The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Nodulated Chickpea Roots and Isolation of Rhizobium Species
2.2. Identification and Phenotypic Characteristics of Isolates
2.3. Detection of IAA and GA in Broth Culture
2.4. Seed Bacterization of Cicer Arietinum L.
2.5. Pot Experiment
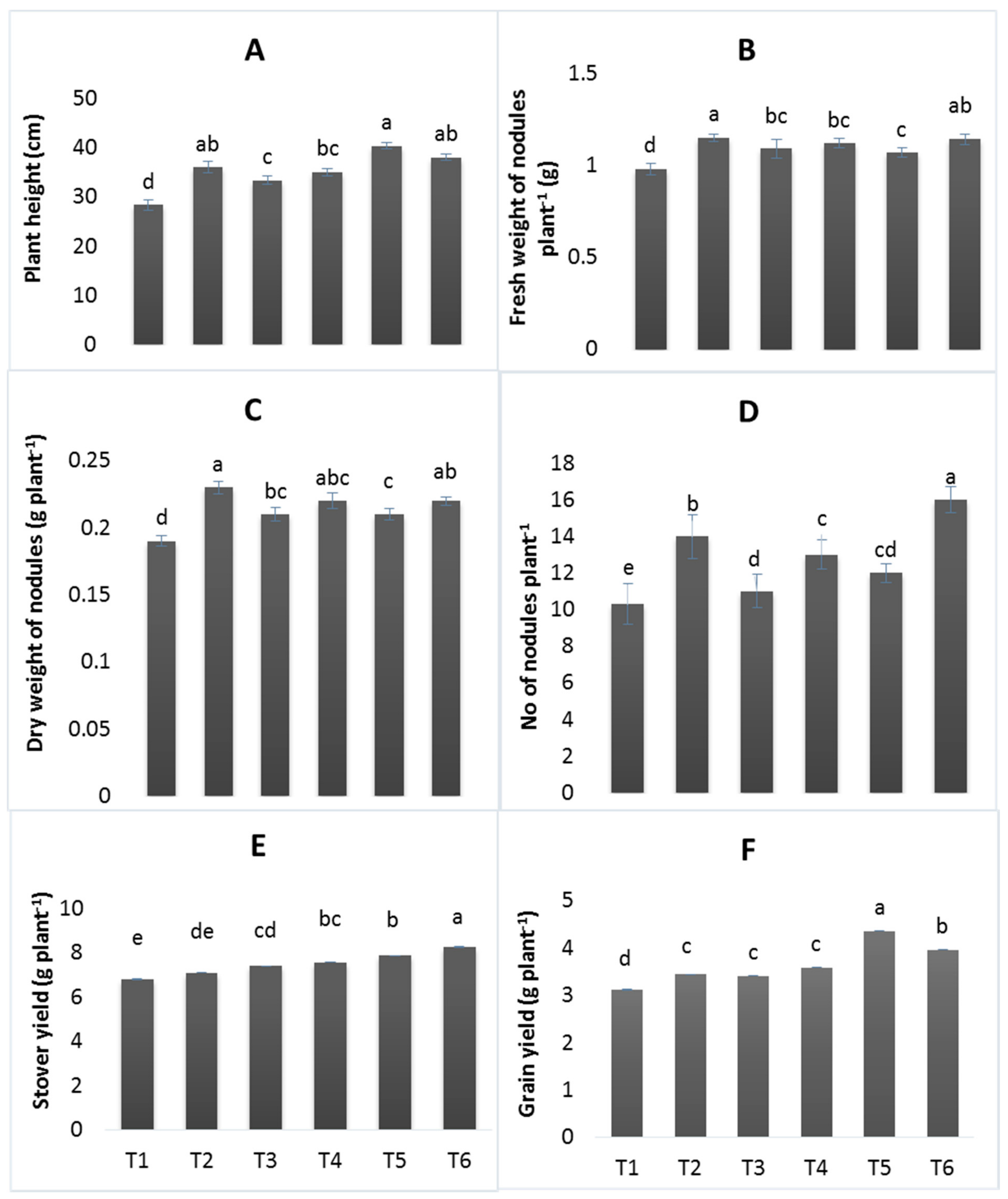
2.6. Agronomic Traits
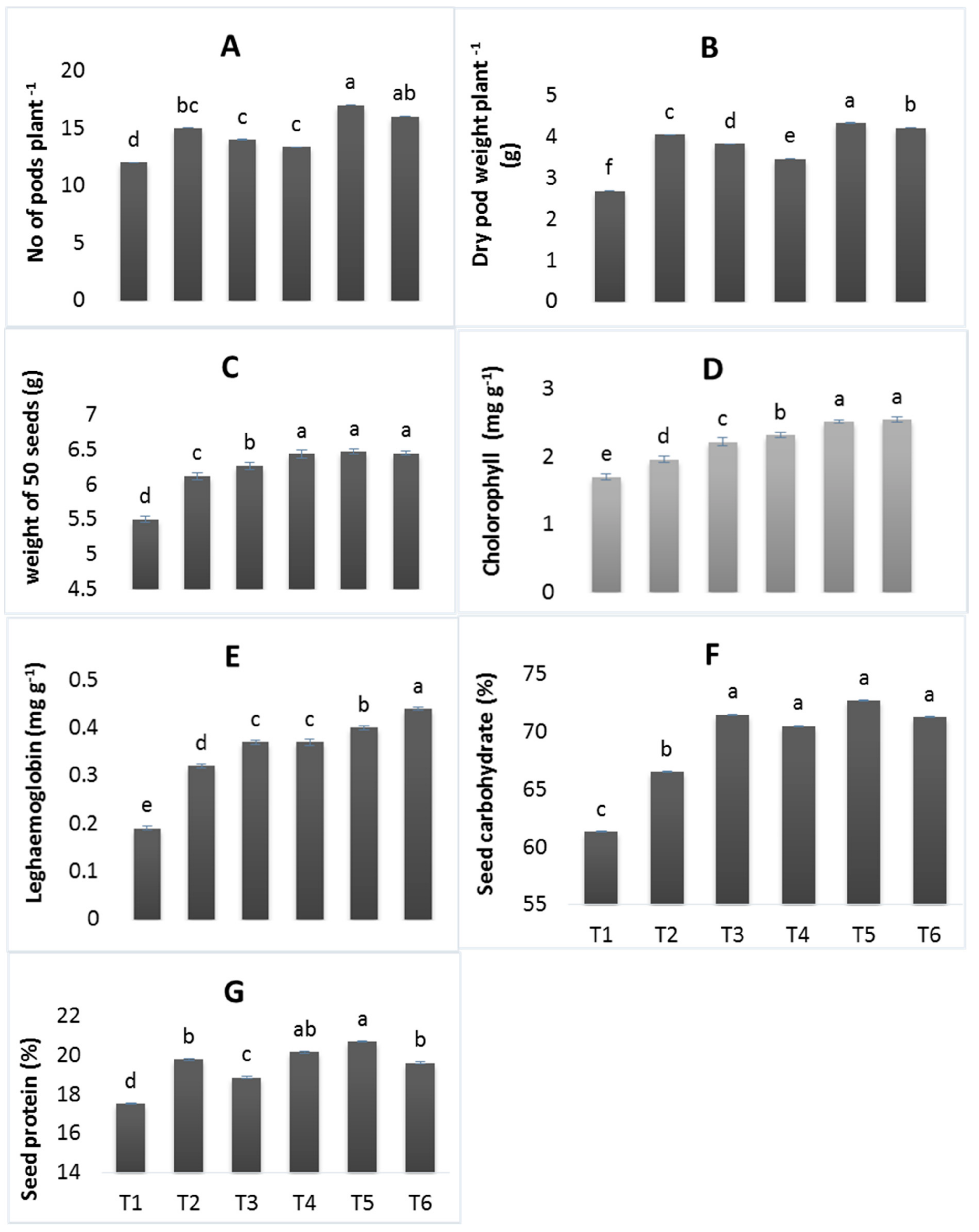
2.7. Physiological Traits
2.8. Physicochemical Analysis of Plant and Soil
2.9. Statistical Analysis
3. Results
3.1. Biochemical Characterization
3.2. Growth and Yield Parameters
3.3. Physiological and Quality Parameters
3.4. Chemical Analysis of Soil and Plant
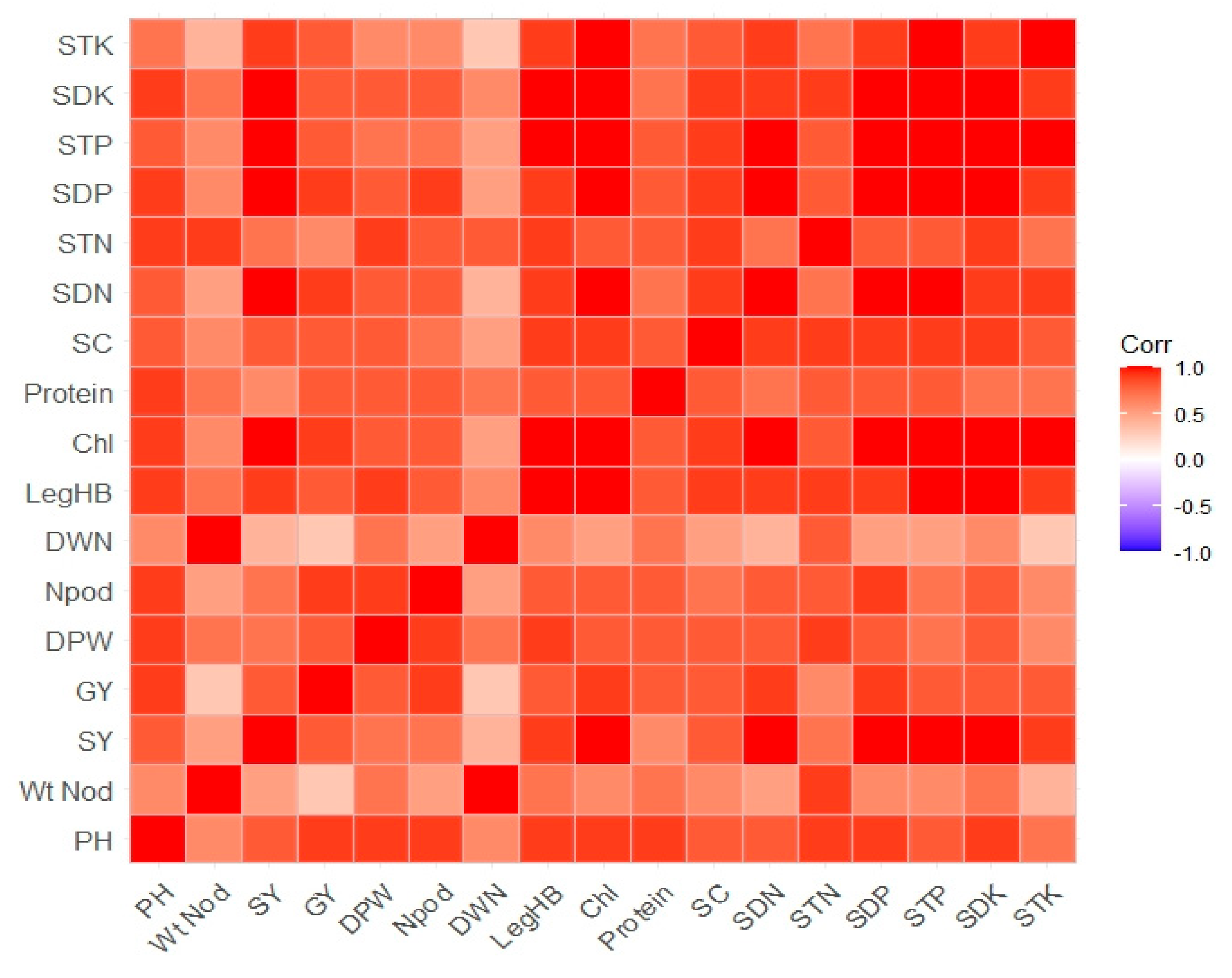
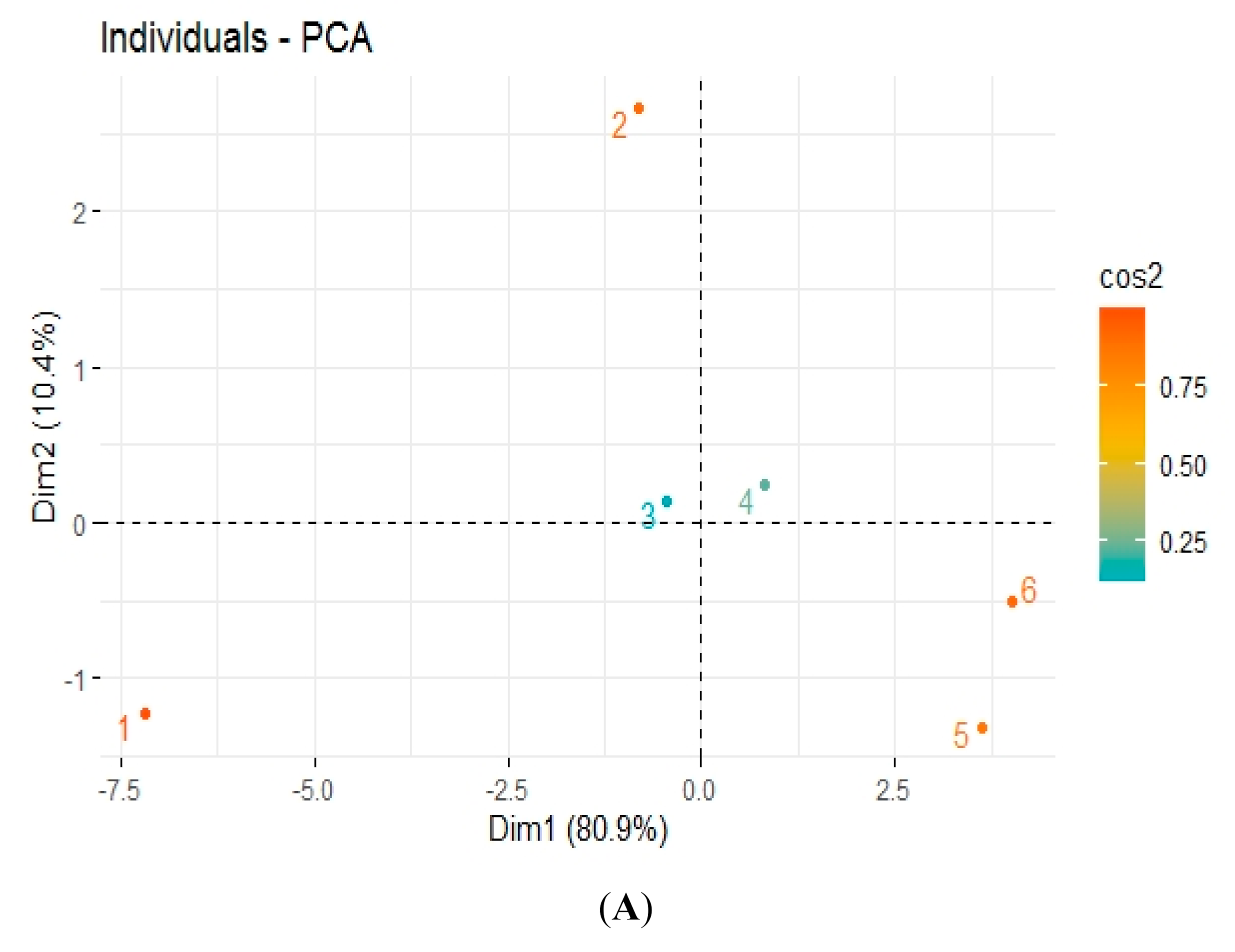
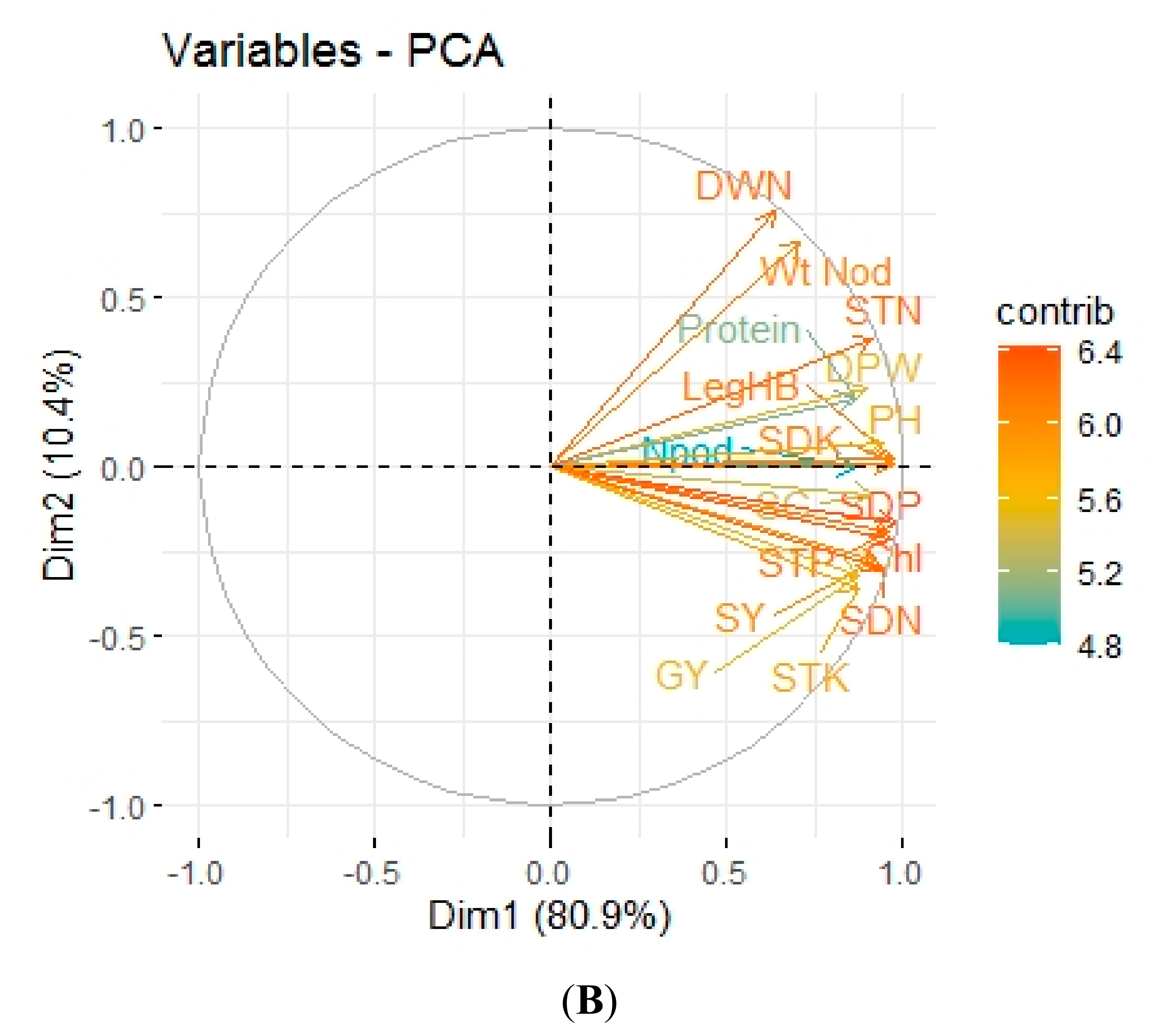
3.5. Pearson Correlation and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wik, M.; Pingali, P.; Broca, S. Background Paper for the World Development Report: Global Agricultural Performance: Past Trends and Future Prospects; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Ahmad, M.; Naseer, I.; Hussain, A.; Zahid, M.M.; Mustafa, A.; Hilger, T.H.; Zahir, Z.A.; Minggang, X. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.). Agronomy 2019, 9, 621. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Mustafa, A.; Hamid, Y.; Saeed, Q.; Xu, M. Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Raza, S.; Zia, M.; Mustafa, A.; Xu, M.; Wang, Y. Evaluating organic materials coating on urea as potential nitrification inhibitors for enhanced nitrogen recovery and growth of maize (Zea mays). Intl. J. Agric. Biol. 2019, 22, 1102–1108. [Google Scholar]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.Z.; Yaseen, M.; Naveed, M.; Wang, X.; Fatima, K.; Saeed, Q.; Mustafa, A. Polymer-Paraburkholderia phytofirmans PsJN coated diammonium phosphate enhanced microbial survival, phosphorous use efficiency, and production of wheat. Agronomy 2020, 10, 1344. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Kazy, S.K.; Saxena, A.K.; Suman, A. Elucidating the diversity and plant growth promoting attributes of wheat (Triticum aestivum) associated acid tolerant bacteria from southern hills zone of India. Natl. J. Life Sci. 2013, 10, 219–226. [Google Scholar]
