Interactive Effects of Nitrogen and Humic Substances Applications on Bioethanol Production from Sweet Sorghum and Combustion Characteristics of Its Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Site History and Crop Culture
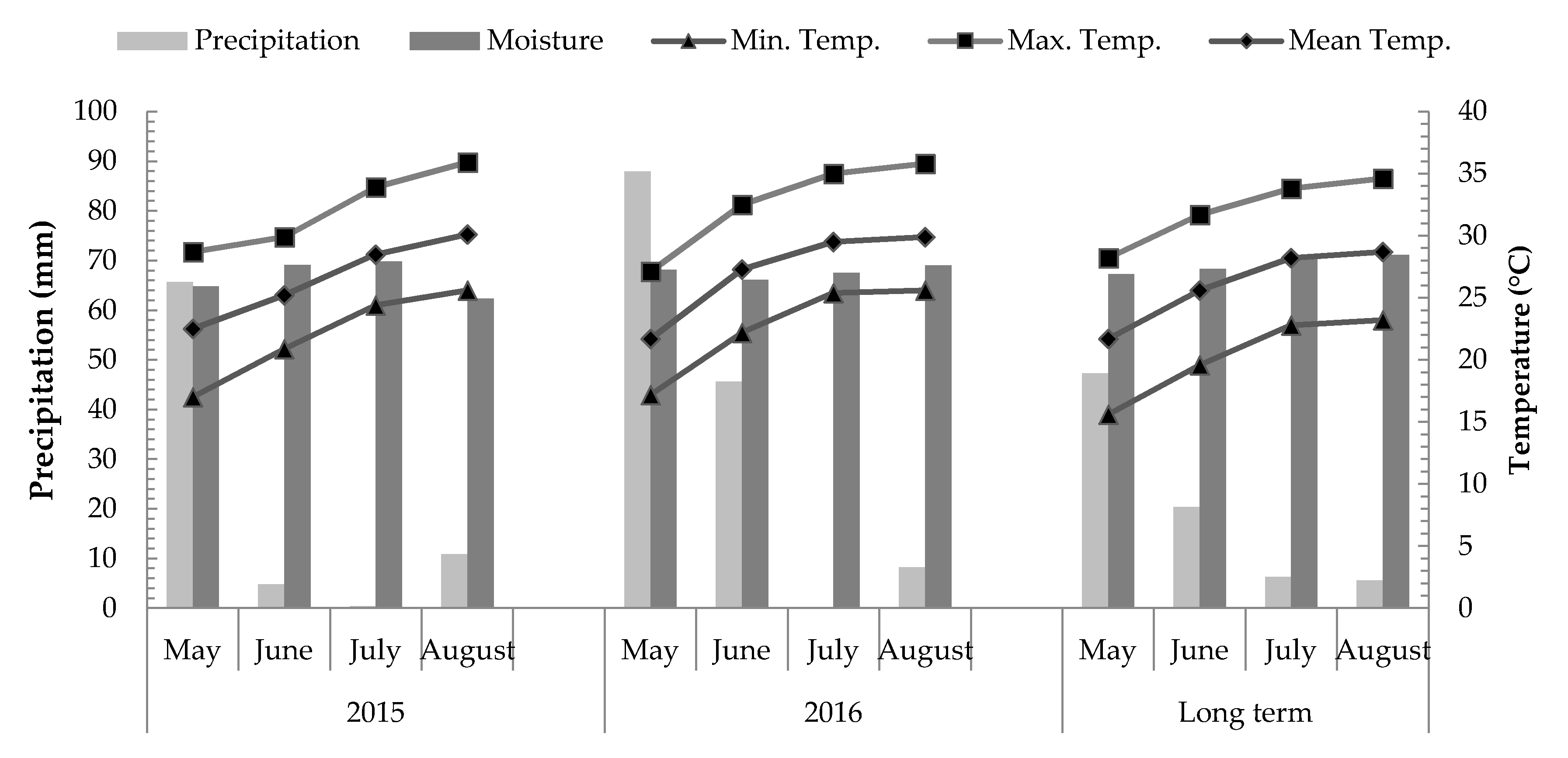
2.2. Sample Preparation, Measurements, and Calculations
× DBY (t ha−1) × 1.11 × 0.85 × 0.51 × 0.85 × 1000/0.79
- 1.11 (conversion factor of sugar from cellulose and hemicellulose);
- 0.85 (process efficiency of sugar from cellulose and hemicellulose);
- 0.51 (conversion factor of sugar from ethanol);
- 0.85 (process efficiency of ethanol from sugar);
- 1000/0.79 (specific gravity of ethanol, g ml−1).
