QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva × Paragon Mapping Population of Bread Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Genotyping
2.2. Phenotyping of Seedling Resistance in Greenhouse
2.3. Adult Plant Resistance and Yield Components in Field Conditions
2.4. Statistical Analysis of Phenotypic Data and QTL Mapping
3. Results
3.1. Phenotyping Variations of Seedling and Adult Plant Resistance in Mapping Population
3.2. Correlations among SR and LR Seedling and Adult Plant Resistance and Influence of APR on the Yield-Related Traits
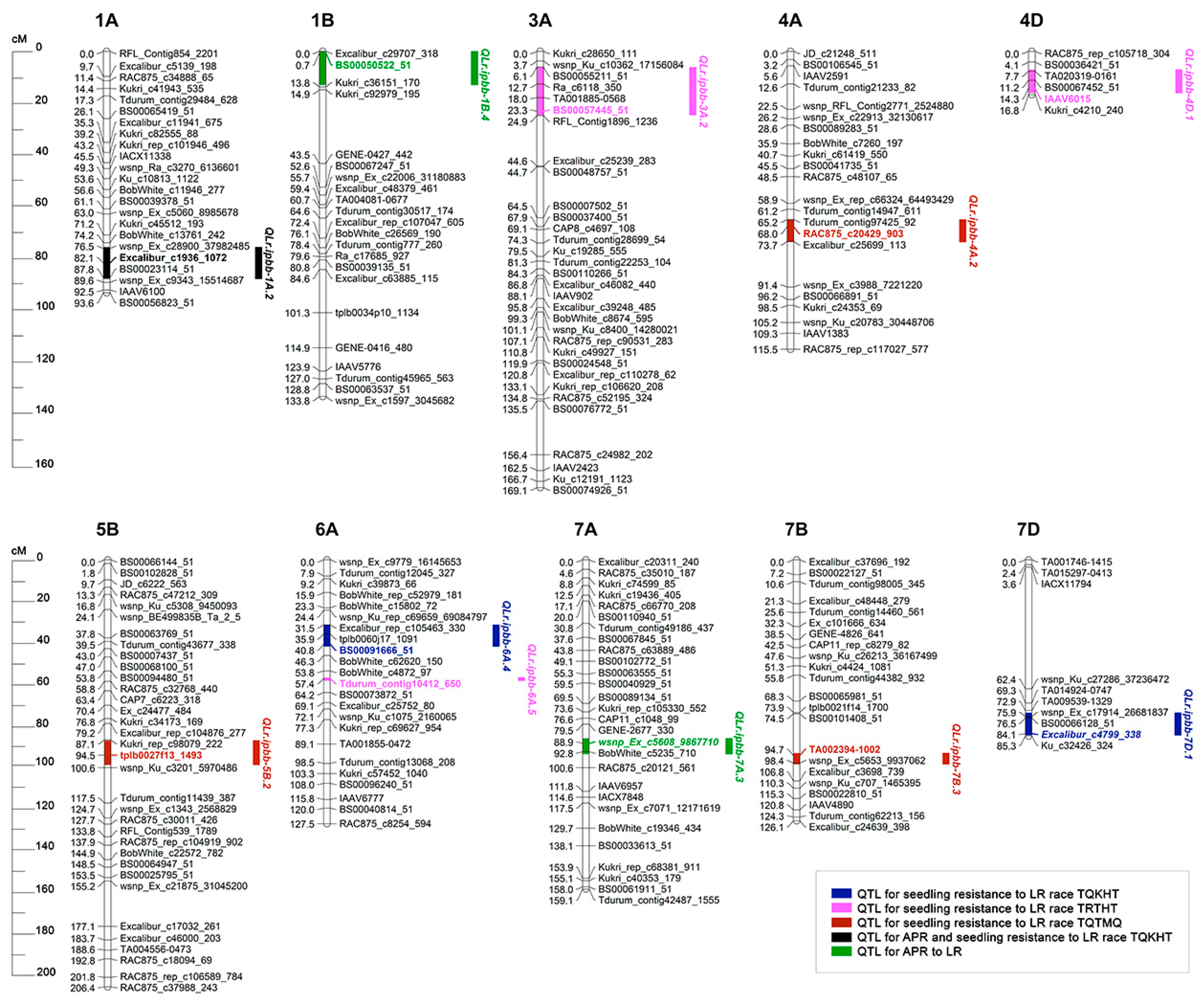
3.3. Identification of QTLs for Seedling and Adult Plant Resistance to LR in the RIL Population
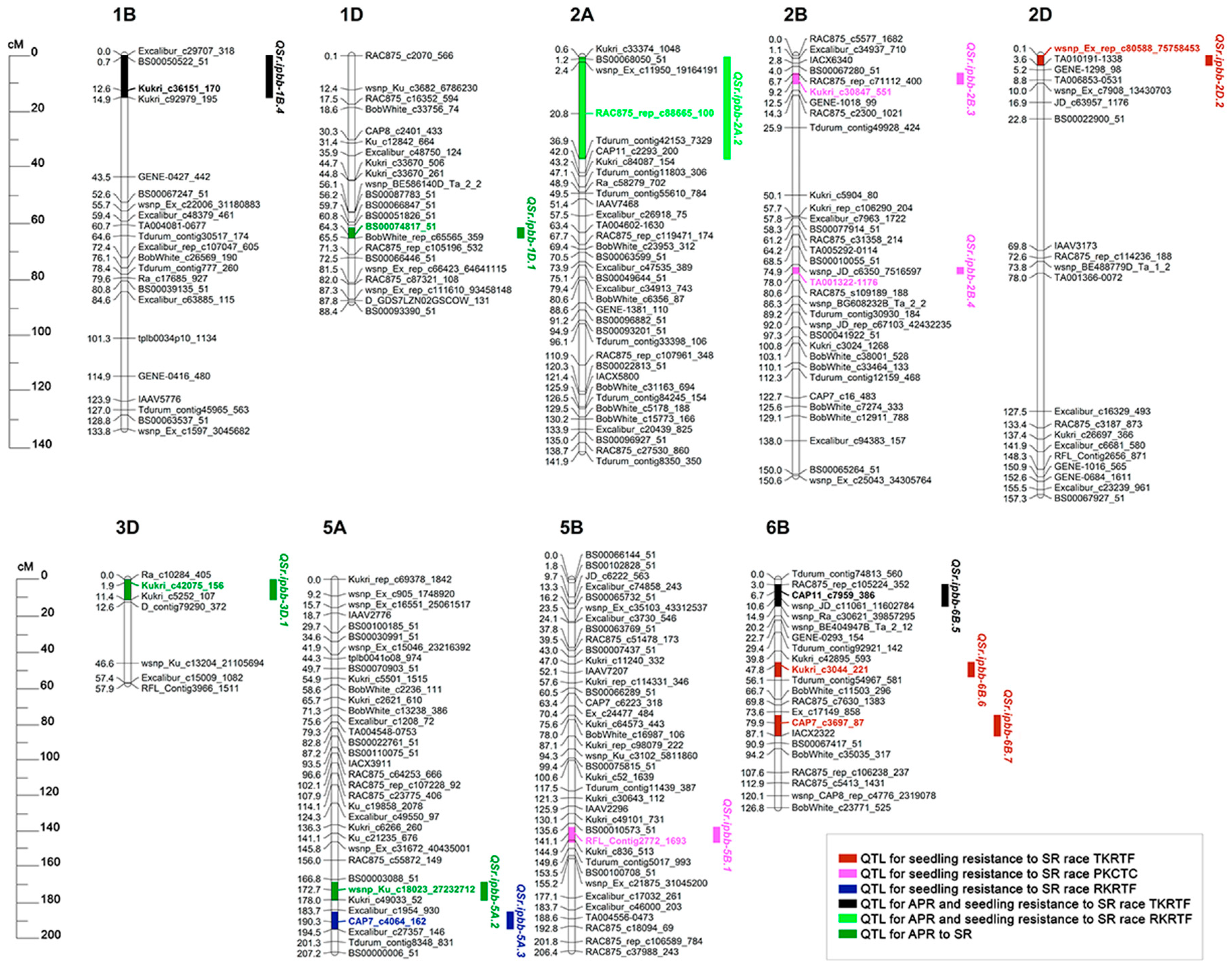
3.4. QTLs for SR Resistance at Seedling and Adult Plant Stages Identified in PA × Par Mapping Population
3.5. Comparison of Identified QTLs with Previous Works and Gene Identification
4. Discussion
4.1. General Resistance of RILs in Studied Environments
4.2. QTL Mapping for Leaf Rust Resistance
4.3. QTLs for Stem Rust
4.4. QTLs Cluster on Chromosome 1B
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/ (accessed on 12 March 2020).