- Mustafa, A.; Naveed, M.; Abbas, T.; Saeed, Q.; Hussain, A.; Ashraf, M.N.; Minggang, X. Growth response of wheat and associated weeds to plant antagonistic rhizobacteria and fungi. Ital. J. Agron. 2019, 14, 191–198. [Google Scholar] [CrossRef]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Shakya, M.S.; Patel, M.M.; Singh, V.B. Knowledge level of chickpea growers about chickpea production technology. Indian Res. J. Ext. Educ. 2008, 8, 65–68. [Google Scholar]
- Jukanti, A.; Gaur, P.M.; Gowda, C.L.; Chibbar, R. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.). Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Umar, W.; Ayub, M.A.; ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and Phosphorus Use Efficiency in Agroecosystems. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 213–257. [Google Scholar]
- Mazid, M.; Khan, T.A.; Mohammad, F. Medicinal Plants of rural India: A review of use by Indian folks. Indo Glob. J. Pharm. Sci. 2012, 2, 286–304. [Google Scholar]
- Kantar, F.; Hafeez, F.Y.; Shivakumar, B.G.; Sundaram, S.P.; Tejera, N.A.; Aslam, A.; Bano, A.; Raja, P. Chickpea: Rhizobium management and nitrogen fixation. In Chickpea Breeding and Management; CABI: London, UK, 2007; pp. 179–192. [Google Scholar]
- Mazid, M.; Zeba, H.K.; Quddusi, S. Significance of Sulphur nutrition against metal induced oxidative stress in plants. J. Stress Physiol. Biochem. 2011, 7, 165–184. [Google Scholar]
- Khattak, S.; Khan, D.F.; Shah, S.H.; Madani, M.S.; Khan, T. Role of Rhizobial inoculation in the production of chickpea crop. Soil Environ. 2006, 25, 143–145. [Google Scholar]
- Ditta, A.; Arshad, M.; Zahir, Z.A.; Jamil, A. Comparative efficacy of rock phosphate enriched organic fertilizer vs. mineral phosphatic fertilizer for nodulation, growth and yield of lentil. Int. J. Agric. Biol. 2015, 17, 589–595. [Google Scholar] [CrossRef]
- Ditta, A.; Khalid, A. Bio-organo-phos: A sustainable approach for managing phosphorus deficiency in agricultural soils. In Organic Fertilizers—From Basic Concepts to Applied Outcomes; Larramendy, M., Soloneski, S., Eds.; InTech: London, UK, 2016; pp. 109–136. [Google Scholar]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Role of biochar and plant growth-promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Shah, M.K.; Naveed, M.; Akhter, M.J. Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J. Microbiol. Biotechnol. 2010, 20, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Stougaard, J. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef]