2.3. Statistical Analysis
3. Results
3.1. Dry Matter Yield and Bioethanol Production
3.2. Bagasse Combustion Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zegada-Lizarazu, W.; Monti, A. Are we ready to cultivate sweet sorghum as a bioenergy feedstock? A review on field management practices. Biomass Bioenergy 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Da Silva, T.M.; de Oliveira, A.B.; de Moura, J.G.; da Trindade Lessa, B.F.; de Oliveira, L.S.B. Potential of Sweet Sorghum Juice as a Source of Ethanol for Semi-arid Regions: Cultivars and Spacing Arrangement Effects. Sugar Tech 2019, 21, 145–152. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A.; Wyman, C.E. Refining sweet sorghum to ethanol and sugar: Economic trade-offs in the context of North China. Bioresour. Technol. 2005, 96, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, R.; Bressani, R.; Rolz, C. The potential of sweet sorghum as a source of ethanol and protein. Energy Sustain. Dev. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Dar, R.A.; Dar, E.A.; Kaur, A.; Phutela, U.G. Sweet sorghum-a promising alternative feedstock for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 4070–4090. [Google Scholar]
- Doggett, H. Sorghum, 2nd ed.; Tropical Agricultural Series; Longman Scientific: Essex, UK, 1988. [Google Scholar]
- Habyarimana, E.; Lorenzoni, C.; Laureti, D.; Di Fonzo, N. Biomass production and drought resistance at the seedling stage and in field conditions in sorghum. Maydica [Sorghum bicolor L.]. Maydica 2002, 47, 305–309. (In Italian) [Google Scholar]
- Rao, M.M.; Govindasamy, R.; Chandrasekaran, S. Effect of humic acid on Sorghum vulgare var. CSH-9. Curr. Sci. 1987, 56, 1273–1276. [Google Scholar]
- Reddy, B.V.S.; Rao, P.S.; Kumar, A.A.; Reddy, P.S.; Rao, P.P.; Sharma, K.K.; Blummel, M.; Reddy, C.R. Sweet sorghum as a biofuel crop where are we now. In Proceedings of the 6th International Biofuels Conference; ICRISAT: Hyderabad, India, 2009; pp. 193–202. [Google Scholar]
- Singh, M.P.; Erickson, J.E.; Sollenberger, L.E.; Woodard, K.R.; Vendramini, J.M.B.; Fedenko, J.R. Mineral composition and biomass partitioning of sweet sorghum grown for bioenergy in the southeastern USA. Biomass Bioenergy 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Goshadrou, A.; Karimi, K.; Taherzadeh, M.J. Bioethanol production from sweet sorghum bagasse by Mucor hiemalis. Ind. Crops Prod. 2011, 34, 1219–1225. [Google Scholar] [CrossRef]
- Goldemberg, J.; Guardabassi, P. The potential for first-generation ethanol production from sugarcane. Biofuels Bioprod. Biorefin. Innov. Sustain. Econ. 2010, 4, 17–24. [Google Scholar] [CrossRef]
- Monti, A.; Venturi, G. Comparison of the energy performance of fibre sorghum, sweet sorghum and wheat monocultures in northern Italy. Eur. J. Agron. 2003, 19, 35–43. [Google Scholar] [CrossRef]
- Lewandowski, I.; Kicherer, A. Combustion quality of biomass: Practical relevance and experiments to modify the biomass quality of Miscanthus × giganteus. Eur. J. Agron. 1997, 6, 163–177. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Angelini, L.G.; Bonari, E. Influence of fertilization and harvest time on fuel quality of giant reed (Arundo donax L.) in Central Italy. Eur. J. Agron. 2010, 32, 219–227. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lewandowski, I. Inter-annual variation in biomass combustion quality traits over five years in fifteen Miscanthus genotypes in south Germany. Fuel Process. Technol. 2014, 121, 47–55. [Google Scholar] [CrossRef]
- Demirbas, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Singh, S.P.; Joshi, Y.P.; Singh, R.K.; Meena, V.S. Influence of nitrogen levels and seed rate on growth, yield and quality of sweet sorghum. Ann. Biol. 2014, 30, 89–93. [Google Scholar]
- Reddy, P.S.; Reddy, B.V.; Ashok Kumar, A.; Rao, P.S. Standardization of nitrogen fertilizer rate for sugar yield optimization in sweet sorghum. J. SAT Agric. Res. 2008, 6, 1–4. [Google Scholar]
