Zeolite and Vermiculite as Inorganic Soil Amendments Modify Shoot-Root Allocation, Mineral Nutrition, Photosystem II Activity and Gas Exchange Parameters of Chestnut (Castanea sativa Mill) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Soil Sampling and Treatments
2.2. Chemical Analyses of Soil Samples and Mixtures
2.3. Plant Growth Data
2.4. Leaf Nutrient Analyses and Total Plant Nutrient Content
2.5. Chlorophyll Fluorescence and Gas Exchange Measurements
2.6. Statistical Analysis
3. Results
3.1. Soil Fertility in the Four Soil Treatments
3.2. Plant Growth
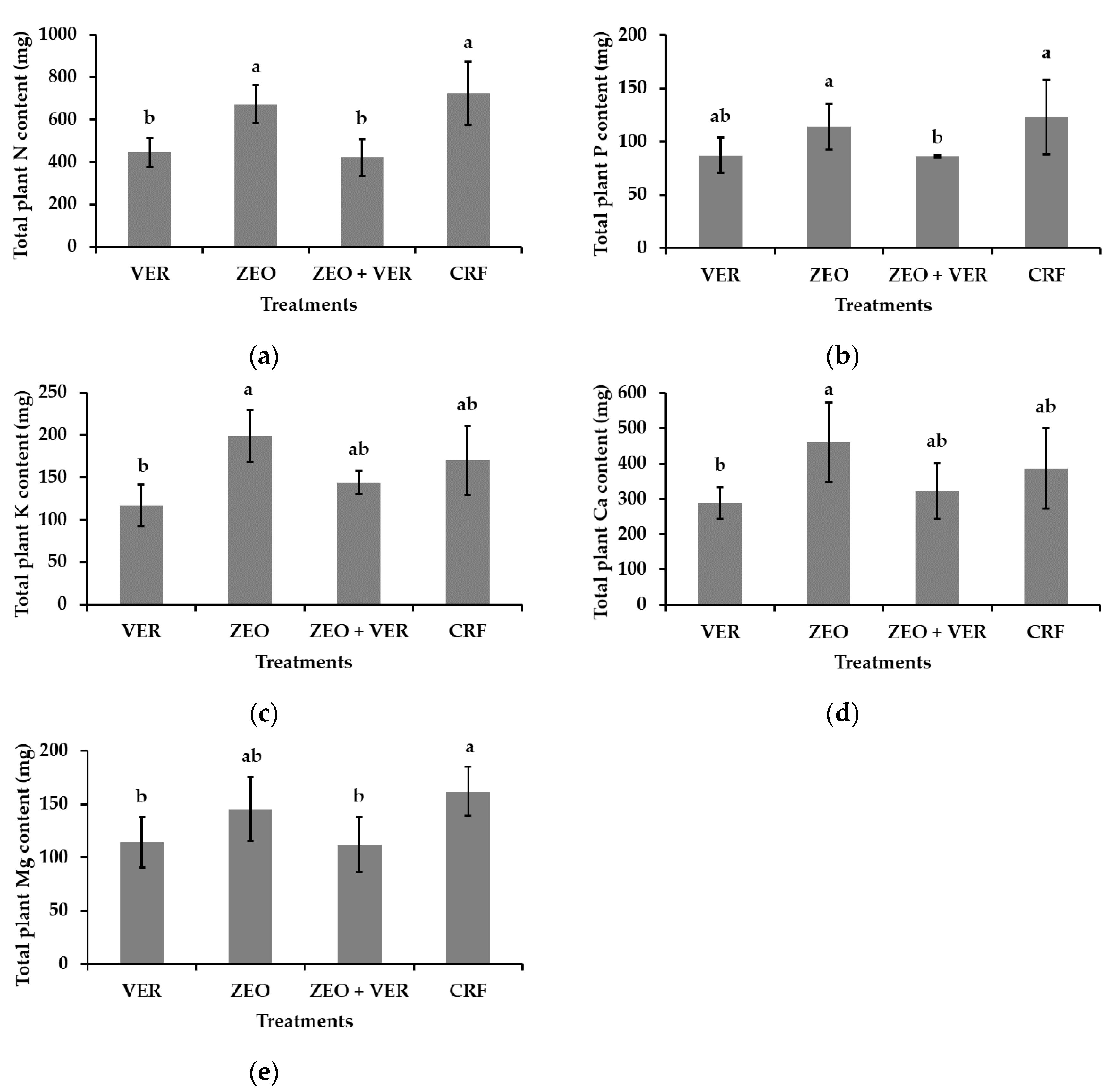
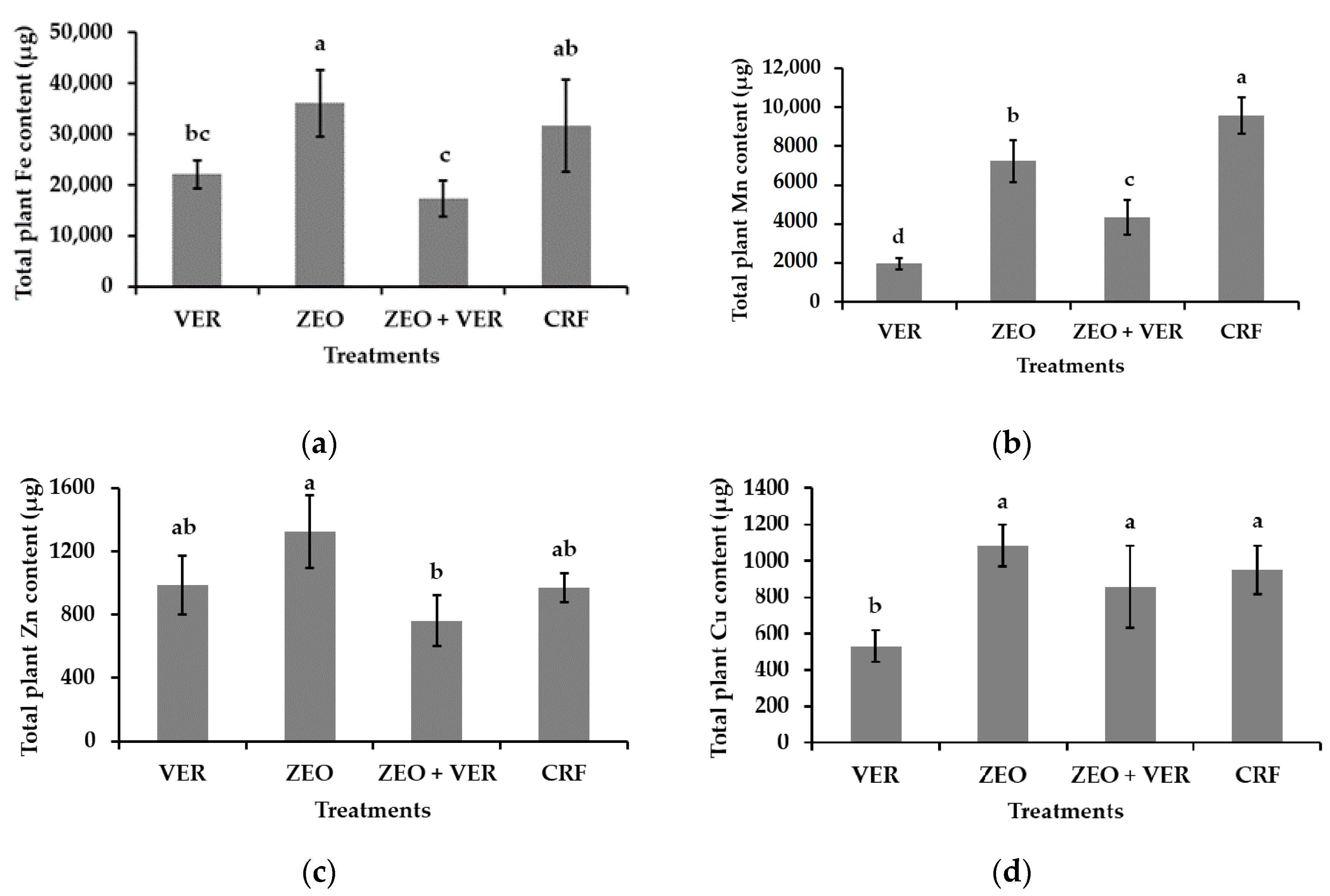
3.3. Leaf Nutrient Concentrations and Total Plant Nutrient Content
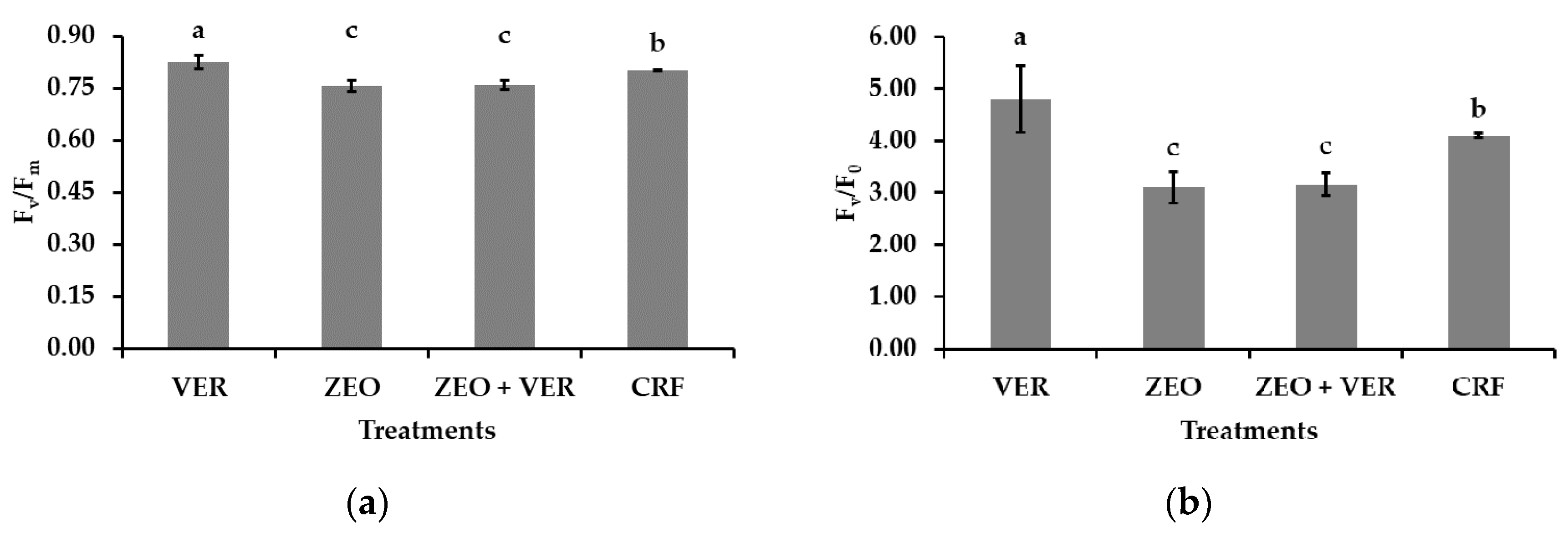
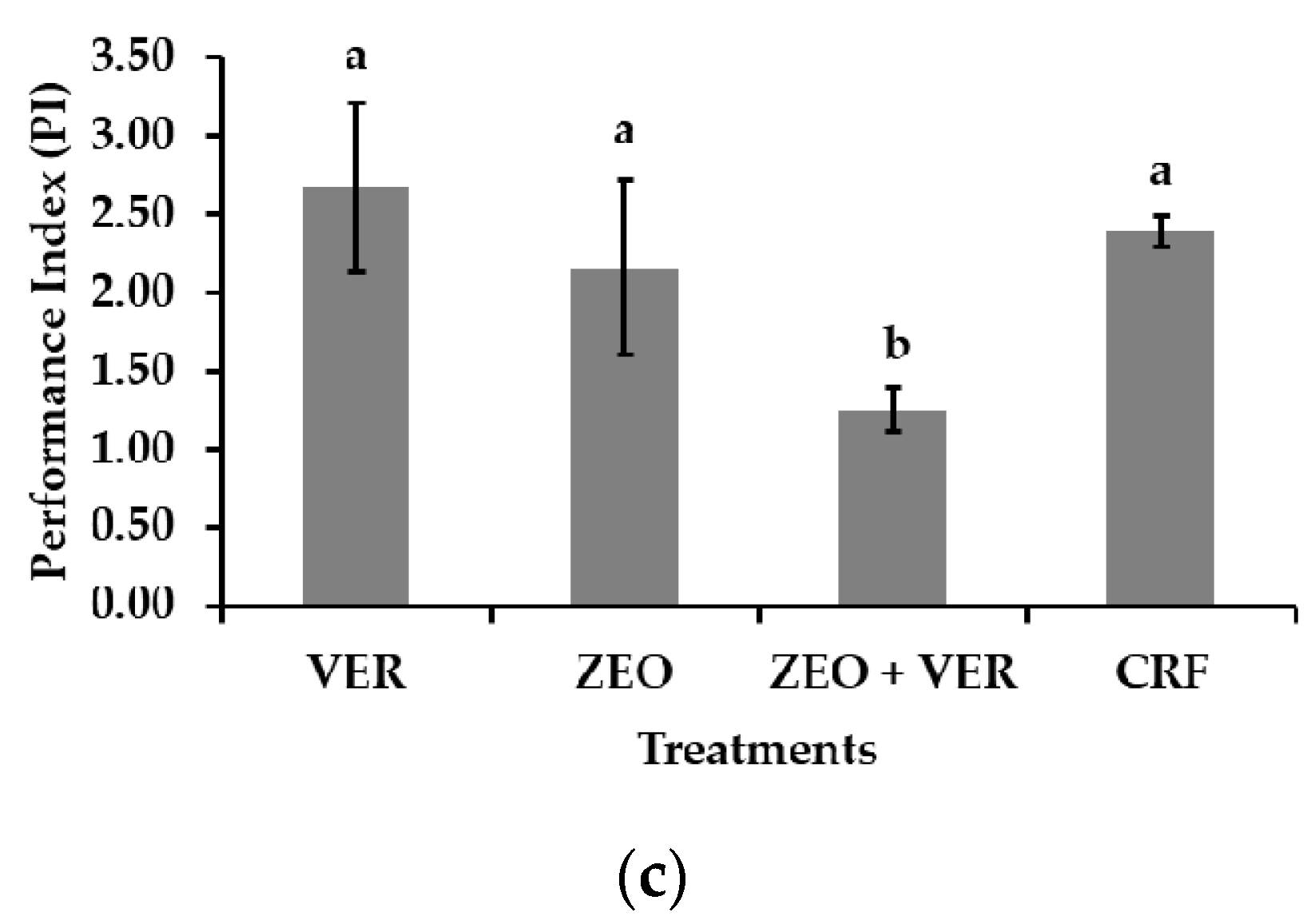
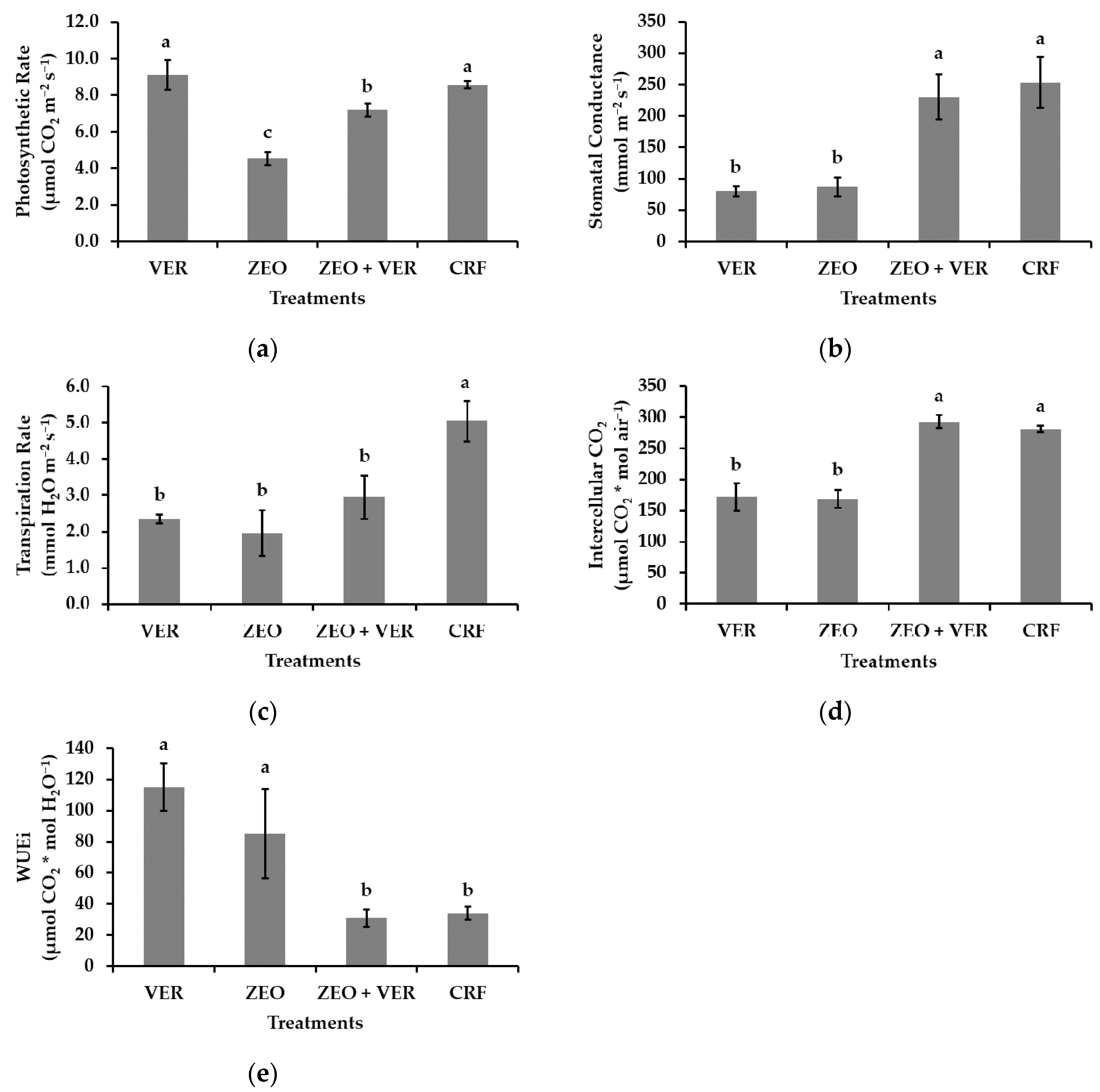
3.4. Photosystem II Activity and Gas Exchange Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrobas, M.; Afonso, S.; Ferreira, I.Q.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.A. Liming and application of nitrogen, phosphorus, potassium and boron on a young plantation of chestnut. Turk. J. Agric. For. 2017, 41, 441–451. [Google Scholar] [CrossRef]
- Portela, E.; Martins, A.; Pires, A.L.; Raimundo, F.; Marques, G. Cap 6—Práticas culturais no souto: O manejo do solo. In Vila Real, Portugal: Programa AGRO 499; Castanheiros; Gomes-Laranjo, J., Ferreira-Cardoso, J., Portela, E., Abreu, C.G., Eds.; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2007; pp. 207–264. (In Portuguese) [Google Scholar]
- Zhang, J.; Li, Y.; Chang, S.X.; Jiang, P.; Zhou, G.; Liu, J.; Wu, J.; Shen, Z. Understory vegetation management affected greenhouse gas emissions and labile organic C pools in an intensively managed Chinese chestnut plantation. Plant Soil 2014, 376, 363–375. [Google Scholar] [CrossRef]