- Giraldo-Carbajo, P.; Barzana, M.E.B.; Manzano-Agugliaro, F.; Giménez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- USDA. Available online: https://www.usda.gov/ (accessed on 17 March 2020).
- Statistics Committee. Ministry of National Economy of the Republic of Kazakhstan. Available online: https://stat.gov.kz/ (accessed on 15 March 2020).
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic Gains in Wheat Breeding and Its Role in Feeding the World. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar]
- Samborski, D.J. Wheat leaf rust. In The Cereal Rusts Vol. II: Diseases, Distribution, Epidemiology and Control; Roelfs, A.P., Bushnell, W.R., Eds.; Academic Press: Orlando, FL, USA, 1985; pp. 39–59. [Google Scholar]
- Koyshybaev, M. Wheat Diseases, 1st ed.; FAO: Ankara, Turkey, 2018; p. 365. [Google Scholar]
- Eversmeyer, M.G.; Kramer, C.L. Epidemiology of Wheat Leaf and Stem Rust in the Central Great Plains of the USA. Annu. Rev. Phytopathol. 2000, 38, 491–513. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Brown, J.K.M. Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant Pathol. 2013, 62, 115–121. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.F.; Xia, X.C. Catalogue of Gene Symbols for Wheat: 2017 Supplement. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf (accessed on 27 July 2020).