- Holou, R.A.; Stevens, G. Impact of nitrogen fertilization and the soil type on the quality and yield of sweet sorghum juice. In Proceedings of the XVI International Plant Nutrition Colloquium, Sacramento, CA, USA, 26–30 August 2009. [Google Scholar]
- Mekdad, A.A.A.; El-Sherif, A.M.A. The Effect of Nitrogen and Potassium Fertilizers on Yield and Quality of Sweet Sorghum Varieties under Arid Regions Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 811–823. [Google Scholar] [CrossRef]
- Tomar, G.S.; Sai, S. Effect of planting density and levels of nitrogen on ethanol production of sweet sorghum (Sorghum bicolor [L.] Moench) varieties. Pharma Innov. J. 2018, 7, 4–7. [Google Scholar]
- Sawargaonkar, G.L.; Patil, M.D.; Wani, S.P.; Pavani, E.; Reddy, B.V.S.R.; Marimuthu, S. Nitrogen response and water use efficiency of sweet sorghum cultivars. Field Crop. Res. 2013, 149, 245–251. [Google Scholar] [CrossRef]
- Maw, M.J.; Houx, J.H., III; Fritschi, F.B. Sweet sorghum ethanol yield component response to nitrogen fertilization. Ind. Crops Prod. 2016, 84, 43–49. [Google Scholar] [CrossRef]
- Turgut, I.; Bilgili, U.; Duman, A.; Acikgoz, E. Production of sweet sorghum (Sorghum bicolor L. Moench) increases with increased plant densities and nitrogen fertilizer levels. Acta Agric. Scand. Sect. B Soil Plant 2005, 55, 236–240. [Google Scholar]
- Adams, C.B.; Erickson, J.E.; Singh, M.P. Investigation and synthesis of sweet sorghum crop responses to nitrogen and potassium fertilization. Field Crop. Res. 2015, 178, 1–7. [Google Scholar] [CrossRef]
- Amaducci, S.; Monti, A.; Venturi, G. Non-structural carbohydrates and fibre components in sweet and fibre sorghum as affected by low and normal input techniques. Ind. Crops Prod. 2004, 20, 111–118. [Google Scholar] [CrossRef]
- Sowiński, J.; Głąb, L. The effect of nitrogen fertilization management on yield and nitrate contents in sorghum biomass and bagasse. Field Crop. Res. 2018, 227, 132–143. [Google Scholar] [CrossRef]
- Tang, C.; Yang, X.; Chen, X.; Ameen, A.; Xie, G. Sorghum biomass and quality and soil nitrogen balance response to nitrogen rate on semiarid marginal land. Field Crop. Res. 2018, 215, 12–22. [Google Scholar] [CrossRef]
- Verlinden, G.; Coussens, T.; De Vliegher, A.; Baert, G.; Haesaert, G. Effect of humic substances on nutrient uptake by herbage and on production and nutritive value of herbage from sown grass pastures. Grass Forage Sci. 2010, 65, 133–144. [Google Scholar] [CrossRef]
- Akinremi, O.O.; Janzen, H.H.; Lemke, R.L.; Larney, F.J. Response of canola, wheat and green beans to leonardite additions. Can. J. Soil Sci. 2000, 80, 437–443. [Google Scholar] [CrossRef]
- Hamad, M.M.; Tantawy, M.F. Effect of different Humic Acids Sources on the Plant Growth, Cal-cium and Iron Utilization by Sorghum. Egypt. J. Soil Sci. 2018, 58, 291–307. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Facanha-Okorokova, A.L.; Facanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H -ATPase activity in maize roorts. Plant Physiol. 2002, 30, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Akinci, S.; Buyukkeskin, T.; Eroglu, A.; Erdogan, B.E. The Effect of Humic Acid on Nutrient Composition in Broad Bean (Vicia faba L.). Roots Not. Sci. Biol. 2009, 1, 81–87. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; El-Greadly, N.H.M.; Helmy, Y.I.; Singer, S.M. Responses of tomato plants to different rates of humic-based fertilizer and NPK fertilization. J. Appl. Sci. Res. 2007, 3, 169–174. [Google Scholar]
- Moghadam, H.R.T.; Khamene, M.K.; Zahedi, H. Effect of humic acid foliar application on growth and quantity of corn in irrigation withholding at different growth stages. Maydica 2014, 59, 124–128. [Google Scholar]
- Ece, A.; Saltali, K.; Eryigit, N.; Uysal, F. The effects of leonardite applications on climbing bean (Phaseolus vulgaris L.) yield and soil properties. J. Agron. 2007, 6, 480–483. [Google Scholar]
- Demir, M.; Noyan, O.F.; Oğuz, I. Leonardit kullanımı ile birlikte azaltılmış azotlu gübre uygulamalarının bitki verim ve toprak özellikleri üzerine etkileri. Sak. Ünive. Fen Edeb. Derg. 2012, 1, 445–455. [Google Scholar]
