Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. The Origin of the Biological Material
2.2. Molecular and Genetic Diversity Analyses
2.3. Genetic and Cluster Analysis
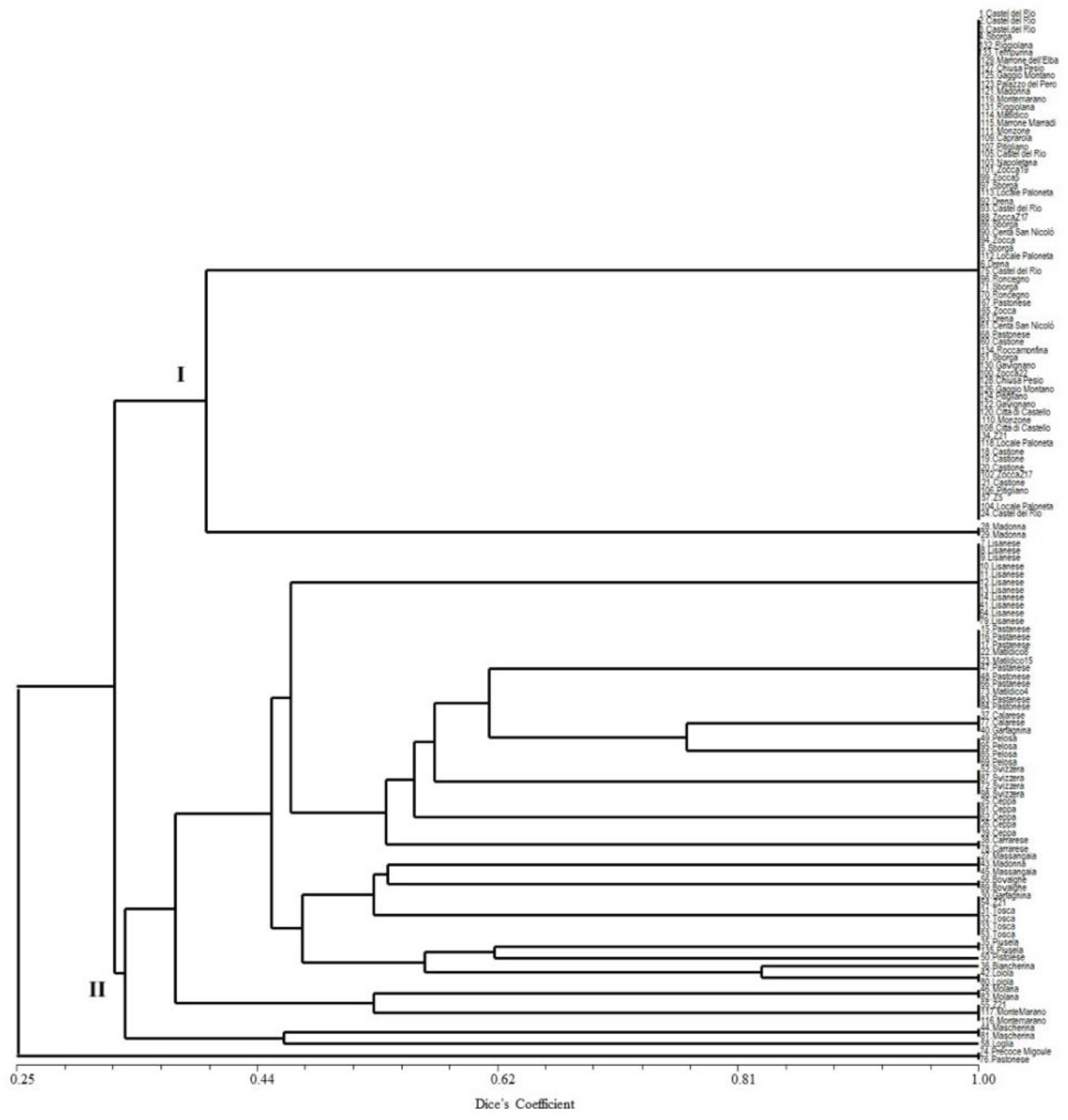
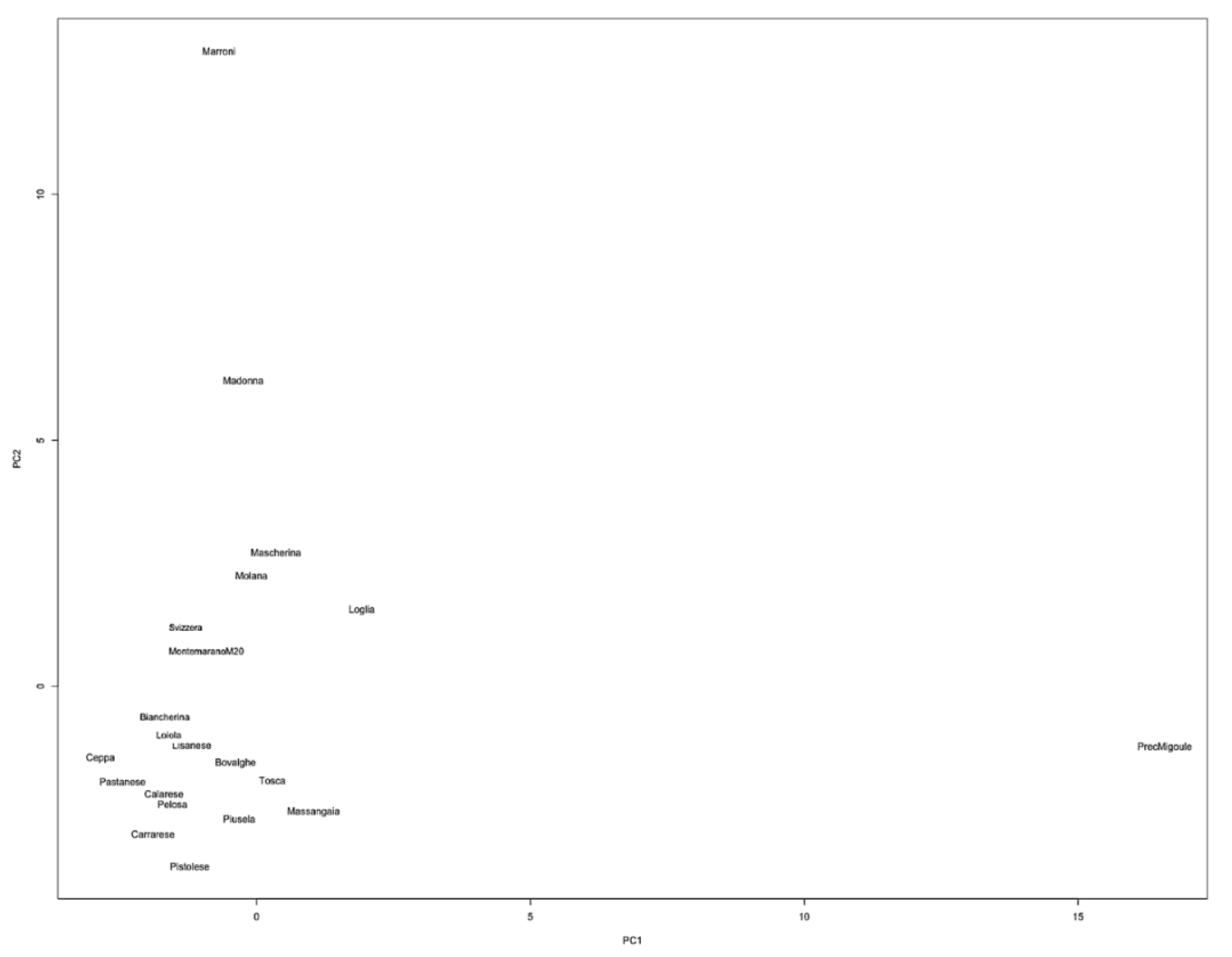
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conedera, M.; Tinner, W.; Krebs, P. Castanea sativa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 78–79. [Google Scholar]
- Krebs, P.; Pezzatti, G.B.; Beffa, G.; Tinner, W.; Conedera, M. Revising the sweet chestnut (Castanea sativa Mill.) refugia history of the last glacial period with extended pollen and macrofossil evidence. Quat. Sci. Rev. 2019, 206, 111–128. [Google Scholar] [CrossRef]
- Kaltenrieder, P.; Procacci, G.; Vannière, B.; Tinner, W. Vegetation and fire history of the euganean Hills (Colli Euganei) as recorded by Late glacial and Holocene sedimentary series from Lago della Costa (northeastern Italy). Holocene 2010, 20, 679–695. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–179. [Google Scholar] [CrossRef]
- Bassi, D.; Marangoni, B. Contributo allo studio varietale del castagno da frutto (Castanea sativa Mill.): Caratteri biometrici e analisi chimico-fisiche dei frutti. Riv. Fruttic. 1984, 6, 43–46. [Google Scholar]
- Borghetti, M.; Giannini, R.; Nocentini, C. Indagini preliminari sulla variazione di alcuni caratteri del frutto in popolazioni di “Marrone Fiorentino”. Monti Boschi 1983, 1, 49–52. [Google Scholar]
- Breviglieri, N. Indagini ed Osservazioni Sulle Migliori Varietà Italiane di Castagno (Castanea sativa Mill.); Centro di Studio sul Castagno: Florence, Italy, 1995; Volume 2, pp. 27–166. [Google Scholar]
- Martín, M.A.; Mattioni, C.; Cherubini, M.; Taurchini, D.; Villani, F. Genetic characterisation of traditional chestnut varieties in Italy using microsatellites (SSRs) markers. Ann. Appl. Biol. 2010, 157, 37–44. [Google Scholar] [CrossRef]
- Mellano, M.G.; Beccaro, G.L.; Donno, D.; Torello Marinoni, D.; Boccacci, P.; Canterino, S.; Cerutti, A.K.; Bounous, P. Castanea spp. biodiversity conservation: Collection and characterization of the genetic diversity of an endangered species. Genet. Resour. Crop. Evol. 2012, 59, 1727–1741. [Google Scholar] [CrossRef]
- Gallesio, G. Pomona Italiana, Ossia Trattato Degli Alberi Fruttiferi; Niccolò Capurbo: Pisa, Italy, 1817. [Google Scholar]