- Ellis, J.G.; Lagudah, E.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef]
- Bariana, H.S.; Hayden, M.J.; Ahmed, N.U.; Bell, J.A.; Sharp, P.J.; McIntosh, R.A. Mapping of durable adult plant and seedling resistances to stripe rust and stem rust diseases in wheat. Aust. J. Agric. Res. 2001, 52, 1247. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Yang, Z.; Yang, Z. Genetic mapping of quantitative trait loci in crops. Crop J. 2017, 5, 175–184. [Google Scholar] [CrossRef]
- Arruda, M.P.; Brown, P.; Brown-Guedira, G.; Krill, A.M.; Thurber, C.; Merrill, K.R.; Foresman, B.J.; Kolb, F.L. Genome-Wide Association Mapping of Fusarium Head Blight Resistance in Wheat using Genotyping-by-Sequencing. Plant Genome 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Tan, C.; Carver, B.F.; Bai, G.; Wang, X.; Bonman, J.M.; Wu, Y.; Hunger, R.; Cowger, C. Identification of powdery mildew resistance loci in wheat by integrating genome-wide association study (GWAS) and linkage mapping. Crop J. 2019, 7, 294–306. [Google Scholar] [CrossRef]
- Cheng, B.; Gao, X.; Cao, N.; Ding, Y.; Gao, Y.; Chen, T.; Xin, Z.; Zhang, L.Y. Genome-wide association analysis of stripe rust resistance loci in wheat accessions from southwestern China. J. Appl. Genet. 2020, 61, 37–50. [Google Scholar] [CrossRef]
- Ollier, M.; Talle, V.; Brisset, A.-L.; Le Bihan, Z.; Duerr, S.; Lemmens, M.; Goudemand, E.; Robert, O.; Hilbert, J.-L.; Buerstmayr, H. QTL mapping and successful introgression of the spring wheat-derived QTL Fhb1 for Fusarium head blight resistance in three European triticale populations. Theor. Appl. Genet. 2020, 133, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Unamba, C.I.N.; Nag, A.; Sharma, R.K. Next Generation Sequencing Technologies: The Doorway to the Unexplored Genomics of Non-Model Plants. Front. Plant Sci. 2015, 6, 6. [Google Scholar] [CrossRef]
- Turuspekov, Y.; Plieske, J.; Ganal, M.; Akhunov, E.; Abugalieva, S. Phylogenetic analysis of wheat cultivars in Kazakhstan based on the wheat 90 K single nucleotide polymorphism array. Plant Genet. Resour. 2015, 15, 29–35. [Google Scholar] [CrossRef]
- Singh, M.; Upadhyaya, H.D. Genetic and Genomic Resources for Grain Cereals Improvement, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 58–59. [Google Scholar]
- Hu, J.; Wang, X.; Zhang, G.; Jiang, P.; Chen, W.; Hao, Y.; Ma, X.; Xu, S.; Jia, J.; Kong, L.; et al. QTL mapping for yield-related traits in wheat based on four RIL populations. Theor. Appl. Genet. 2020, 133, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, K.; Singh, B.; Grewal, S.; Dwivedi, N.; Alqarawi, A.A.; Allah, E.A.; Ahmad, P.; Singh, N.K. Analysis of genetic control and QTL mapping of essential wheat grain quality traits in a recombinant inbred population. PLoS ONE 2019, 14, e0200669. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ. 2019, 42, 2540–2553. [Google Scholar] [CrossRef]
- Emebiri, L.C.; Tan, M.K.; El-Bouhssini, M.; Wildman, O.; Jighly, A.; Tadesse, W.; Ogbonnaya, F.C. QTL mapping identifies a major locus for resistance in wheat to Sunn pest (Eurygaster integriceps) feeding at the vegetative growth stage. Theor. Appl. Genet. 2017, 130, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Amalova, A.Y.; Yermekbayev, K.A.; Griffiths, S.; Abugalieva, S.I.; Turuspekov, Y.K.; Centre, U.K.J.I. Phenotypic variation of common wheat mapping population Pamyati Azieva x Paragon in south-east of Kazakhstan. Int. J. Biol. Chem. 2019, 12, 11–17. [Google Scholar] [CrossRef]
- Genievskaya, Y.; Amalova, A.; Ydyrys, A.; Sarbayev, A.; Griffiths, S.; Abugalieva, S.; Turuspekov, Y. Resistance of common wheat (Triticum aestivum L.) mapping population Pamyati Azieva × Paragon to leaf and stem rusts in conditions of south-east Kazakhstan. Eurasian J. Ecol. 2019, 61, 14–23. [Google Scholar]
- Genievskaya, Y.; Fedorenko, Y.; Sarbayev, A.; Amalova, A.; Abugalieva, S.; Griffiths, S.; Turuspekov, Y. Identification of QTLs for resistance to leaf and stem rusts in bread wheat (Triticum aestivum L.) using a mapping population of ‘Pamyati Azieva × Paragon’. Vavilov J. Genet. Breed. 2019, 23, 887–895. [Google Scholar] [CrossRef]
- ADAPTAWHEAT. Available online: https://www.jic.ac.uk/adaptawheat/ (accessed on 12 April 2020).