- Limpens, E.; Franken, C.; Smit, P.; Willemse, J.; Bisseling, T.; Geurts, R. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 2003, 302, 630–633. [Google Scholar] [CrossRef]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Gronlund, M.; Stougaard, J. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef]
- Gleason, C.; Chaudhuri, S.; Yang, T.; Munoz, A.; Poovaiah, B.W.; Oldroyd, G.E. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 2006, 441, 1149–1152. [Google Scholar] [CrossRef]
- Tirichine, L.; Imaizumi-Anraku, H.; Yoshida, S.; Murakami, Y.; Madsen, L.H.; Miwa, H.; Stougaard, J. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 2006, 441, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, S.B.; Tajini, F.; Trabelsi, M.; Aouani, M.E.; Mhamdi, R. Competition for nodule formation between introduced strains of Mesorhizobium cicer and the native populations of rhizobia nodulating chickpea (Cicer arietinum L.) in Tunisia. World J. Microb. Biotechnol. 2007, 23, 1195–1201. [Google Scholar] [CrossRef]
- Maekawa, T.; Maekawa-Yoshikawa, M.; Takeda, N.; Imaizumi-Anraku, H.; Murooka, Y.; Hayashi, M. Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J. 2009, 58, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Hussain, A.; Naveed, M.; Ditta, A.; Nazli, Z.; Sattar, A. Response of okra (Abelmoschus esculentus L.) to soil and foliar applied L-tryptophan. Soil Environ. 2016, 35, 76–84. [Google Scholar]
- Gana, A.S. The role of synthetic growth hormones in crop multiplication and improvement. Afr. J. Biotechnol. 2010, 10, 10330–10334. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Durán, D.; Boulila, F.; Ahnia, H.; Boulila, A.; Temprano, F.; Palacios, F.M.; Imperial, J.; Ruiz-Argüeso, T.; Rey, L. Diversity of Bradyrhizobium strains nodulating Lupinus micranthus on both sides of the Western Mediterranean: Algeria and Spain. Syst. Appl. Microbiol. 2016, 39, 266–274. [Google Scholar] [CrossRef]