- Antonaroli, R.; Bagnaresi, U.; Bassi, D. Indagini sulla variazione di alcuni caratteri morfologici in popolazioni di castagno da frutto nella provincia di Bologna. Monti Boschi 1984, 2, 47–50. [Google Scholar]
- Bagnaresi, U.; Bassi, D.; Casini, E.; Conticini, L.; Magnani, G.P. Contributo Alla Individuazione Delle Cultivar Di Castagno Tosco-Emiliane. In Atti del Convegno “Giornata del Castagno”; Caprese Michelangelo (Arezzo): Tuscany, Italy, 1977; pp. 165–234. [Google Scholar]
- Neri, L.; Dimitri, G.; Sacchetti, G. Chemical composition and antioxidant activity of cured chestnuts from three sweet chestnut (Castanea sativa Mill.) ecotypes from Italy. J. Food Compos. Anal. 2010, 23, 23–29. [Google Scholar] [CrossRef]
- Fideghelli, C. Aspetti pomologici e qualitativi dei materiali di propagazione. I Georgofili. 2016, 2, 55–61. [Google Scholar]
- Lucchi, E.; Frascaroli, F.; Maresi, G.; Ferretti, F.; Viaggi, D.; Pezzi, G. Gestione dei castagneti, realtà e prospettive. Ecoscienza 2016, 1, 72–73. [Google Scholar]
- Pezzi, G.; Maresi, G.; Conedera, M.; Ferrari, C. Woody species composition of chestnut stands in the Northern Apennines: The result of 200 years of changes in land use. Landsc. Ecol. 2011, 26, 1463–1476. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Costa, R.; Ramos-Cabrer, A.M.; Ribeiro, C.; Serra da Silva, C.; Manzano, G.; Barreneche, T. Variation in grafted European chestnut and hybrids by microsatellites reveals two main origins in the Iberian Peninsula. Tree Genet. Genomes 2010, 6, 701–715. [Google Scholar] [CrossRef]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages and reveals a large admixture amongst varieties of different geographic origin. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef]
- Liang, W.; Dondini, L.; De Franceschi, P.; Paris, R.; Sansavini, S.; Tartarini, S. Genetic diversity, population structure and construction of a core collection of apple cultivars from Italian germplasm. Plant Mol. Biol. Rep. 2015, 33, 458–473. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Feugey, L.; Poncet, C.; Lateur, M.; Houben, P.; Ordidge, M.; et al. Analysis of the genetic diversity and structure across a wide range of germplasm reveals prominent gene flow in apple at the European level. BMC Plant Biol. 2016, 16, 130. [Google Scholar] [CrossRef]