- Yermekbayev, K.; Turuspekov, Y.; Ganal, M.; Plieske, J.; Griffiths, S. Construction and utilization of the hexaploid map Pamyati Azieva × Paragon. In Proceedings of PlantGen (Plant Genetics, Genomics, Bioinformatics and Biotechnology), Almaty, Kazakhstan, 29 May–2 June 2017; Turuspekov, Y., Abugalieva, S., Eds.; Institute of Plant Biology and Biotechnology: Almaty, Kazakhstan, 2017. [Google Scholar]
- Chu, C.-G.; Friesen, T.L.; Xu, S.S.; Faris, J.D.; Kolmer, J.A. Identification of novel QTLs for seedling and adult plant leaf rust resistance in a wheat doubled haploid population. Theor. Appl. Genet. 2009, 119, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, Z.A.; Jin, Y.; Bender, C.M.; Herselman, L.; Prins, R. Seedling resistance to stem rust race Ug99 and marker analysis for Sr2, Sr24 and Sr31 in South African wheat cultivars and lines. Euphytica 2011, 186, 15–23. [Google Scholar] [CrossRef]
- Spanic, V.; Rouse, M.N.; Kolmer, J.A.; Anderson, J.A. Leaf and stem seedling rust resistance in wheat cultivars grown in Croatia. Euphytica 2014, 203, 437–448. [Google Scholar] [CrossRef]
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of physiologic races of Puccinia graminis var. tritici. US Agric. Res. Serv. 1962, 617, 1–53. [Google Scholar]
- Rsaliyev, A.; Rsaliyev, S.S. Principal approaches and achievements in studying race composition of wheat stem rust. Vavilov J. Genet. Breed. 2019, 22, 967–977. [Google Scholar] [CrossRef]
- Roelfs, A.P. An International System of Nomenclature for Puccinia graminis f. sp. tritici. Phytopathology 1988, 78, 526. [Google Scholar] [CrossRef]
- Long, D.L.; Kolmer, J.A. A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 1989, 79, 525–529. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Ordoñez, M.E. Genetic Differentiation of Puccinia triticina Populations in Central Asia and the Caucasus. Phytopathology 2007, 97, 1141–1149. [Google Scholar] [CrossRef][Green Version]
- Kolmer, J.A.; Kabdulova, M.G.; Mustafina, M.A.; Zhemchuzhina, N.S.; Dubovoy, V. Russian populations of Puccinia triticina in distant regions are not differentiated for virulence and molecular genotype. Plant Pathol. 2014, 64, 328–336. [Google Scholar] [CrossRef]
- Schachtel, G.A.; Dinoor, A.; Herrmann, A.; Kosman, E. Comprehensive Evaluation of Virulence and Resistance Data: A New Analysis Tool. Plant Dis. 2012, 96, 1060–1063. [Google Scholar] [CrossRef]
- Mains, E.B.; Jackson, H.S. Physiologic specialization in the leaf rust of wheat Puccinia triticiana Erikss. Phytopathology 1926, 16, 89–120. [Google Scholar]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A Diagrammatic Scale for Estimating Rust Intensity on Leaves and Stems of Cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Roelfs, A.; Singh, R.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico City, Mexico, 1992; p. 45. [Google Scholar]
- Zhang, D.; Bowden, R.; Bai, G. A method to linearize Stakman infection type ratings for statistical analysis. In Proceedings of the Borlaug Global Rust Initiative 2011 Technical Workshop, Saint Paul, MN, USA, 13–16 June 2011. [Google Scholar]
- Windows QTL Cartographer V2.5. Available online: https://brcwebportal.cos.ncsu.edu/qtlcart/WQTLCart.htm (accessed on 9 February 2020).
- Rong, J.; Feltus, F.A.; Waghmare, V.N.; Pierce, G.J.; Chee, P.W.; Draye, X.; Saranga, Y.; Wright, R.J.; Wilkins, T.A.; May, O.L.; et al. Meta-analysis of Polyploid Cotton QTL Shows Unequal Contributions of Subgenomes to a Complex Network of Genes and Gene Clusters Implicated in Lint Fiber Development. Genetics 2007, 176, 2577–2588. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- EnsemblPlant. Triticum Aestivum. Available online: https://plants.ensembl.org/Triticum_aestivum/Info/Index (accessed on 27 February 2020).
- BLAST Tool. Triticum Aestivum. Available online: https://plants.ensembl.org/Triticum_aestivum/Tools/Blast (accessed on 2 March 2020).
- UniProt. Available online: https://www.uniprot.org/ (accessed on 2 March 2020).