- Corredoira, E.; Valladares, S.; Vieitez, A.M.; Ballester, A. Improved germination of somatic embryos and plant recovery of European chestnut. In Vitro Cell. Dev. Biol. Plant 2008, 44, 307–315. [Google Scholar] [CrossRef]
- Cuenca, B.; Sanchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa x C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tiss Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Larson, C.G.; Hasbun, R.; Paz Jofre, M.; Sanchez-Olate, M.; Darcy Rios, L. Effect of genotype and cytokines source at the initiation stage in vitro culture of adult tissue of Castanea sativa Mill. Gayana Bot. 2017, 74, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Serdar, U.; Dermirsoy, H. Non-destructive leaf area estimation in chestnut. Sci. Hortic. 2006, 108, 227–230. [Google Scholar] [CrossRef]
- Silvanini, A.; Dall’Asta, C.; Morrone, L.; Cirlini, M.; Beghe, D.; Fabbri, A.; Ganino, T. Altitude effects on fruit morphology and flour composition of two chestnut cultivars. Sci. Hortic. 2014, 176, 311–318. [Google Scholar] [CrossRef]
- Guzman, M.; Urrestarazu, M.; Romero, L. Active and total Fe in Castanea sativa and their relation to other nutrients. J. Plant Nutr. 1986, 9, 909–921. [Google Scholar] [CrossRef]
- Zysset, M.; Brunner, I.; Frey, B.; Blaser, P. Response of European chestnut to varying Calcium/Aluminum ratios. J. Environ. Qual. 1996, 25, 702–708. [Google Scholar] [CrossRef]
- Eprikashvili, L.; Zautashvili, M.; Kordzakhia, T.; Pirtskhalava, N.; Dzagania, M.; Rubashvili, I.; Tsitsishvili, V. Intensification of bio-productivity of agricultural cultures by adding natural zeolites and brown coals into soils. Ann. Agrar. Sci. 2016, 14, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Chatzistathis, T.; Papadakis, I.E.