- Bhattarai, G.; Mehlenbacher, S.A. In silico development and characterization of tri-nucleotide simple sequence repeat markers in hazelnut (Corylus avellana L.). PLoS ONE 2017, 12, e0178061. [Google Scholar] [CrossRef]
- Ferradini, N.; Lancioni, H.; Torricelli, R.; Russi, L.; Dalla Ragione, I.; Cardinali, I.; Marconi, G.; Gramaccia, M.; Concezzi, L.; Achilli, A.; et al. Characterization and phylogenetic analysis of ancient Italian landraces of pear. Front. Plant Sci. 2017, 8, 751. [Google Scholar] [CrossRef]
- Baccichet, I.; Foria, S.; Messina, R.; Peccol, E.; Losa, A.; Fabro, M.; Gori, G.; Zandigiacomo, P.; Cipriani, G.; Testolin, R. Genetic and ploidy diversity of pear (Pyrus spp.) germplasm of Friuli-Venezia Giulia, Italy. Genet. Resour. Crop. Evol. 2020, 67, 83–96. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Bischofberger, Y.; Conedera, M.; Piattini, P.; Crovadore, J.; Chablais, R.; Rudow, A.; Hatt, S.; Ramos-Cabrer, A.M.; Barreneche, T.; et al. Reservoir of the European chestnut diversity in Switzerland. Biodivers. Conserv. 2020. [Google Scholar] [CrossRef]
- Beghè, D.; Ganino, T.; Dall’Asta, C.; Silvanini, A.; Cirlini, M.; Fabbri, A. Identification and characterization of ancient Italian chestnut using nuclear microsatellite markers. Sci. Hortic. 2013, 161, 50–57. [Google Scholar] [CrossRef]
- Quintana, J.; Contreras, A.; Merino, I.; Vinuesa, A.; Orozco, G.; Ovalle, F.; Gomez, L. Genetic characterization of chestnut (Castanea sativa Mill.) orchards and 75 traditional nut varieties in El Bierzo, a glacial refuge and major cultivation site in northwestern Spain. Tree Genet. Genomes 2015, 11. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Díaz-Hernández, M.B.; Martín, L.M.; Martín, Á. Database of European chestnut cultivars and definition of a core collection using simple sequence repeats. Tree Genet. Genomes 2017, 13, 114. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Díaz-Hernández, B.; Martín, L.M.; Robles-Loma, A.; Cáceres, Y.; Martín, A. Instant domestication process of European chestnut cultivars. Ann. Appl. Biol. 2019, 174, 74–85. [Google Scholar] [CrossRef]
- Bouffartigue, C.; Debille, S.; Fabreguette, O.; Ramos Cabrer, A.; Pereira-Lorenzo, S.; Flutre, T.; Harvengt, L. Genetic diversity and structure of sweet chestnut (Castanea sativa Mill.) in France: At the intersection between Spain and Italy. bioRxiv 2019, 792259. [Google Scholar] [CrossRef]