- Collard, B.C.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Genievskaya, Y.; Abugalieva, S.; Rsaliyev, A.; Turuspekov, Y. Genome-wide association mapping for resistance to leaf, stem, and yellow rusts of bread wheat in conditions of South Kazakhstan. PeerJ 2020. submitted work under review. [Google Scholar]
- Aoun, M.; Breiland, M.; Turner, M.K.; Loladze, A.; Chao, S.; Xu, S.S.; Ammar, K.; Anderson, J.A.; Kolmer, J.A.; Acevedo, M. Genome-Wide Association Mapping of Leaf Rust Response in a Durum Wheat Worldwide Germplasm Collection. Plant Genome 2016, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-X.; Barbier, H.; Rouse, M.N.; Singh, S.; Singh, R.P.; Bhavani, S.; Huerta-Espino, J.; Sorrells, M.E. A consensus map for Ug99 stem rust resistance loci in wheat. Theor. Appl. Genet. 2014, 127, 1561–1581. [Google Scholar] [CrossRef]
- Hart, G.E.; Gale, M.D.; McIntosh, R.A. Linkage maps of Triticum aestivum (hexaploid wheat, 2n = 42, genomes A, B & D) and T. tauschii (2n = 14, genome D). In Progress in Genome Mapping of Wheat and Related Species, Proceedings of the 3rd Publ Wkshp Int Triticeae Mapping Initiative, Mexico City, Mexico, 22–26 September 1992; Hoisington, D., McNab, A., Eds.; CIMMYT: Mexico City, Mexico, 1993; pp. 32–46. [Google Scholar]
- Periyannan, S.; Milne, R.J.; Figueroa, M.; Lagudah, E.; Dodds, P.N. An overview of genetic rust resistance: From broad to specific mechanisms. PLoS Pathog. 2017, 13, e1006380. [Google Scholar] [CrossRef]
- Figlan, S.; Terefe, T.G.; Shimelis, H.; Tsilo, T.J. Adult plant resistance to leaf rust and stem rust of wheat in a newly developed recombinant inbred line population. S. Afr. J. Plant Soil 2017, 35, 111–119. [Google Scholar] [CrossRef]
- Tadesse, W.; Reents, H.J.; Hsam, S.L.K.; Zeller, F.J. Relationship of seedling and adult plant resistance and evaluation of wheat germplasm against tan spot (Pyrenophora tritici-repentis). Genet. Resour. Crop Evol. 2010, 58, 339–346. [Google Scholar] [CrossRef]
- Niks, R.E.; Qi, X.; Marcel, T.C. Quantitative Resistance to Biotrophic Filamentous Plant Pathogens: Concepts, Misconceptions, and Mechanisms. Annu. Rev. Phytopathol. 2015, 53, 445–470. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Herrera, L.A.; Govindan, V.; Stangoulis, J.C.R.; Hao, Y.; Singh, R.P. QTL Mapping of Grain Zn and Fe Concentrations in Two Hexaploid Wheat RIL Populations with Ample Transgressive Segregation. Front. Plant Sci. 2017, 8, 1800. [Google Scholar] [CrossRef]
- Asif, M.; Yang, R.C.; Navabi, A.; Iqbal, M.; Kamran, A.; Lara, E.P.; Randhawa, H.; Pozniak, C.; Spaner, D. Mapping QTL, selection differentials, and the effect of Rht-B1 under organic and conventionally managed systems in the Attila × CDC Go spring wheat mapping population. Crop Sci. 2015, 55, 1129–1142. [Google Scholar] [CrossRef]
- Tian, Z.M.; Zhang, L.P.; Sun, Q.L.; Shan, F.H.; Tian, Z.H. Research review on wheat QTLs. Inn. Mong. Agric. Sci. Technol. 2007, 3, 68–71. [Google Scholar]
- Bokore, F.E.; Knox, R.E.; Cuthbert, R.D.; Pozniak, C.J.; McCallum, B.D.; N’Diaye, A.; Depauw, R.M.; Campbell, H.L.; Munro, C.; Singh, A.; et al. Mapping quantitative trait loci associated with leaf rust resistance in five spring wheat populations using single nucleotide polymorphism markers. PLoS ONE 2020, 15, e0230855. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Anderson, J.A.; Flor, J.M. Chromosome Location, Linkage with Simple Sequence Repeat Markers, and Leaf Rust Resistance Conditioned by Gene Lr63 in Wheat. Crop Sci. 2010, 50, 2392–2395. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat Rusts, An Atlas of Resistance Genes; CSIRO Publications: Melbourne, Australia, 1995; pp. 93–99. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.F.; Appels, R.; Xia, X.C. Catalogue of gene symbols for wheat: 2011 supplement. Annu. Wheat Newsl. 2011, 57, 303–321. [Google Scholar]