- Chen, J.; Hu, M.; Ma, H.; Wang, Y.; Wang, E.T.; Zhou, Z.; Gu, J. Genetic diversity and distribution of bradyrhizobia nodulating peanut in acid-neutral soils in Guangdong Province. Syst. Appl. Microbiol. 2016, 39, 418–427. [Google Scholar]
- Dall’Agnol, R.F.; Bournaud, C.; Faria, S.M.; Béna, G.; Moulin, L.; Hungria, M. Genetic diversity of symbiotic Paraburkholderia species isolated from nodules of Mimosa pudica (L.) and Phaseolus vulgaris (L.) grown in soils of the Brazilian Atlantic Forest (Mata Atlântica). FEMS Microbiol. 2017, 93, 1–15. [Google Scholar] [CrossRef]
- Costa, E.M.; Guimarães, A.A.; Carvalho, T.S.; Rodrigues, T.L.; Ribeiro, P.R.A.; Lebbe, L.; Willems, A.; Moreira, F.M.S. Bradyrhizobium forestalis sp. nov., an efficient nitrogen-fixing bacterium isolated from nodules of forest legume species in the Amazon. Arch. Microbiol. 2018, 200, 743–752. [Google Scholar] [CrossRef]
- Rajendran, G.; Singh, F.; Desai, A.J.; Archana, G. Enhanced growth and nodulation of pigeon pea by coinoculation of Bacillus strains along with Rhizobium sp. Bioresour. Technol. 2008, 99, 4544–4550. [Google Scholar] [CrossRef]
- Saeed, Z.; Naveed, M.; Imran, M.; Bashir, M.A.; Sattar, A.; Mustafa, A.; Hussain, A.; Xu, M. Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 2019, 14, e0213016. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.; Sadowsky, M.J.; Keyser, H.H. Proposed minimal standards for the description of new genera of roots and stem nodulating bacteria. Syst. Bacteriol. 1991, 41, 582–587. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Yousaf, S.; Pastar, M.; Afzal, M.; Sessitsch, A. The endophyte Enterobacter sp. FD17: A maize growth enhancer selected based on rigorous testing of plant beneficial traits and colonization characteristics. Biol. Fertil Soils 2014, 50, 249–262. [Google Scholar] [CrossRef]
- Kumari, B.S.; Ram, M.R.; Mallaih, K.V. Studies on nodulation, biochemical analysis and protein profiles of Rhizobium isolates from Indigofera species. Malays. J. Microbiol. 2009, 133–139. [Google Scholar]
- Vincent, J.M. A Manual of Practical Study of Root Nodule Bacteria; Blackwell Scientific Publication: Oxford, UK, 1970. [Google Scholar]