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a controlled release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv. ‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Elfalleh, W.; Belayadi, H.; Haddad, M. Effect of date palm waste compost on forage alfalfa growth, yield, seed yield and minerals uptake. Int. J. Recycl. Org. Waste Agric. 2018, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G.; Moutsopoulou, M.; Stefanoudaki, E. Application of olive mill wastewater to a Cretan olive orchard: Effects on soil properties, plant perfromance and the environment. Agric. Ecosyst. Environ. 2010, 138, 293–298. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Koutsos, T. Olive mill wastewater as a source of organic matter, water and nutrients for restoration of degraded soils and for crops managed with sustainable systems. Agric. Water Manag. 2017, 190, 55–64. [Google Scholar] [CrossRef]
- Paskovic, I.; Bronic, J.; Subotic, B.; Pecina, M.; Perica, S.; Palcic, I.; Herak Custic, M. Impact of zeolite fertilization on radicchio mineral composition and nutritive value. J. Food Agric. Environ. 2013, 11, 498–502. [Google Scholar]
- Doostikhah, N.; Panahpour, E.; Nadian, H.; Gholami, A. Tomato (Lycopersicon esculentum L.) nutrient and lead uptake affected by zeolite and DTPA in a lead-polluted soil. Plant Biol. 2020, 22, 317–322. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Effects of vermiculite, nanoclay and zeolite on ammonium transport through saturated sandy loam soil: Column experiments and modeling approaches. Catena 2019, 176, 170–180. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Miao, Q.; Yu, B.; Xu, L.; Cui, Z. Combining mineral amendments improves wheat yield and soil properties in a coastal saline area. Agronomy 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Assimakopoulou, A.; Dimitroulia, D.; Kosmidis, S.; Doula, M.K. Growth, yield and nutrient status of pepper plants grown on a soil substrate with olive mill waste sludge and natural zeolite addition. J. Plant Nutr. 2020, 43, 629–640. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Akbari, L.; Shirani, H. Effects of co-application of zeolites and vermicompost on speciation and phytoavailability of cadmium, lead and zinc in a contaminated soil. Commun. Soil Sci. Plant Anal. 2017, 48, 262–273. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Tzanakakis, V.; Giannakoula, A.; Psoma, P. Inorganic and organic amendments affect soil fertility, nutrition, photosystem II activity and fruit weight, and may enhance the sustainability of Solanum lycopersicon L. (cv. ‘Mountain Fresh’) crop. Sustainability 2020, 12, 9028. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. In Agronomy Monograph, Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Agronomy Monograph, Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 539–547. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphorus. In Agronomy Monograph, Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 595–615. [Google Scholar]
- Thomas, G.W. Exchangeable cations. Methods of soil analysis. In Agronomy Monograph, Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Chyla, M.A.; Żyrnicki, W. Determination of metal concentrations in animal hair by the ICP method Biological. Biol. Trace Elem. Res. 2000, 75, 187–194. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; Division of Agricultural Sciences, University of California: Riverside, CA, USA, 1961; p. 309. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence a Signature of Photosynthesis; Papageorgiou, G., Govindjee, C., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Basri, M.H.; Abdu, A.; Jusop, S.; Ahmed, O.H.; Abdul-Hamid, H.; Kusno, M.A.; Zainal, B.; Senin, A.L.; Junejo, N. Effects of mixed organic and inorganic fertilizers