- Selim, E.M.; El-Neklawy, A.S.; El-Ashry, S.M. Beneficial effects of humic substances on soil fertility to fertigated potato grown on sandy soil. Libyan Agric. Res. Cent. J. Int. 2010, 1, 255–262. [Google Scholar]
- Magdi, T.A.; Selim, E.M.; El-Ghamry, A.M. Integrated effects of bio and mineral fertilizers and humic substances on growth, yield and nutrient contents of fertigated cowpea (Vigna unguiculata L.) grown on sandy soils. J. Agron. 2011, 10, 34–39. [Google Scholar]
- Nazli, R.I.; Kusvuran, A.; Inal, I.; Demirbas, A.; Tansi, V. Effects of different organic materials on forage yield and quality of silage maize (Zea mays L.). Turk. J. Agric. For. 2014, 38, 23–31. [Google Scholar] [CrossRef]
- Almodares, A.; Hadi, M.R.; Ranjbar, M.; Taheri, R. The effects of nitrogen treatments, cultivars and harvest stages on stalk yield and sugar content in sweet sorghum. Asian J. Plant Sci. 2007, 6, 423–426. [Google Scholar]
- Thivierge, M.N.; Chantigny, M.H.; Bélanger, G.; Seguin, P.; Bertrand, A.; Vanasse, A. Response to nitrogen of sweet pearl millet and sweet sorghum grown for ethanol in eastern Canada. Bioenergy Res. 2015, 8, 807–820. [Google Scholar] [CrossRef]
- Akin, A. The Effects of Some Summer Pruning and Humic Substance Applications on the Nutritional Value of ‘Alphonse Lavallée’ Grape Cultivar. Erwerbs-Obstbau 2018, 60, 271–274. [Google Scholar] [CrossRef]
- Gezgin, S.; Dursun, N.; Yilmaz, G. Bitki Yetiştiriciliğinde humik ve fulvik asit kaynağı olan Tki-humas kullanımı. Sak. Üniv. Fen Edeb. Derg. 2012, 1, 159–163. [Google Scholar]
- Wortmann, C.S.; Liska, A.J.; Ferguson, R.B.; Lyon, D.J.; Klein, R.N.; Dweikat, I. Dryland performance of sweet sorghum and grain crops for biofuel in Nebraska. Agron. J. 2010, 102, 319–326. [Google Scholar] [CrossRef]
- Teetor, V.H.; Duclos, D.V.; Wittenberg, E.T.; Young, K.M.; Chawhuaymak, J.; Riley, M.R.; Ray, D.T. Effects of planting date on sugar and ethanol yield of sweet sorghum grown in Arizona. Ind. Crops Prod. 2011, 34, 1293–1300. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. 2. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Hodgson, E.M.; Lister, S.J.; Bridgwater, A.V.; Clifton-Brown, J.; Donnison, I.S. Genotypic and environmentally derived variation in the cell wall composition of Miscanthus in relation to its use as a biomass feedstock. Biomass Bioenergy 2010, 34, 652–660. [Google Scholar] [CrossRef]
- Institution of Japan Energy (Ed.) Biomass Handbook; Hua, Z.Z.; Shi, Z.P., Translators; Chemistry Industry Press: Beijing, China, 2006; pp. 166–167, 172. [Google Scholar]
- Zhao, Y.L.; Dolat, A.; Steinberger, Y.; Wang, X.; Osman, A.; Xie, G.H. Biomass yield and changes in chemical composition of sweet sorghum cultivars grown for biofuel. Field Crop. Res. 2009, 111, 55–64. [Google Scholar] [CrossRef]
- Kurai, T.; Morey, S.R.; Wani, S.P.; Watanabe, T. Efficient rates of nitrogenous fertiliser for irrigated sweet sorghum cultivation during the post-rainy season in the semi-arid tropics. Eur. J. Agron. 2015, 71, 63–72. [Google Scholar] [CrossRef]
- Erickson, J.E.; Woodard, K.R.; Sollenberger, L.E. Optimizing sweet sorghum production for biofuel in the southeastern USA through nitrogen fertilization and top removal. Bioenergy Res. 2012, 5, 86–94. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Mantineo, M.; Testa, G. Water and nitrogen balance of sweet sorghum (Sorghum bicolor moench (L.)) cv. Keller under semi-arid conditions. Ind. Crops Prod. 2012, 36, 329–342. [Google Scholar] [CrossRef]
- Carpenter-Boggs, L.; Pikul, J.L.; Vigil, M.F.; Riedell, W.E. Soil nitrogen mineralization influenced by crop rotation and nitrogen fertilization. Soil Sci. Soc. Am. J. 2000, 64, 2038–2045. [Google Scholar] [CrossRef]
- Villaseñor, D.; Zagal, E.; Stolpe, N.; Hirzel, J. Relationship between mineralized nitrogen during anaerobic incubations and residual effect of nitrogen fertilization in two rice paddy soils in Chile. Chil. J. Agric. Res. 2015, 75, 98–104. [Google Scholar] [CrossRef]
- Cai, A.; Xu, H.; Shao, X.; Zhu, P.; Zhang, W.; Xu, M.; Murphy, D.V. Carbon and nitrogen mineralization in relation to soil particle-size fractions after 32 years of chemical and manure application in a continuous maize cropping system. PLoS ONE 2016, 11, e0152521. [Google Scholar] [CrossRef][Green Version]