- Flores-Vargas, R.D.; O’Hara, G.W. Isolation and characterization of rhizosphere bacteria with potential for biological of weeds in vineyards. J. Appl. Microbiol. 2006, 100, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Arshad, M.; Martens, W.T.; Frankenberger, J.R. Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 1992, 147, 207–215. [Google Scholar] [CrossRef]
- Holbrook, A.A.; Edge, W.L.W.; Bailey, F. Spectrophotometric Method for Determination of Gibberellic Acid in Gibberellins; ACS Publications: Washington, DC, USA, 1961; pp. 159–167. [Google Scholar]
- Arnon, I.D. Copper enzymes in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 11–15. [Google Scholar] [CrossRef]
- Sadasivan, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International: New Delhi, India, 2008. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India: New Delhi, India, 1967; pp. 183–192. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics—A Biometrical Approach, 3rd ed.; McGraw-Hill Book International Co.: Singapore, 1997. [Google Scholar]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef]
- Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.S.; Mubeen, F.; Aziz, O.; Qian, Z.; Ijaz, R.; Tu, S. Rock phosphate enriched organic fertilizer with phosphate solubilizing microorganisms improves nodulation, growth and yield of legumes. Commun. Soil Sci. Plant Anal. 2018, 49, 2715–2725. [Google Scholar] [CrossRef]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of rock phosphate enriched composts increases nodulation, growth and yield of chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Khalid, A.; Mehmood, S.; Rizwan, M.S.; Mubeen, F.; Imtiaz, M. Integrated effect of algal biochar and plant growth promoting rhizobacteria on physiology and growth of maize under deficit irrigations. J. Soil Sci. Plant Nutr. 2020, 20, 346–356. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Solaiman, A.R.M.; Hossain, K.M.; Hossain, T. Effects of Rhizobium inoculants, nitrogen, phosphorus and molybdenum on nodulation, yield and seed protein in pea. Korean J. Crop. Sci. 2005, 50, 112–119. [Google Scholar]
- Nishita, G.; Joshi, N.C. Growth and yield response of chickpea (Cicer arietinum) to seed inoculation with Rhizobium sp. Nat. Sci. 2010, 8, 232–236. [Google Scholar]
