. 132–188. [Google Scholar]
- Høgh-Jensen, H.; Schjoerring, J.K. Measurement of biological dinitrogen fixation in grassland: Comparison of the enriched 15N dilution and the natural 15N abundance methods at different nitrogen application rates and defoliation frequencies. Plant Soil. 1994, 166, 153–163. [Google Scholar] [CrossRef]
- Hansen, J.; Vinther, F. Spatial variability of symbiotic N2 fixation in grass-white clover pastures estimated by the 15N isotope dilution method and the natural 15N abundance method. Plant Soil 2001, 230, 257–266. [Google Scholar] [CrossRef]
- Chalk, P.M.; Inácio, C.D.T.; Balieiro, F.C.; Rouws, J.R.C. Do techniques based on 15N enrichment and 15N natural abundance give consistent estimates of the symbiotic dependence of N2-fixing plants? Plant Soil 2015, 399, 415–426. [Google Scholar] [CrossRef]
- Deguchi, S.; Shimazaki, Y.; Uozumi, S.; Tawaraya, K.; Kawamoto, H.; Tanaka, O. White clover living mulch increases the yield of silage corn via arbuscular mycorrhizal fungus colonization. Plant Soil 2007, 291, 291–299. [Google Scholar] [CrossRef]
- Deguchi, S.; Uozumi, S.; Touno, E.; Kaneko, M.; Tawaraya, K. Arbuscular mycorrhizal colonization increases phosphorus uptake and growth of corn in a white clover living mulch system. Soil Sci. Plant Nutr. 2012, 58, 169–172. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Graham, J.H.; Syvertsen, J.P.; Drouillard, D.L. Carbon Economy of Sour Orange in Relation to Mycorrhizal Colonization and Phosphorus Status. Ann. Bot. 1993, 71, 1–10. [Google Scholar] [CrossRef]
- Graham, J.H.; Duncan, L.W.; Eissenstat, D.M. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997, 135, 335–343. [Google Scholar] [CrossRef][Green Version]
- Sanders, I.R. Specificity in the Arbuscular Mycorrhizal Symbiosis. Biogeogr. Mycorrhizal Symbiosis 2002, 157, 415–437. [Google Scholar] [CrossRef]
- Jalonen, R.; Nygren, P.; Sierra, J. Transfer of nitrogen from a tropical legume tree to an associated fodder grass via root exudation and common mycelial networks. Plant Cell Environ. 2009, 32, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Okon, I.; Osonubi, O.; Sanginga, N. Vesicular-arbuscular mycorrhiza effects on Gliricidia sepium and Senna siamea in a fallowed alley cropping system. Agrofor. Syst. 1996, 33, 165–175. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Verdú, M.; Querejeta, J.I.; Sortibrán, L.; Valiente-Banuet, A. Soil fungi promote nitrogen transfer among plants involved in long-lasting facilitative interactions. Perspect. Plant Ecol. Evol. Syst. 2016, 18, 45–51. [Google Scholar] [CrossRef]
- Rasmussen, J.; Eriksen, J.; Jensen, E.S.; Esbensen, K.H.; Høgh-Jensen, H. In situ carbon and nitrogen dynamics in ryegrass-clover mixtures: Transfers, deposition and leaching. Soil Biol. Biochem. 2007, 39, 804–815. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Høgh-Jensen, H. Interactions between white clover and ryegrass under contrasting nitrogen availability: N2 fixation, N fertilizer recovery, N transfer and water use efficiency. Plant Soil 1997, 197, 187–199. [Google Scholar]
- Martinez, J.M.; Bañuls, J.; Quiñones, A.; Martin, B.; Primo-Millo, E.; Legaz, F. Fate and transformations of 15N labelled nitrogen applied in spring to Citrus trees. J. Hortic. Sci. Biotechnol. 2002, 77, 361–367. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Mattos Junior, D.; Quaggio, J.A.; Cantarella, H.; Trivelin, P.C.O. Nitrogen-15 uptake and distribution in two citrus species. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 156–159. [Google Scholar]
- Legaz, F.; Serna, M.D.; Primo-Millo, E. Mobilization of the reserve N in citrus. Plant Soil 1995, 173, 205–210. [Google Scholar] [CrossRef]
| Treatments | Shoot δ15N(‰) | Nodule Numbers | Nodule Fresh Weight (g) | %Nfix | Nfix (mg/pot) |
|---|---|---|---|---|---|
| CK | 1.25 ± 0.14 a | – | – | – | – |
| +Rhi | 0.71 ± 0.13 b | 126 ± 10 b | 2.27 ± 0.02 a | 31.28 ± 5.43 a | 710.44 ± 100.19 a |