- Barker, A.V.; Bryson, G.M. Section II, Essential elements-macronutrients. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 21–43. [Google Scholar]
- Prasad, R.; Power, J.F. Section VIII, Nitrogen, in Soil Fertility Management for Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 1997; pp. 115–169. [Google Scholar]
- Koca. Potential Use of Preplant Soil Mineral Nitrogen Content in First Crop Corn Fertilization Programe in Cukurova Region. Master’s Dissertation, Cukurova University, Adana, Turkey, 2013. [Google Scholar]
- Pamiralan, H.; Gok, M. Bakla Vejetasyonu Altında Nitrifikasyon İnhibitörü Uygulamasının Nodülasyon, Nodül, Kök ve Kök Üstünde Toplam Azot ve Toprakta Nmin Değerlerine Etkisi. Sdü Ziraat Fakültesi Derg. 2018, 3, 519–527. [Google Scholar]
- Kizil, S.; Tansi, V.; Avci, M. An investigation on determining the most suitable nitrogen and zinc fertilizer doses for the main crops of Sorghum-Sudangrass Hybrids(Sorghum bicolor × Sorghum sudanense) in the Cukurova Region (Turkey). Biol. Divers. Conserv. 2010, 3, 117–122. [Google Scholar]
- Ozkan, A.; Ulger, A.C. Çukurova Ekolojik Koşullarında Değişik Azot Dozu Uygulamalarının İki Cin Mısırı (Zea mays L. everta Sturt.) Çeşidinde Tane Verimi ve Bazı Tarımsal Özelliklere Etkisi. Yüzüncü Yıl Üniv. Tarım Bilimleri Derg. 2011, 21, 198–208. [Google Scholar]
- Iptas, S.; Brohi, A.R. Effect of nitrogen rate and stubble height on dry matter yield, crude protein content and crude protein yield of a sorghum–sudangrass hybrid [Sorghum bicolor (L.) Moench × Sorghum sudanense (Piper) Stapf.] in the three-cutting system. J. Agron. Crop Sci. 2003, 189, 227–232. [Google Scholar] [CrossRef]
- Matsuyama, N. The effect of ample nitrogen fertilizer on cell-wall materials and its significance to rice blast disease. Jpn. J. Phytopathol. 1975, 41, 56–61. [Google Scholar] [CrossRef]
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: Evidence from solid-state nuclear magnetic resonance. Plant Physiol. 2015, 168, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C.; et al. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Ogden, M.; Hoefgen, R.; Roessner, U.; Persson, S.; Khan, G. Feeding the Walls: How Does Nutrient Availability Regulate Cell Wall Composition? Int. J. Mol. Sci. 2018, 19, 2691. [Google Scholar] [CrossRef] [PubMed]
- Blummel, M.; Zerbini, E.; Reddy, B.V.S.; Hash, C.T.; Bidinger, F.; Ravi, D. Improving the production and utilization of sorghum and pearl millet as livestock feed: Methodological problems and possible solutions. Field Crop. Res. 2003, 84, 123–142. [Google Scholar] [CrossRef]
- Sher, A.; Hassan, F.U.; Ali, H.; Hussain, M.; Sattar, A. Enhancing forage quality through appropriate nitrogen dose, seed rate and harvest stage, in sorghum cultivars grown in Pakistan. Grassl. Sci. 2017, 63, 15–22. [Google Scholar] [CrossRef]
- Chen, Y.; Aviad, T. Effect of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences: Selected Readings; MacCarthy, P., Ed.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 161–186. [Google Scholar]
- Tan, K.H. Section VIII, Agronomic importance of humic matter. In Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Ahmed, A.H.; Nesiem, M.R.; Hewedy, A.M.; Sallam, H. Effect of some simulative compounds on growth, yield and chemical composition of snap bean plants grown under calcareous soil conditions. J. Am. Sci. 2010, 6, 552–569. [Google Scholar]
- Kumar, S.R.; Shrotria, P.K.; Deshmukh, J.P. Characterizing nutrient management effect on yield of sweet sorghum genotypes. World J. Agric. Sci. 2008, 4, 787–789. [Google Scholar]
- Uchino, H.; Watanabe, T.; Ramu, K.; Sahrawat, K.L.; Marimuthu, S.; Wani, S.P.; Ito, O. Effects of nitrogen application on sweet sorghum (Sorghum bicolor (L.) Moench) in the semi-arid tropical zone of India. Jpn. Agric. Res. Q. JARQ 2013, 47, 65–73. [Google Scholar] [CrossRef][Green Version]