application on soil properties and the growth of Kenaf (Hibiscus cannabinus L.) cultivated on Bris soils. Am. J. Appl. Sci. 2013, 10, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Litaor, M.I.; Katz, L.; Shenker, M. The influence of compost and zeolite co-addition on the nutrients’ status and plant growth in intensively cultivated Mediterranean soils. Soil Use Manag. 2017, 33, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Nanos, G.; Tsintirakou, I. Fertilization of Castanea sativa Mill. Agric. Livest. Farming Agrotypos Publ. 2013, 74. (In Greek) [Google Scholar]
- El-Jaoual, T.; Cox, D.A. Manganese toxicity in plants. J. Plant Nutr. 1998, 21, 353–386. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Rengel, Z.; Liu, H.; Zhao, P. Excess Zn-induced changes in physiological parameters and expression levels of TaZips in two wheat genotypes. Environ. Exp. Bot. 2020, 177, 104133. [Google Scholar] [CrossRef]
- Mahmoodabadi, M.R.; Ronaghi, A.M.; Khayyat, M.; Hadarbadi, G. Effects of zeolite and cadmium on growth and chemical composition of soybean (Glycine max L.). Trop. Subtrop. Agroecosyst. 2009, 10, 515–521. [Google Scholar]
- Ozbahce, A.; Tari, A.F.; Gönülal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2015, 61, 615–626. [Google Scholar] [CrossRef]
- Iskander, A.L.; Khald, E.M.; Sheta, A.S. Zinc and manganese sorption behavior by natural zeolite and bentonite. Ann. Agric. Sci. 2011, 56, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Najafi-Ghiri, M.; Rahimi, T. Zinc uptake by spinach (Spinacia oleracea L.) as affected by Zn application rate, zeolite and vermicompost. Compost Sci. Util. 2016, 24, 203–207. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I. How soil nutrient availability influences plant biomass and how biomass stimulation alleviates heavy metal toxicity in soils: The cases of nutrient use efficient genotypes and phytoremediators, respectively. In Biomass Now-Cultivation and Utilization; Matovic, D.M., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 427–448. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.D.; Shao, X.H.; Zhang, Y.C.; Zhang, H.; Hou, M.M. Effects of K fertilizer application on photosynthesis and seedling growth of sweet potato under drought stress. J. Food Agric. Environ. 2012, 10, 487–491. [Google Scholar]
- Chen, Y.; Liu, L.; Guo, Q.; Zhu, Z.; Zhang, L. Effects of water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biol. Res. 2016, 49, 12. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, W.H.; Wang, Y. The K+ channel KZM2 is involved in stomatal movement by modulating inward K+ currents in maize guard cells. Plant J. 2017, 92, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [Green Version]
- Saykhul, A.; Chatzistathis, T.; Chatzissavvidis, C.; Koundouras, S.; Therios, I.; Dimassi, K. Potassium utilization efficiency of three olive cultivars grown in a hydroponic system. Sci. Hortic. 2013, 162, 55–62. [Google Scholar] [CrossRef]
| Amendment | pH | Organic Matter (%) | NO3−-N | P | K | Ca | Mg | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||||||
| Zeolite | 7.28 | 0.00 | 9.36 | 1.87 | 14,984 | 16050 | 922 | 0.57 | 1.12 | 0.24 | 0.05 |
| Vermiculite | 8.83 | 0.18 | 6.58 | 2.53 | 445 | 1727 | 146 | 14.44 | 1.92 | 0.33 | 0.47 |
| Treatment (Soil Amendment) | pH | Organic C (%) | Organic Matter (%) | N (%) | C/N | P (mg/100 g) | K (cmol kg−1) | Ca (cmol kg−1) | Mg (cmol kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| VER | 5.59 | 7.91 | 13.64 | 0.48 | 16.48 | 1.95 | 0.80 | 10.28 | 3.88 |
| ZEO | 5.16 | 7.77 | 13.39 | 0.40 | 19.43 | 1.51 | 14.62 | 13.95 | 3.99 |
| ZEO + VER | 4.98 | 7.85 | 13.53 | 0.45 | 17.44 | 1.34 | 8.27 | 12.89 | 3.57 |