- Martín, M.A.; Alvarez, J.B.; Mattioni, C.; Cherubini, M.; Villani, F.; Martín, L.M. Identification and characterization of traditional chestnut varieties of southern Spain using morphological and simple sequence repeat (SSRs) markers. Ann. Appl. Biol. 2009, 154, 389–398. [Google Scholar] [CrossRef]
- Maguire, T.L.; Collins, G.G.; Sedgley, M. A modified CTAB DNA extraction procedure for plants belonging to the family proteaceae. Plant Mol. Biol. Rep. 1994, 12, 106–109. [Google Scholar] [CrossRef]
- Marinoni, D.; Akkak, A.; Bounous, G.; Edwards, K.J.; Botta, R. Development and characterization of microsatellite markers in Castanea sativa (Mill.). Mol. Breed. 2003, 11, 127–136. [Google Scholar] [CrossRef]
- Buck, E.J.; Hadonou, M.; James, C.J.; Blakesley, D.; Russell, K. Isolation and characterization of polymorphic microsatellites in European chestnut (Castanea sativa Mill.). Mol. Ecol. Notes 2003, 3, 239–241. [Google Scholar] [CrossRef]
- Gobbin, D.; Hohl, L.; Conza, L.; Jermini, M.; Gessler, C.; Conedera, M. Microsatellite-based characterization of the Castanea sativa cultivar heritage of southern Switzerland. Genome 2007, 50, 1089–1103. [Google Scholar] [CrossRef]
- Kampfer, S.; Lexer, C.; Glossl, J.; Steinkellner, H. Characterization of (GA)(n) microsatellite loci from Quercus robur. Hereditas 1998, 129, 183–186. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc Numerical Taxonomy and Multivariate Analysis System (Version 1.80); Applied Biostatistics; Exeter Publishing: Setauket, NY, USA, 1992. [Google Scholar]
- Fideghelli, C. Atlante dei Fruttiferi Autoctoni Italiani; Crea—Centro di frutticoltura: Roma, Italy, 2016. [Google Scholar]
- Urrestarazu, J.; Royo, J.B.; Santesteban, L.G.; Miranda, C. Evaluating the Influence of the Microsatellite Marker Set on the Genetic Structure Inferred in Pyrus communis L. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Lourenço Costa, R.M.; Ramos-Cabrer, A.M.; Ciordia-Ara, M.; Marques Ribeiro, C.A.; Borges, O.; Barreneche, T. Chestnut cultivar diversification process in the Iberian Peninsula, Canary Islands, and Azores. Genome 2011, 54, 301–315. [Google Scholar] [CrossRef]
- Martín, M.A.; Mattioni, C.; Molina, J.R.; Alvarez, J.A.; Cherubini, M.; Herrera, M.A.; Villani, F.; Martín, L.M. Landscape genetics structure of chestnut (Castanea sativa Mill.) in Spain. Tree Genet. Genomes 2012, 8, 127–136. [Google Scholar] [CrossRef]