- Holou, R.A.; Stevens, G. Juice, sugar, and bagasse response of sweet sorghum (Sorghum bicolor (L.) Moench cv. M81E) to N fertilization and soil type. GCB Bioenergy 2012, 4, 302–310. [Google Scholar] [CrossRef]
- Merlo, L.; Ghisi, R.; Passera, C.; Rascio, N. Effects of humic substances on carbohydrate metabolism of maize leaves. Can. J. Plant Sci. 1991, 71, 419–425. [Google Scholar] [CrossRef]
- Govindasmy, R.; Chandrasekaran, S. Effect of humic acids on the growth, yield and nutrient content of sugarcane. Sci. Total Environ. 1992, 117, 575–581. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.Z.; Hussain, F.; Akhtar, M.E.; Gurmani, A.R.; Khan, S. Effect of humic acid on the growth, yield, nutrient composition, photosynthetic pigment and total sugar contents of peas (Pisum sativum L.). J. Chem. Soc. Pak. 2013, 35, 206–211. [Google Scholar]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Nazli, R.I.; Tansi, V. Influences of nitrogen fertilization and harvest time on combustion quality of four perennial grasses in a semi-arid Mediterranean climate. Ind. Crops Prod. 2019, 128, 239–247. [Google Scholar] [CrossRef]
- Chen, Y.; De Nobili, M.; Aviad, T. Section IV. Stimulatory effects of humic substances on plant growth. In Soil Organic Matter in Sustainable Agriculture; Magdoff, F., Weil, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 103–129. [Google Scholar]
- Tipping, E. Cation Binding by Humic Substances; Cambridge University Press: Cambridge, UK, 2002; Volume 12. [Google Scholar]
- Kulikova, N.A.; Stepanova, E.V.; Koroleva, O.V. Mitigating activity of humic substances: Direct influence on biota. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2005; pp. 285–309. [Google Scholar]
- Garcia, A.C.; Santos, L.A.; de Souza, L.G.A.; Tavares, O.C.H.; Zonta, E.; Gomes, E.T.M.; Mina, J.M.G.; Berbara, R.L.L. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J. Plant Physiol. 2016, 192, 56–63. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Shawkat, A.; et al. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef]
- Afifi, M.M.I.; El-Sayed, G.A.M.; Manal, A.; El-Gamal, H.; Massoud, O.N. Synergistic effect of biofertilizers containing N-fixer, P and K solubilizers and humic substances on Sorghum bicolor productivity. Middle East J. Appl. Sci. 2014, 4, 1065–1074. [Google Scholar]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Katkat, A.V.; Çelik, H.; Turan, M.A.; Asik, B.B. Effects of soil and foliar applications of humic substances on dry weight and mineral nutrients uptake of wheat under calcareous soil conditions. Aust. J. Basic Appl. Sci. 2009, 3, 1266–1273. [Google Scholar]
- Rashad, R.T.; Hussien, R.A. Studying the Solubility, Availability, and Uptake of Silicon (Si) from Some Ore Minerals in Sandy Soil. Sains Tanah J. Soil Sci. Agroclimatol. 2018, 15, 69–82. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 2. Combustion. Bioresour. Technol. 2009, 100, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.C.; Li, W.Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Barnthaler, G. Chemical properties of solid biofuels–significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.L.; Miles, T.R. Combustion properties of biomass. Fuel Proc. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
| Property | Depth (0–30 cm) |
|---|---|
| Texture | Loam |
| Organic matter (%) | 0.81 |
| pH | 8 |
| Salt (mmhos cm−1) | 0.01 |
| Lime (%) | 11.11 |
| Available P (mg kg−1) | 25 |
| Available K (mg kg−1) | 1992 |
| Available Fe (mg kg−1) | 4.34 |
| Available Zn (mg kg−1) | 0.14 |
| Available Cu (mg kg−1) | 7.14 |
| Available Mn (mg kg−1) | 2.14 |
| Measurements | Y | N | Y × N | HS | Y × HS | N × HS | Y × N × HS |
|---|---|---|---|---|---|---|---|
| DMY | ** | ** | ns | * | ns | ns | ns |
| FSY (t ha−1) | ** | ** | ns | ** | ns | ns | ns |
| JR (%) | ns | ** | ns | ns | ns | ns | ns |
| JY (kg ha−1) | ** | ** | ns | ** | ns | ns | ns |
| Brix (%) | ns | ns | ns | ns | ns | ns | ns |
| SY (kg ha−1) | ** | ** | ns | ** | ns | ns | ns |
| JEY (L ha−1) | ** | ** | ns | ** | ns | ns | ns |
| DBY (t ha−1) | ** | ** | ns | ns | ns | ns | ns |
| Lignin (%) | ** | ** | ** | ** | ** | ** | ** |