| +AMF | 1.19 ± 0.09 a | – | – | – | – |
| +Rhi + AMF | 0.703 ± 0.11 b | 168 ± 12 a | 2.38 ± 0.02 a | 34.85 ± 6.94 a | 923.38 ± 167.40 a |
| Treatments | White Clover Root (%) | Citrus Root (%) |
|---|---|---|
| CK | 1.48 ± 0.14 b | 1.35 ± 0.45 b |
| +Rhi | 1.52 ± 0.75 b | 1.41 ± 0.10 b |
| +AMF | 66.85 ± 0.71 a | 19.29 ± 4.91 a |
| +Rhi + AMF | 68.74 ± 6.70 a | 23.41 ± 4.82 a |
| Treatments | White Clover | Citrus | ||
|---|---|---|---|---|
| Aboveground | Belowground | Aboveground | Belowground | |
| CK | 3.78 ± 0.07 a | 2.61 ± 0.14 a | 2.31 ± 0.03 b | 1.81 ± 0.03 b |
| +Rhi | 3.88 ± 0.09 a | 2.65 ± 0.06 a | 2.30 ± 0.10 b | 1.93 ± 0.21 ab |
| +AMF | 3.92 ± 0.31 a | 2.52 ± 0.11 a | 2.53 ± 0.05 a | 1.88 ± 0.08 ab |
| +Rhi + AMF | 3.99 ± 0.18 a | 2.65 ± 0.08 a | 2.59 ± 0.08 a | 2.034 ± 0.07 a |
| Treatments | White Clover | Citrus | |||||
|---|---|---|---|---|---|---|---|
| Leaf | Root | Total | Leaf | Stem | Root | Total | |
| N content (mg per pot) | |||||||
| CK | 1756.96 ± 139.98 b | 473.77 ± 6.72 a | 2273.72 ± 134.21 b | 670.24 ± 32.79 b | 272.15 ± 24.23 a | 643.92 ± 44.92 b | 1586.32 ± 72.54 c |
| +Rhi | 1923.91 ± 145.58 b | 363.66 ± 13.32 bc | 2287.57 ± 138.57 b | 711.76 ± 91.02 b | 302.27 ± 13.55 a | 716.34 ± 49.76 b | 1730.36 ± 114.77 bc |
| +AMF | 2119.29 ± 74.23 ab | 406.35 ± 41.17 ab | 2525.64 ± 93.24 ab | 870.92 ± 24.52 ab | 288.85 ± 28.11 a | 876.74 ± 37.79 ab | 1976.52 ± 82.21 b |
| +Rhi + AMF | 2341.23 ± 37.15 a | 315.75 ± 9.45 c | 2656.97 ± 42.68 a | 1048.12 ± 14.78 a | 322.88 ± 25.14 a | 990.05 ± 12.51 a | 2361.06 ± 53.60 a |
| 15N atom % excess | |||||||
| CK | 0.49 ± 0.10 a | 0.29 ± 0.03 a | – | 0.00075 ± 0.00012 c | 0.00027 ± 0.00014 c | 0.0012 ± 0.0007 a | – |
| +Rhi | 0.43 ± 0.11 a | 0.26 ± 0.05 ab | – | 0.00072 ± 0.00022 c | 0.00005 ± 0.00003 c | 0.0013 ± 0.0006 a | – |
| +AMF | 0.38 ± 0.11 a | 0.22 ± 0.03 c | – | 0.010 ± 0.00067 b | 0.0066 ± 0.0007 b | 0.0016 ± 0.0001 a | – |
| +Rhi + AMF | 0.41 ± 0.03 a | 0.27 ± 0.03 ab | – | 0.013 ± 0.0029 a | 0.0095 ± 0.0031 a | 0.0016 ± 0.0002 a | – |
| 15N content (mg per pot) | |||||||
| CK | 8.67 ± 2.63 a | 0.79 ± 0.16 a | 9.45 ± 2.80 a | 0.0077 ± 0.0067 c | 0.0030 ± 0.0015 c | 0.0073 ± 0.0035 c | 0.018 ± 0.006 c |
| +Rhi | 8.13 ± 1.24 a | 0.80 ± 0.18 a | 8.92 ± 1.39 a | 0.0050 ± 0.0012 c | 0.0032 ± 0.0007 c | 0.0099 ± 0.0050 ab | 0.018 ± 0.003 c |
| +AMF | 8.12 ± 2.39 a | 0.65 ± 0.18 a | 8.78 ± 1.93 a | 0.087 ± 0.009 b | 0.019 ± 0.006 b | 0.013 ± 0.0006 ab | 0.12 ± 0.01 b |
| +Rhi + AMF | 9.38 ± 0.68 a | 0.88 ± 0.16 a | 10.27 ± 0.61 a | 0.13 ± 0.04 a | 0.030 ± 0.008 a | 0.016 ± 0.005 a | 0.18 ± 0.04 a |
| Treatments | Root | Stem | Leaf | Total |
|---|---|---|---|---|
| CK | 1.82 ± 0.51 b | 0.78 ± 0.27 b | 1.75 ± 0.70 b | 4.36 ± 0.75 b |
| +Rhi | 2.31 ± 0.57 ab | 0.83 ± 0.07 b | 1.31 ± 0.22 b | 4.81 ± 0.79 b |
| +AMF | 4.03 ± 0.54 a | 5.74 ± 0.93 a | 26.58 ± 3.86 a | 36.34 ± 5.11 a |
| +Rhi + AMF | 4.10 ± 0.71 a | 7.78 ± 1.09 a | 34.34 ± 5.21 a | 46.23 ± 5.79 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; He, X.; Zhang, X.; Yang, Y.; Liu, R.; Shi, S.; Shi, X.; Zhang, Y. A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks. Agronomy 2021, 11, 32. https://doi.org/10.3390/agronomy11010032
Fang L, He X, Zhang X, Yang Y, Liu R, Shi S, Shi X, Zhang Y. A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks. Agronomy. 2021; 11(1):32. https://doi.org/10.3390/agronomy11010032
Chicago/Turabian StyleFang, Linfa, Xinhua He, Xueliang Zhang, Yehua Yang, Rui Liu, Songmei Shi, Xiaojun Shi, and Yuting Zhang. 2021. "A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks" Agronomy 11, no. 1: 32. https://doi.org/10.3390/agronomy11010032
APA StyleFang, L., He, X., Zhang, X., Yang, Y., Liu, R., Shi, S., Shi, X., & Zhang, Y. (2021). A Small Amount of Nitrogen Transfer from White Clover to Citrus Seedling via Common Arbuscular Mycorrhizal Networks. Agronomy, 11(1), 32. https://doi.org/10.3390/agronomy11010032