- Marinoni, D.T.; Akkak, A.; Beltramo, C.; Guaraldo, P.; Boccacci, P.; Bounous, G.; Ferrara, A.M.; Ebone, A.; Viotto, E.; Botta, R. Genetic and morphological characterization of chestnut (Castanea sativa Mill.) germplasm in Piedmont (north-western Italy). Tree Genet. Genomes 2013, 9, 1017–1030. [Google Scholar] [CrossRef]
- Piccioli, L. Monografia del Castagno; Tipografia di S. Landi, Firenze; Kessinger Publishing: Montana, MT, USA, 1902; pp. 25–28. [Google Scholar]
- Antonaroli, R.; Bassi, D. Le varietà dell’Emilia-Romagna. Il Divulgatore XXII 1999, 10, 5–34. [Google Scholar]
| Locus | k | N | Ho | He | PIC |
|---|---|---|---|---|---|
| CsCAT41 | 10 | 20 | 0.750 | 0.763 | 0.710 |
| CsCAT16 | 9 | 20 | 0.900 | 0.840 | 0.795 |
| CsCAT6 | 8 | 20 | 0.900 | 0.823 | 0.773 |
| CsCAT1 | 9 | 20 | 0.750 | 0.710 | 0.662 |
| CsCAT3 | 16 | 20 | 0.850 | 0.917 | 0.885 |
| QrZAG96 | 6 | 20 | 0.750 | 0.731 | 0.674 |
| EMCs15 | 6 | 20 | 0.600 | 0.521 | 0.473 |
| EMCs38 | 13 | 20 | 0.750 | 0.842 | 0.801 |
| EMCs2 | 3 | 20 | 0.750 | 0.668 | 0.577 |
| EMCs22 | 7 | 20 | 0.750 | 0.760 | 0.703 |
| CsCAT2 | 11 | 20 | 0.800 | 0.831 | 0.794 |
| CsCAT17 | 8 | 20 | 0.750 | 0.827 | 0.780 |
| CsCAT14 | 7 | 20 | 1.000 | 0.787 | 0.730 |
| CsCAT15 | 5 | 20 | 0.650 | 0.573 | 0.499 |
| CsCAT8 | 9 | 20 | 0.900 | 0.827 | 0.779 |
| OAL | 5 | 20 | 0.350 | 0.319 | 0.300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandri, S.; Krznar, M.; Ajolfi, D.; Ramos Cabrer, A.M.; Pereira-Lorenzo, S.; Dondini, L. Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy). Agronomy 2020, 10, 1319. https://doi.org/10.3390/agronomy10091319
Alessandri S, Krznar M, Ajolfi D, Ramos Cabrer AM, Pereira-Lorenzo S, Dondini L. Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy). Agronomy. 2020; 10(9):1319. https://doi.org/10.3390/agronomy10091319
Chicago/Turabian StyleAlessandri, Sara, Mihaela Krznar, Dario Ajolfi, Ana María Ramos Cabrer, Santiago Pereira-Lorenzo, and Luca Dondini. 2020. "Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy)" Agronomy 10, no. 9: 1319. https://doi.org/10.3390/agronomy10091319
APA StyleAlessandri, S., Krznar, M., Ajolfi, D., Ramos Cabrer, A. M., Pereira-Lorenzo, S., & Dondini, L. (2020). Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy). Agronomy, 10(9), 1319. https://doi.org/10.3390/agronomy10091319