| Cellulose (%) | ns | ** | ** | ** | ** | ** | ** |
| Hemicellulose (%) | ** | ** | ** | ** | ** | ** | ** |
| LEY (L ha−1) | ** | ** | ns | * | ns | ns | ns |
| TEY (L ha−1) | * | ** | ns | * | ns | ns | ns |
| Bagasse N (g kg−1) | ** | ** | ** | ** | ** | ** | ** |
| Bagasse P (g kg−1) | ** | ** | ns | ** | ns | ** | ** |
| Bagasse K (g kg−1) | ns | ** | ns | ** | ns | ** | ns |
| Bagasse Ca (g kg−1) | ns | ns | ns | ns | ns | ns | ns |
| Bagasse Mg (g kg−1) | ns | ns | ** | ** | ** | ** | ** |
| Bagasse S (g kg−1) | * | * | ** | ** | * | ** | ns |
| Bagasse Si (g kg−1) | * | ** | ns | ** | ** | ** | ns |
| Bagasse Moisture (%) | ** | ns | ns | ns | ns | ns | ns |
| Bagasse Ash (%) | ns | ** | ns | ** | ns | ** | ns |
| Bagasse LHV (MJ kg−1) | ns | ** | ** | ** | ** | ** | ** |
| HS Levels (L ha−1) | N Levels (kg ha−1) | |||
|---|---|---|---|---|
| 100 | 150 | 200 | Mean | |
| 0 | 7.7 | 10.5 | 10.7 | 9.7 b |
| 15 | 10.7 | 10.9 | 10.9 | 10.8 a |
| 30 | 10.6 | 9.8 | 11.3 | 10.6 ab |
| 45 | 10.2 | 11.5 | 12.2 | 11.3 a |
| Mean | 9.8 b | 10.7 a | 11.3 a | |
| HS Levels (L ha−1) | N Levels (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | Mean | 100 | 150 | 200 | Mean | |
| FSY (t ha−1) | JR (%) | |||||||
| 0 | 28.4 | 39.7 | 42.3 | 36.8 b | 21.43 | 24.80 | 24.41 | 23.55 |
| 15 | 39.3 | 39.9 | 44.5 | 41.2 a | 25.93 | 27.59 | 23.81 | 25.77 |
| 30 | 41.0 | 44.1 | 46.9 | 44.0 a | 24.14 | 25.30 | 26.43 | 25.29 |
| 45 | 36.8 | 41.9 | 49.2 | 42.6 a | 22.38 | 23.77 | 25.68 | 23.94 |
| Mean | 36.4 c | 41.4 b | 45.7 a | 23.47 b | 25.37 a | 25.08 a | ||
| JY (t ha−1) | Brix (%) | |||||||
| 0 | 6.2 | 10.0 | 10.2 | 8.8 b | 16.42 | 16.40 | 16.22 | 16.34 |
| 15 | 10.3 | 11.1 | 10.6 | 10.7 a | 17.37 | 16.83 | 16.87 | 17.02 |
| 30 | 10.1 | 11.2 | 12.5 | 11.3 a | 16.82 | 16.08 | 16.27 | 16.39 |
| 45 | 8.1 | 9.9 | 12.7 | 10.2 a | 16.35 | 16.42 | 16.30 | 16.36 |
| Mean | 8.7 c | 10.6 b | 11.5 a | 16.74 | 16.43 | 16.41 | ||
| SY (kg ha−1) | JEY (L ha−1) | |||||||
| 0 | 772.2 | 1220.0 | 1242.5 | 1078.2 b | 448.7 | 708.8 | 721.9 | 626.4 b |
| 15 | 1327.7 | 1382.6 | 1334.3 | 1348.2 a | 771.4 | 803.3 | 775.2 | 783.3 a |
| 30 | 1268.2 | 1353.8 | 1493.6 | 1371.9 a | 736.8 | 787.6 | 867.8 | 797.1 a |
| 45 | 999.7 | 1215.4 | 1539.2 | 1251.4 a | 580.8 | 706.1 | 894.3 | 727.1 a |
| Mean | 1092.0 b | 1292.9 a | 1402.4 a | 634.4 b | 751.2 a | 814.8 a | ||
| DBY (t ha−1) | Lignin (%) | |||||||
| 0 | 4.7 | 6.7 | 7.0 | 6.1 | 11.15 k | 11.66 i | 13.12 b | 11.97 b |
| 15 | 6.7 | 6.8 | 7.6 | 7.1 | 11.33 j | 11.86 h | 12.79 d | 11.99 b |
| 30 | 6.7 | 6.8 | 7.3 | 6.9 | 12.39 f | 13.41 a | 12.92 c | 12.90 a |
| 45 | 6.3 | 6.8 | 7.8 | 6.9 | 10.59 l | 12.56 e | 12.18 g | 11.78 c |
| Mean | 6.1 c | 6.8 b | 7.4 a | 11.36 c | 12.37 b | 12.75 a | ||
| Cellulose (%) | Hemicellulose (%) | |||||||
| 0 | 42.15 k | 44.56 f | 45.06 d | 43.92 c | 26.41 e | 27.02 b | 24.45 j | 25.96 b |
| 15 | 46.10 b | 44.42 g | 47.08 a | 45.86 a | 25.53 h | 27.46 a | 26.55 d | 26.51 a |
| 30 | 44.74 e | 46.07 b | 45.60 c | 45.47 b | 24.60 i | 24.23 k | 25.91 f | 24.91 d |
| 45 | 43.06 j | 43.36 i | 43.36 i | 43.50 d | 25.73 g | 26.85 c | 24.25 k | 25.61 c |
| Mean | 44.01 c | 44.60 b | 45.46 a | 25.57 b | 26.39 a | 25.29 c | ||
| LEY (L ha−1) | TEY (L ha−1) | |||||||
| 0 | 1650.4 | 2468.8 | 2510.4 | 2209.9 b | 2099.1 | 3177.6 | 3232.3 | 2836.3 b |
| 15 | 2501.0 | 2533.4 | 2910.1 | 2648.1 a | 3272.4 | 3336.6 | 3685.3 | 3431.4 a |
| 30 | 2415.6 | 2459.6 | 2705.8 | 2527.0 a | 3152.4 | 3246.2 | 3573.6 | 3324.1 a |
| 45 | 2229.5 | 2470.0 | 2743.1 | 2480.9 ab | 2810.3 | 3176.2 | 3637.3 | 3207.9 a |
| Mean | 2199.1 c | 2483.0 b | 2717.3 a | 2833.5 c | 3234.1 b | 3532.1 a | ||
| HS Levels (L ha−1) | N Levels (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | Mean | 100 | 150 | 200 | Mean | |
| N (g kg−1) | P (g kg−1) | |||||||
| 0 | 4.15 g | 4.22 fg | 4.75 c | 4.37 c | 0.55 c | 0.60 b | 0.50 d | 0.55 b |
| 15 | 4.45 e | 4.70 cd | 4.90 b | 4.68 b | 0.50 d | 0.55 c | 0.45 e | 0.50 c |
| 30 | 4.25 f | 4.75 c | 5.05 a | 4.68 b | 0.60 b | 0.40 f | 0.50 d | 0.50 c |