- Ogutcu, H.; Kasimoglu, C.; Elkocae, E. Effects of rhizobium strains isolated from wild chickpeas on the growth and symbiotic performance of chickpeas (Cicer arietinum L.) under salt stress. Turk. J. Agric. For. 2010, 34, 361–371. [Google Scholar]
- Kumar, A.; Biswas, T.K.; Singh, N.; Lal, E.P. Effect of gibberellic acid on growth, quality and yield of tomato (Lycopersicon esculentum Mill.). IOSR J. Agric. Vet. Sci. 2014, 7, 28–30. [Google Scholar] [CrossRef]
- Nabi, A.J.; Hasan, M.N.; Alam, M.M.; Islam, M.S.; Islam, M.R. Responses of gibberellic acid (GA3) on growth and yield of cowpea cv. BARI Falon-1 (Vigna unguiculate L.). J. Environ. Sci. Nat. Res. 2014, 7, 7–12. [Google Scholar]
- Thakare, U.; Patil, N.; Malpathak, N. Performance of chickpea under the influence of gibberellic acid and oxygenated peptone during germination. Adv. Biosci. Biotechnol. 2011, 2, 40–45. [Google Scholar] [CrossRef]
- Solaimalai, A.; Sivakumar, C.; Anbumani, S.; Suresh, T.; Arulmurugan, K. Role of plant growth regulators in rice production: A review. Agric. Rev. 2001, 22, 33–40. [Google Scholar]
- Rao, K.L.; Reddy, N.; Mahalakshmi, P.J.R. Effect of PGR and micronutrient on flower absorption, pod setting and yield of chickpea. Annals Plant Physiol. 2005, 19, 14–17. [Google Scholar]
- Neelima, A.; Ranjana, R.; Kaur, J. Alleviation of normal and late sown chickpea (Cicer arietinum L.) yield through foliar application of bio regulators. Environ. Ecol. 2006, 24S, 147–176. [Google Scholar]
- Fatima, Z.; Bano, A.; Aslam, M. Effect of plant growth regulators and Rhizobium inoculum on N2 fixation and yield of chickpea. In Proceedings of the 7th International Symposium of Nitrogen Fixation with Non-Legume, Faisalabad, Pakistan, 16–21 October 1996; Kluwer Academic Publisher: Dordrecht, The Netherlands; Boston, MA, USA, 1998; pp. 103–106. [Google Scholar]
- Akbari, N.; Barani, M.; Ahmadi, H. Effect of gibberellic acid (GA3) on agronomic traits of green gram (Vigna radiate L. Wilczek) irrigated with different levels of saline water. World Appl. Sci. J. 2008, 5, 199–203. [Google Scholar]
- Zaman, S.; Mazid, M.A.; Kabir, G. Effect of Rhizobium inoculation on nodulation, yield and yield traits of chickpea (Cicer arietinum L.) in four different soils of greater Rajshahi. J. Life Earth Sci. 2011, 6, 45–50. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, H.C.; Parihar, S.K.S. Effect of growth regulators on quality parameters in Chickpea (Cicer arietinum). Farm. Sci. J. 2003, 12, 156–157. [Google Scholar]