| Control | 4.61 | 7.79 | 13.42 | 0.34 | 22.85 | 1.06 | 0.48 | 9.23 | 3.63 |
| Treatment (Soil Amendment) | Fe | Mn | Zn | Cu |
|---|---|---|---|---|
| mg kg−1 | ||||
| VER | 28.18 | 6.90 | 0.89 | 0.36 |
| ZEO | 26.06 | 6.64 | 0.71 | 0.25 |
| ZEO + VER | 22.10 | 12.05 | 0.68 | 0.30 |
| Control | 24.84 | 7.00 | 0.63 | 0.33 |
| Treatment | LEAF (g) | STEM (g) | ROOT (g) | Total Plant Biomass (g) | Shoot/Root | Root/Total Plant Biomass | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | |
| VER | (25.68 ± 2.44) b | (10.59 ± 1.46) b | (23.63 ± 3.81) b | (10.84 ± 1.95) b | (75.59 ± 12.01) b | (18.43 ± 3.65) b | (127.77 ± 19.06) b | (40.95 ± 4.27) b | (0.63 ± 0.05) ab | (1.15 ± 0.19) ab | (0.62 ± 0.05) ab | (0,47 ± 0.06) ab |
| ZEO | (27.53 ± 5.58) ab | (10.60 ± 1.12) b | (30.77 ± 5.54) ab | (14.00 ± 2.77) ab | (109.37 ± 12.44) a | (31.23 ± 6.02) a | (180.61 ± 30.05) a | (57,44 ± 6.67) a | (0.53 ± 0.06) bc | (0.96 ± 0.14) b | (0.65 ± 0.05) a | (0.52 ± 0.05) a |
| ZEO + VER | (21.71 ± 3.90) b | (9.05 ± 1.27) b | (20.87 ± 4.45) b | (9.69 ± 1.63) b | (93.63 ± 9.97) ab | (22.30 ± 4.84) ab | (136.21 ± 25.17) ab | (41.74 ± 6.04) b | (0.48 ± 0.06) c | (0.81 ± 0.16) b | (0.68 ± 0.07) a | (0.53 ± 0.07) a |
| CRF | (35.32 ± 5.89) a | (15.08 ± 2.20) a | (38.33 ± 7.92) a | (17.71 ± 3.97) a | (85.69 ± 10.11) b | (21.00 ± 4.50) ab | (151.19 ± 22.38) ab | (50.21 ± 7.42) ab | (0.82 ± 0.16) a | (1.53 ± 0.24) a | (0.55 ± 0.04) b | (0.40 ± 0.04) b |
| Nutrients | Treatment | ||||
|---|---|---|---|---|---|
| VER | ZEO | ZEO + VER | CRF | ||
| N | % D.W. | (2.12 ± 0.28) ab | (2.10 ± 0.39) ab | (1.95 ± 0.26) b | (2.47 ± 0.24) a |
| P | (0.38 ± 0.04) a | (0.33 ± 0.06) a | (0.35 ± 0.05) a | (0.33 ± 0.07) a | |
| K | (0.49 ± 0.05) b | (0.65 ± 0.08) a | (0.61 ± 0.06) a | (0.56 ± 0.06) ab | |
| Ca | (1.12 ± 0.14) b | (1.54 ± 0.18) a | (1.47 ± 0.16) a | (1.17 ± 0.21) ab | |