- Mago, R.; Verlin, D.; Zhang, P.; Bansal, U.; Bariana, H.; Jin, Y.; Ellis, J.; Hoxha, S.; Dundas, I. Development of wheat–Aegilops speltoides recombinants and simple PCR-based markers for Sr32 and a new stem rust resistance gene on the 2S#1 chromosome. Theor. Appl. Genet. 2013, 126, 2943–2955. [Google Scholar] [CrossRef]
- Singh, R. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev. Perspect. Agric. Veter-Sci. Nutr. Nat. Resour. 2006, 1, 1–13. [Google Scholar] [CrossRef]
- Singh, R.P.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Bariana, H.; Bansal, U.; McCallum, B.; Hiebert, C.; Bhavani, S.; Singh, S.; Lan, C.X.; et al. Lr34/Yr18/Sr57/Pm38/Bdv1/Ltn1 confers slow rusting, adult plant resistance to Puccinia graminis tritici. In Proceedings of the 13th International Cereal Rusts and Powdery Mildews Conference, Beijing, China, 28 August–1 September 2012. [Google Scholar]
- Singh, R.P.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.X.; Basnet, B.R.; Bhavani, S.; Lagudah, E.S. Pleiotropic gene Lr46/Yr29/Pm39/Ltn2 confers slow rusting, adult plant resistance to wheat stem rust fungus. In Proceedings of the Borlaug Global Rust Initiative, New Delhi, India, 19–22 August 2013. [Google Scholar]
- Herrera-Foessel, S.A.; Singh, R.P.; Lillemo, M.; Huerta-Espino, J.; Bhavani, S.; Singh, S.; Lan, C.; Calvo-Salazar, V.; Lagudah, E.S. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 2014, 127, 781–789. [Google Scholar] [CrossRef] [PubMed]
| Disease (Pathogen) | Race | Avirulent (Effective) Genes | Virulent (Ineffective) Genes |
|---|---|---|---|
| LR (Puccinia recondita Rob. ex Desm f. sp. tritici) | TQKHT | Lr24, 26, 3ka, 19, 25 | Lr1, 2a, 2c, 3, 9, 16, 11, 17, 30, 20, 29, 2b, 3bg, 14a, 15 |
| TRTHT | Lr24, 19, 25 | Lr1, 2a, 2c, 3, 9, 16, 26, 3ka, 11, 17, 30, 20, 29, 2b, 3bg, 14a, 15 | |
| TQTMQ | Lr24, 26, 20, 25, 14a, 15 | Lr1, 2a, 2c, 3, 9, 16, 3ka, 11, 17, 30, 19, 29, 2b, 3bg | |
| SR (Puccinia graminis Pers. f. sp. tritici Eriks. & E. Henn.) | TKRTF | Sr11, 30, 24, 31 | Sr5, 21, 9e, 7b, 6, 8a, 9g, 36, 9b, 17, 9a, 9d, 10, 38, Tmp, McN |
| PKCTC | Sr21, 11, 36, 9b, 30, 24, 31, 38 | Sr5, 9e, 7b, 6, 8a, 9g, 17, 9a, 9d, 10, Tmp, McN | |
| RKRTF | Sr9e, 11, 30, 24, 31 | Sr5, 21, 7b, 6, 8a, 9g, 36, 9b, 17, 9a, 9d, 10, 38, Tmp, McN |
| KRIAPI | |||||
|---|---|---|---|---|---|
| March | April | May | June | July | |
| Temperature (°C) | 8.2 | 12.8 | 17.0 | 22.3 | 27.0 |
| Precipitation (mm) | 27.3 | 168.4 | 39.3 | 72.7 | 22.6 |
| RIBSP | |||||
| March | April | May | June | July | |
| Temperature (°C) | 13.0 | 17.0 | 24.0 | 29.0 | 33.0 |
| Precipitation (mm) | 1.0 | 2.3 | 1.8 | 0.9 | 0.1 |
| Env. | Plant-Growth Stage | Race (Disease) | Parents IT 1 | RILs IT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA | Par | Mean | Range | R (%) | MR (%) | MS (%) | S (%) | |||
| GH | Seedling | TQTMQ (LR) | 6 (3) | 5 (3-) | 6.1 ± 0.9 | 5–8 | 0 | 0 | 89.8 | 10.2 |
| TQKHT (LR) | 8 (4-) | 6 (3) | 5.9 ± 1.0 | 4–9 | 0 | 3.1 | 91.8 | 5.1 | ||
| TRTHT (LR) | 6 (3) | 6 (3) | 5.9 ± 0.8 | 4–8 | 0 | 1.0 | 96.9 | 2.1 | ||
| Seedling | TKRTF (SR) | 8 (4-) | 8 (4-) | 7.0 ± 1.4 | 5–9 | 0 | 0 | 45.9 | 54.1 | |
| PKCTC (SR) | 5 (3-) | 8 (4-) | 5.4 ± 1.8 | 1–9 | 1.0 | 22.4 | 58.2 | 18.4 | ||
| RKRTF (SR) | 6 (3) | 5 (3-) | 6.5 ± 1.4 | 5–9 | 0 | 0 | 64.3 | 35.7 | ||