- Afrin, S. Effects of GA3 and IAA Alone and in Combination on BARI Tomato-14 (Lycopersicon esculentum Mill). Master’s Thesis, Jagannath University, Dhaka, Bangladesh, 2015; p. 93. [Google Scholar]
- Jain, R.K.; Jain, A.K.; Gera, V.K. Effect of growth regulators on leghaemoglobin synthase in chickpea nodules. Legume Res. 2008, 31, 303–305. [Google Scholar]
- Kaur, S.; Gupta, A.K.; Kaur, N. Seed priming increases crop yield possibly by modulating enzymes of sucrose metabolism in chickpea. J. Agron. Crop. Sci. 2005, 191, 81–97. [Google Scholar] [CrossRef]
- Gupta, B.; Shrivastava, G.K.; Annu, V. Response of plant growth regulators on nutrient uptake and protein yield of chickpea under vertisols of Chhattisgarh. Environ. Ecol. 2007, 25, 100–102. [Google Scholar]
- Idris, E.E.; Iglesias, D.J.; Talón, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]

| Characteristics | Rhizobial Isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | |
| Phenotypic and physiological characterization | ||||||||||
| Colony color | White | Milky white | Milky white | Milky white | Milky white | Milky white | Milky white | Milky white | Milky white | Milky white |
| Shape | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular |
| Size (mm) | 2.5 | 3.1 | 2.1 | 1.8 | 3.0 | 2–3 | 2.6 | 2–4 | 2–4 | 2.5 |
| Opacity | Transparent | Transparent | Transparent | Translucent | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent |
| Gram reaction | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Bacterium shape | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod | Rod |
| Motility test | Motile | Motile | Motile | Motile | Motile | Motile | Motile | Motile | Motile | Motile |
| Bacterial growth conditions a,b | ||||||||||
| NaCl | ||||||||||
| 0.5% | ++ | + | + | ++ | ++ | ++ | ++ | ++ | + | + |
| 1.0% | ++ | + | + | + | ++ | ++ | ++ | ++ | ++ | ++ |
| 1.5% | + | + | + | + | + | ++ | ++ | ++ | ++ | + |
| 2.0% | + | ++ | + | + | ++ | + | ++ | ++ | ++ | + |
| 2.5% | ++ | ++ | ++ | ++ | + | + | ++ | + | + | ++ |
| pH | ||||||||||