| Mg | (0.49 ± 0.06) a | (0.45 ± 0.06) a | (0.53 ± 0.09) a | (0.57 ± 0.08) a | |
| Fe | mg kg−1 | (207 ± 29.08) a | (202 ± 30.46) a | (243 ± 34.27) a | (190 ± 25.17) a |
| Mn | (83 ± 9.96) b | (380 ± 50.11) a | (337 ± 47.17) a | (405 ± 60.44) a | |
| Zn | (35 ± 3.27) a | (24 ± 2.80) b | (24 ± 4.55) b | (21 ± 3.12) b | |
| Cu | (18 ± 2.98) c | (27 ± 4.06) b | (38 ± 5.88) a | (27 ± 3.70) b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzistathis, T.; Papaioannou, E.; Giannakoula, A.; Papadakis, I.E. Zeolite and Vermiculite as Inorganic Soil Amendments Modify Shoot-Root Allocation, Mineral Nutrition, Photosystem II Activity and Gas Exchange Parameters of Chestnut (Castanea sativa Mill) Plants. Agronomy 2021, 11, 109. https://doi.org/10.3390/agronomy11010109
Chatzistathis T, Papaioannou E, Giannakoula A, Papadakis IE. Zeolite and Vermiculite as Inorganic Soil Amendments Modify Shoot-Root Allocation, Mineral Nutrition, Photosystem II Activity and Gas Exchange Parameters of Chestnut (Castanea sativa Mill) Plants. Agronomy. 2021; 11(1):109. https://doi.org/10.3390/agronomy11010109
Chicago/Turabian StyleChatzistathis, Theocharis, Evgenia Papaioannou, Anastasia Giannakoula, and Ioannis E. Papadakis. 2021. "Zeolite and Vermiculite as Inorganic Soil Amendments Modify Shoot-Root Allocation, Mineral Nutrition, Photosystem II Activity and Gas Exchange Parameters of Chestnut (Castanea sativa Mill) Plants" Agronomy 11, no. 1: 109. https://doi.org/10.3390/agronomy11010109
APA StyleChatzistathis, T., Papaioannou, E., Giannakoula, A., & Papadakis, I. E. (2021). Zeolite and Vermiculite as Inorganic Soil Amendments Modify Shoot-Root Allocation, Mineral Nutrition, Photosystem II Activity and Gas Exchange Parameters of Chestnut (Castanea sativa Mill) Plants. Agronomy, 11(1), 109. https://doi.org/10.3390/agronomy11010109