| RIBSP | Adult | LR | 8 (30S) | 6 (50MS) | 3.8 ± 1.4 | 0–9 | 38.8 | 11.2 | 29.6 | 20.4 |
| SR | 9 (80S) | 8 (40S) | 6.6 ± 2.9 | 1–9 | 1.0 | 23.5 | 18.4 | 57.1 | ||
| KRIAPI | LR | 6 (40MS) | 6 (40MS) | 6.6 ± 1.7 | 2–9 | 0 | 9.2 | 64.3 | 26.5 | |
| SR | 0 (0) | 1 (10R) | 1.8 ± 2.3 | 0–8 | 56.1 | 26.5 | 18.4 | 1.0 | ||
| Factor | df | LR | SR | ||||
|---|---|---|---|---|---|---|---|
| MeanS | F | p | MeanS | F | p | ||
| Geno | 97 | 2.506 | 1.630 | 0.001 | 5.380 | 2.679 | 8.39 × 10–11 |
| Race | 2 | 5.420 | 3.525 | 0.031 | 136.940 | 68.180 | <2.00 × 10–16 |
| Geno: Race | 194 | 1.396 | 0.908 | 0.766 | 4.540 | 2.262 | 1.20 × 10–10 |
| Residuals | 294 | 1.537 | 2.010 | ||||
| LR TQTMQ | LR TQKHT | LR TRTHT | APR LR RIBSP | APR LR KRIAPI | SR TKRTF | SR PKCTC | SR RKRTF | APR SR RIBSP | |
|---|---|---|---|---|---|---|---|---|---|
| LR TQKHT | 0.193 * | ||||||||
| LR TRTHT | 0.207 * | 0.283 ** | |||||||
| APR LR RIBSP | 0.008 ns | 0.122 ns | 0.154 ns | ||||||
| APR LR KRIAPI | 0.251 ** | −0.028 ns | 0.192 * | −0.124 ns | |||||
| SR TKRTF | −0.152 ns | 0.000 ns | 0.141 ns | 0.088 ns | 0.228 * | ||||
| SR PKCTC | −0.150 ns | 0.021 ns | 0.058 ns | 0.081 ns | −0.009 ns | −0.019 ns | |||
| SR RKRTF | −0.202 * | −0.006 ns | 0.091 ns | −0.125 ns | 0.069 ns | 0.050 ns | 0.110 ns | ||
| APR SR RIBSP | −0.071 ns | 0.096 ns | 0.197 * | 0.130 ns | 0.038 ns | −0.075 ns | −0.040 ns | −0.029 ns | |
| APR SR KRIAPI | 0.127 ns | 0.040 ns | 0.117 ns | 0.018 ns | 0.245 ** | −0.006 ns | 0.182 * | 0.015 ns | −0.043 ns |
| TKW RIBSP | YP RIBSP | TKW KRIAPI | YP KRIAPI | |
|---|---|---|---|---|
| LR RIBSP | −0.175 ** | −0.252 ** | – | – |
| SR RIBSP | −0.490 ** | −0.474 ** | – | – |
| LR KRIAPI | – | – | −0.055 ns | −0.200 * |
| SR KRIAPI | – | – | 0.134 ns | −0.151 ns |
| Race | Env. | QTL | Flanking Markers (Left–Right) | Chr. | CI (cM) | Peak (cM) | Max. LOD | R2 (%) | Add. Effect 1 | Allele 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| TQTMQ | GH | QLr.ipbb-4A.2 | IAAV7104–Excalibur_c25699_113 | 4A | 65.2–73.9 | 68.0 | 3.3 | 12.1 | −0.47 | PA |
| GH | QLr.ipbb-5B.2 | Kukri_rep_c98079_222–BS00075815_51 | 5B | 87.4–99.2 | 94.5 | 4.3 | 15.2 | −0.57 | PA | |
| GH | QLr.ipbb-7B.3 | BS00023023_51–wsnp_Ex_c5653_9937062 | 7B | 93.5–98.9 | 94.7 | 4.9 | 15.7 | −0.79 | PA | |
| TQKHT | GH | QLr.ipbb-6A.4 | Excalibur_rep_c105463_330–Ku_c37893_495 | 6A | 31.5–42.0 | 40.8 | 8.6 | 25.7 | 0.63 | Paragon |
| GH | QLr.ipbb-7D.1 | Kukri_c16416_647–BS00062644_51 | 7D | 74.0–84.9 | 84.1 | 4.2 | 11.6 | 0.41 | Paragon | |
| TRTHT | GH | QLr.ipbb-3A.2 | Excalibur_c74666_291–RFL_Contig1896_1236 | 3A | 6.2–24.7 | 23.3 | 5.1 | 16.2 | 0.46 | Paragon |
| GH | QLr.ipbb-4D.1 | TA020319-0161–BS00022436_51 | 4D | 7.1–16.2 | 14.3 | 3.7 | 12.3 | −0.39 | PA | |
| GH | QLr.ipbb-6A.5 | BobWhite_c30930_192–BS00022992_51 | 6A | 56.9–58.5 | 57.4 | 4.8 | 15.1 | 0.44 | Paragon | |
| APR | RIBSP | QLr.ipbb-1B.4 | Excalibur_c29707_318–Kukri_c36151_170 | 1B | 0–13.0 | 0.7 | 3.2 | 13.6 | 1.29 | Paragon |
| APR | RIBSP | QLr.ipbb-7A.3 | Ra_c4601_2417–CAP7_c7296_88 | 7A | 86.4–94.0 | 88.9 | 3.4 | 11.7 | 1.18 | Paragon |
| TQKHT/APR | GH/KRIAPI | QLr.ipbb-1A.2 | RAC875_c12348_720–BS00022824_51 | 1A | 75.9–88.2 | 82.1 | 4.6 | 16.0 | 0.86 | Paragon |
| Race | Env. | QTL | Flanking Markers (Left–Right) | Chr. | CI (cM) | Peak (cM) | Max. LOD | R2 (%) | Add. Effect * | Allele * |
|---|---|---|---|---|---|---|---|---|---|---|
| TKRTF | GH | QSr.ipbb-2D.2 | wsnp_Ex_rep_c80588_75758453–TA010191-1338 | 2D | 0–3.7 | 0.1 | 3.0 | 10.0 | 0.43 | Paragon |