| 4 | − | + | + | − | + | + | + | + | + | + |
| 5 | + | + | + | + | + | + | + | + | − | + |
| 6 | + | + | + | + | + | + | + | + | + | + |
| 7 | + | + | + | + | + | + | + | + | + | + |
| 8 | + | + | + | + | + | + | + | − | + | − |
| 9 | − | − | + | + | + | − | + | + | + | + |
| Carbon utilization potential | ||||||||||
| GPA assay | + | − | + | + | + | + | + | + | + | + |
| Lactose Assay | − | + | − | − | − | − | − | − | − | − |
| Biochemical Characterization | ||||||||||
| Gelatin Hydrolysis | − | − | − | − | − | − | − | − | − | − |
| Fluorescence assay | − | − | − | − | − | − | − | − | − | − |
| Triple sugar Ion | + | + | + | + | + | + | + | + | + | + |
| Lipase | − | + | + | + | + | + | + | − | − | + |
| Lysine | + | + | + | + | + | + | − | + | − | + |
| Citrate | + | + | + | + | + | + | + | − | + | + |
| Urease | + | + | + | + | + | + | + | + | + | − |
| Starch Hydrolysis | − | − | − | + | + | + | − | − | + | + |
| Bromothymol blue | + | + | + | + | + | + | + | + | + | + |
| Auxin (IAA equivalents µg mL−1) | 4.34 | 5.67 | 6.86 | 4.23 | 3.01 | 2.34 | 4.45 | 5.68 | 4.21 | 2.47 |
| Gibbrellic acid (mg L−1) | 5.37 | 8.34 | 4.87 | 11.36 | 7.42 | 7.87 | 6.11 | 8.75 | 8.99 | 7.01 |
| Biolog identification and similarity index % | ||||||||||
| Mesorhizobium ciceri | 76 | 80 | 74 | 76 | 74 | 73 | 72 | 78 | 76 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.M. The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. https://doi.org/10.3390/agronomy11010105
Rafique M, Naveed M, Mustafa A, Akhtar S, Munawar M, Kaukab S, Ali HM, Siddiqui MH, Salem MZM. The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy. 2021; 11(1):105. https://doi.org/10.3390/agronomy11010105
Chicago/Turabian StyleRafique, Munazza, Muhammad Naveed, Adnan Mustafa, Saleem Akhtar, Muneeb Munawar, Sadia Kaukab, Hayssam M. Ali, Manzer H. Siddiqui, and Mohamed Z. M. Salem. 2021. "The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.)" Agronomy 11, no. 1: 105. https://doi.org/10.3390/agronomy11010105
APA StyleRafique, M., Naveed, M., Mustafa, A., Akhtar, S., Munawar, M., Kaukab, S., Ali, H. M., Siddiqui, M. H., & Salem, M. Z. M. (2021). The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy, 11(1), 105. https://doi.org/10.3390/agronomy11010105