| 45 | 4.65 d | 4.85 b | 5.05 a | 4.85 a | 0.65 a | 0.60 b | 0.50 d | 0.58 a |
| Mean | 4.38 c | 4.63 b | 4.94 a | 0.58 a | 0.54 b | 0.49 c | ||
| K (g kg−1) | Ca (g kg−1) | |||||||
| 0 | 7.95 d | 8.35 c | 7.55 e | 7.95 c | 1.05 | 1.20 | 1.10 | 1.12 |
| 15 | 8.05 d | 8.50 c | 8.35 c | 8.30 b | 1.10 | 1.05 | 1.25 | 1.13 |
| 30 | 9.20 ab | 9.00 b | 7.80 de | 8.67 a | 1.25 | 1.15 | 1.20 | 1.20 |
| 45 | 8.50 c | 9.35 a | 8.10 d | 8.63 a | 1.20 | 1.15 | 1.10 | 1.15 |
| Mean | 8.43 b | 8.80 a | 7.94 c | 1.15 | 1.14 | 1.16 | ||
| Mg (g kg−1) | S (g kg−1) | |||||||
| 0 | 0.65 d | 0.65 d | 0.61 e | 0.64 c | 0.57 c | 0.56 c | 0.56 c | 0.56 d |
| 15 | 0.65 d | 0.65 d | 0.70 c | 0.67 b | 0.56 c | 0.57 c | 0.57 c | 0.57 c |
| 30 | 0.75 b | 0.64 d | 0.76 b | 0.72 a | 0.50 d | 0.66 a | 0.60 b | 0.59 b |
| 45 | 0.71 c | 0.81 a | 0.60 e | 0.70 a | 0.67 a | 0.65 a | 0.61 a | 0.64 a |
| Mean | 0.69 | 0.69 | 0.67 | 0.58 b | 0.61 a | 0.59 b | ||
| Si (g kg−1) | Ash (g kg−1) | |||||||
| 0 | 5.45 d | 5.45 d | 5.65 d | 5.52 b | 18.16 ij | 20.50 fg | 19.37 gh | 19.34 d |
| 15 | 6.45 c | 6.55 c | 6.65 c | 6.55 a | 19.30 hi | 22.50 de | 21.36 ef | 21.06 c |
| 30 | 7.85 a | 6.40 c | 5.40 c | 6.55 a | 27.03 b | 23.36 cd | 18.06 j | 22.82 b |
| 45 | 6.60 c | 7.20 b | 5.40 d | 6.40 a | 24.07 b | 28.70 a | 19.00 hij | 23.92 a |
| Mean | 6.59 a | 6.40 b | 5.78 c | 22.14 b | 23.77 a | 19.45 c | ||
| Moisture (%) | LHV (MJ kg−1) | |||||||
| 0 | 71.02 | 72.27 | 71.96 | 71.75 | 17.96 d | 17.89 e | 18.10 c | 17.98 a |
| 15 | 71.17 | 71.16 | 71.11 | 71.14 | 17.38 i | 18.19 a | 18.13 b | 17.90 b |
| 30 | 71.50 | 72.12 | 72.01 | 71.88 | 17.95 d | 17.60 h | 17.85 f | 17.80 c |
| 45 | 70.44 | 72.24 | 70.76 | 71.15 | 17.74 g | 17.84 f | 17.38 i | 17.65 d |
| Mean | 71.03 | 71.95 | 71.46 | 17.76 b | 17.88 a | 17.87 a | ||
| Field Experiment | Region | N Levels (kg ha−1) | Parameters | Optimum N Level (kg ha−1) | Optimum Yield |
|---|---|---|---|---|---|
| Maw et al. [26] | Midwestern USA | 0, 56, 112, 168, 224 | SY | 112 | 1217 kg ha−1 |
| JY | 112 | 10,294 kg ha−1 | |||
| JEY | 112 | 692 L ha−1 | |||
| DBY | 112 | 17.6 t ha−1 | |||
| Singh et al. [20] | Uttarakhand, India | 0, 60, 120, 180 | SY | 120 | 2130 kg ha−1 |
| JEY | 120 | 6461.11 L ha−1 | |||
| Kurai et al. [55] | Patancheru, India | 0, 63, 90, 150 | SY | 90 | 5300 kg ha−1 |
| JEY | 90 | 2900 L ha−1 | |||
| Thivierge et al. [46] | Quebec, Canada | 0, 40, 80, 120, 160 | JEY | 80 | 865–1539 L ha−1 |
| Tang et al. [31] | Northern China | 0, 60, 120, 240 | JEY | 120 | 3089 L ha−1 |
| DMY | 60 | 8.6 t ha−1 | |||
| Erickson et al. [56] | Florida, USA | 45, 90, 135, 180 | DMY | 45 | 15 t ha−1 |
| Cosentino et al. [57] | Southern Italy | 0, 60, 120, 180 | DMY | 0 | 17.9 t ha−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazli, R.I.; Tansi, V.; Gulnaz, O.; Kafkas, E.; Kusvuran, A.; Ozturk, H.H.; Bostan Budak, D. Interactive Effects of Nitrogen and Humic Substances Applications on Bioethanol Production from Sweet Sorghum and Combustion Characteristics of Its Bagasse. Agronomy 2020, 10, 1397. https://doi.org/10.3390/agronomy10091397
Nazli RI, Tansi V, Gulnaz O, Kafkas E, Kusvuran A, Ozturk HH, Bostan Budak D. Interactive Effects of Nitrogen and Humic Substances Applications on Bioethanol Production from Sweet Sorghum and Combustion Characteristics of Its Bagasse. Agronomy. 2020; 10(9):1397. https://doi.org/10.3390/agronomy10091397
Chicago/Turabian StyleNazli, Recep Irfan, Veyis Tansi, Osman Gulnaz, Ebru Kafkas, Alpaslan Kusvuran, Hasan Huseyin Ozturk, and Dilek Bostan Budak. 2020. "Interactive Effects of Nitrogen and Humic Substances Applications on Bioethanol Production from Sweet Sorghum and Combustion Characteristics of Its Bagasse" Agronomy 10, no. 9: 1397. https://doi.org/10.3390/agronomy10091397
APA StyleNazli, R. I., Tansi, V., Gulnaz, O., Kafkas, E., Kusvuran, A., Ozturk, H. H., & Bostan Budak, D. (2020). Interactive Effects of Nitrogen and Humic Substances Applications on Bioethanol Production from Sweet Sorghum and Combustion Characteristics of Its Bagasse. Agronomy, 10(9), 1397. https://doi.org/10.3390/agronomy10091397