| GH | QSr.ipbb-6B.6 | wsnp_Ex_c12618_20079758–wsnp_Ex_c1249_2399894 | 6B | 46.0–54.3 | 47.8 | 4.2 | 14.1 | −0.57 | PA | |
| GH | QSr.ipbb-6B.7 | wsnp_Ku_c43368_50890819–wsnp_Ku_c4910_8793327 | 6B | 75.5–87.3 | 79.9 | 4.7 | 19.2 | −0.66 | PA | |
| PKCTC | GH | QSr.ipbb-2B.3 | RAC875_rep_c71112_400–Kukri_c1175_1577 | 2B | 6.3–10.4 | 9.2 | 3.9 | 12.2 | −0.63 | PA |
| GH | QSr.ipbb-2B.4 | BS00041323_51–RAC875_c32503_134 | 2B | 75.9–78.3 | 78.0 | 2.9 | 8.9 | 0.54 | Paragon | |
| GH | QSr.ipbb-5B.1 | RAC875_rep_c114200_428–wsnp_RFL_Contig1548_762547 | 5B | 137.6–146.3 | 141.1 | 4.9 | 14.7 | 0.96 | Paragon | |
| RKRTF | GH | QSr.ipbb-5A.3 | BS00036851_51–Excalibur_c27357_146 | 5A | 184.7–194.4 | 190.3 | 6.8 | 24.7 | 0.72 | Paragon |
| APR | KRIAPI | QSr.ipbb-1D.1 | BS00051826_51–BobWhite_rep_c65565_359 | 1D | 61.7–65.5 | 64.3 | 5.0 | 18.0 | 2.24 | Paragon |
| RIBSP | QSr.ipbb-3D.1 | Ra_c10284_405–Kukri_c5252_107 | 3D | 0–11.4 | 1.9 | 3.1 | 11.3 | −0.99 | PA | |
| KRIAPI | QSr.ipbb-5A.2 | BS00089076_51–CAP11_c2623_196 | 5A | 168.2–178.5 | 172.7 | 3.0 | 10.8 | 0.72 | Paragon | |
| TKRTF/APR | GH/KRIAPI | QSr.ipbb-1B.4 | Excalibur_c29707_318–Kukri_c92979_195 | 1B | 0–15.2 | 12.6 | 4.2 | 15.7 | 0.59/–0.77 | Paragon/PA |
| RKRTF/APR | GH/RIBSP | QSr.ipbb-2A.2 | Kukri_c33374_1048–Tdurum_contig42153_5854 | 2A | 0.5–37.2 | 30.8 | 5.2 | 39.1 | −1.45 | PA |
| TKRTF/APR | GH/RIBSP | QSr.ipbb-6B.5 | RAC875_rep_c105224_352–Kukri_rep_c107077_360 | 6B | 2.7–15.1 | 6.7 | 4.6 | 17.3 | −0.56/1.26 | PA/Paragon |
| # | Trait | Type of Resistance (Race) | QTL | Reference QTL | Candidate Genes |
|---|---|---|---|---|---|
| 1 | LR | TQKHT/APR | QLr.ipbb-1A.2 | QLr.ipbb-1A.1 [51] | - |
| 2 | APR | QLr.ipbb-1B.4 | - | Lr21 [47] | |
| 3 | TRTHT | QLr.ipbb-3A.2 | - | Lr63, Lr66 [52] | |
| 4 | TQKHT | QLr.ipbb-6A.4 | QLr.ipbb-6A.1 [51] | - | |
| 5 | TRTHT | QLr.ipbb-6A.5 | QLr.ipbb-6A.2 [51] | - | |
| 6 | TQTMQ | QLr.ipbb-7B.3 | QLr.ipbb-7B.1 [51] | Lr14 [52] | |
| 1 | SR | TKRTF/APR | QSr.ipbb-1B.4 | - | Sr31 [53] |
| 2 | APR | QSr.ipbb-1D.1 | - | Sr18 [54] | |
| 3 | RKRTF/APR | QSr.ipbb-2A.2 | - | Sr32-2A [53] | |
| 4 | PKCTC | QSr.ipbb-2B.3 | - | Sr32-2B, Sr39 [53] | |
| 5 | PKCTC | QSr.ipbb-2B.4 | QSR.IPBB-2B [26] | Sr36 [53] | |
| 6 | TKRTF | QSr.ipbb-2D.2 | - | Sr32-2D [53] | |
| 7 | TKRTF/APR | QSr.ipbb-6B.5 | QSR.IPBB-6B.1 [27], QSr.ipbb-6B.3 [51] | - | |
| 8 | TKRTF | QSr.ipbb-6B.7 | QSR.IPBB-6B.2 [27], QSr.ipbb-6B.4 [51] | Sr11 [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genievskaya, Y.; Abugalieva, S.; Rsaliyev, A.; Yskakova, G.; Turuspekov, Y. QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva × Paragon Mapping Population of Bread Wheat. Agronomy 2020, 10, 1285. https://doi.org/10.3390/agronomy10091285
Genievskaya Y, Abugalieva S, Rsaliyev A, Yskakova G, Turuspekov Y. QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva × Paragon Mapping Population of Bread Wheat. Agronomy. 2020; 10(9):1285. https://doi.org/10.3390/agronomy10091285
Chicago/Turabian StyleGenievskaya, Yuliya, Saule Abugalieva, Aralbek Rsaliyev, Gulbahar Yskakova, and Yerlan Turuspekov. 2020. "QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva × Paragon Mapping Population of Bread Wheat" Agronomy 10, no. 9: 1285. https://doi.org/10.3390/agronomy10091285
APA StyleGenievskaya, Y., Abugalieva, S., Rsaliyev, A., Yskakova, G., & Turuspekov, Y. (2020). QTL Mapping for Seedling and Adult Plant Resistance to Leaf and Stem Rusts in Pamyati Azieva × Paragon Mapping Population of Bread Wheat. Agronomy, 10(9), 1285. https://doi.org/10.3390/agronomy10091285





