1. Introduction
Toxigenic fungal species occur across the globe, wherever maize is grown. Most of the papers on this subject deal with direct breeding for resistance to one pathogen. As most toxin contamination is due to susceptible hybrids, the issue revolves around how susceptible hybrids could be excluded from commercial production. Often, more than ten
Fusarium species contaminate maize, with differing ecological needs and toxin profiles [
1].
Fusarium graminearum and
F. verticillioides are the dominant
Fusarium species in Hungary.
Fusarium graminearum causes severe epidemics for a period of 1–2 years every decade.
Fusarium culmorum occurs rarely in ears, but it is highly aggressive, and its heat demand is lower, therefore, it functions better in cooler seasons.
Fusarium verticillioides causes an epidemic nearly every year [
2], which should be treated in the future [
3]. In 2014, a national epidemic was recorded, resulting in a loss of about 300 million USD in crop and animal husbandry. All three species participated in the damage, and of them, deoxynivalenol (DON) was the most severe. For this reason, we need to find approaches to develop resistance to all three
Fusarium species mentioned. It is known that natural infection and species mixtures do not provide an alternative [
1]. Therefore, artificial inoculation should support breeding work. The variation in
F. graminearum is high in terms of aggressiveness and toxin production [
4]. This is also true for
F. culmorum [
5,
6,
7]. The ear rot and toxin relations are severely influenced by the ecological conditions [
8]. In spite of the significance of breeding [
1], progress has been slow [
9]. Our question is whether the resistance to different toxigenic fungi is connected to or follows different patterns.
Two components of resistance have been described in maize resistance to ear rot fungi [
9,
10]: Kernel resistance, KR (Type I), which occurs after inoculation in the middle of the ear, and silk channel resistance, SR (Type II), which occurs after inoculation of the silk or silk channel. Munkvold and Dejardins [
11] concluded that kernel infection via silk mediation is more important than kernel infection through seeds, stalks, crowns, and roots. Therefore, silk inoculation is considered the best approach for artificial inoculation.
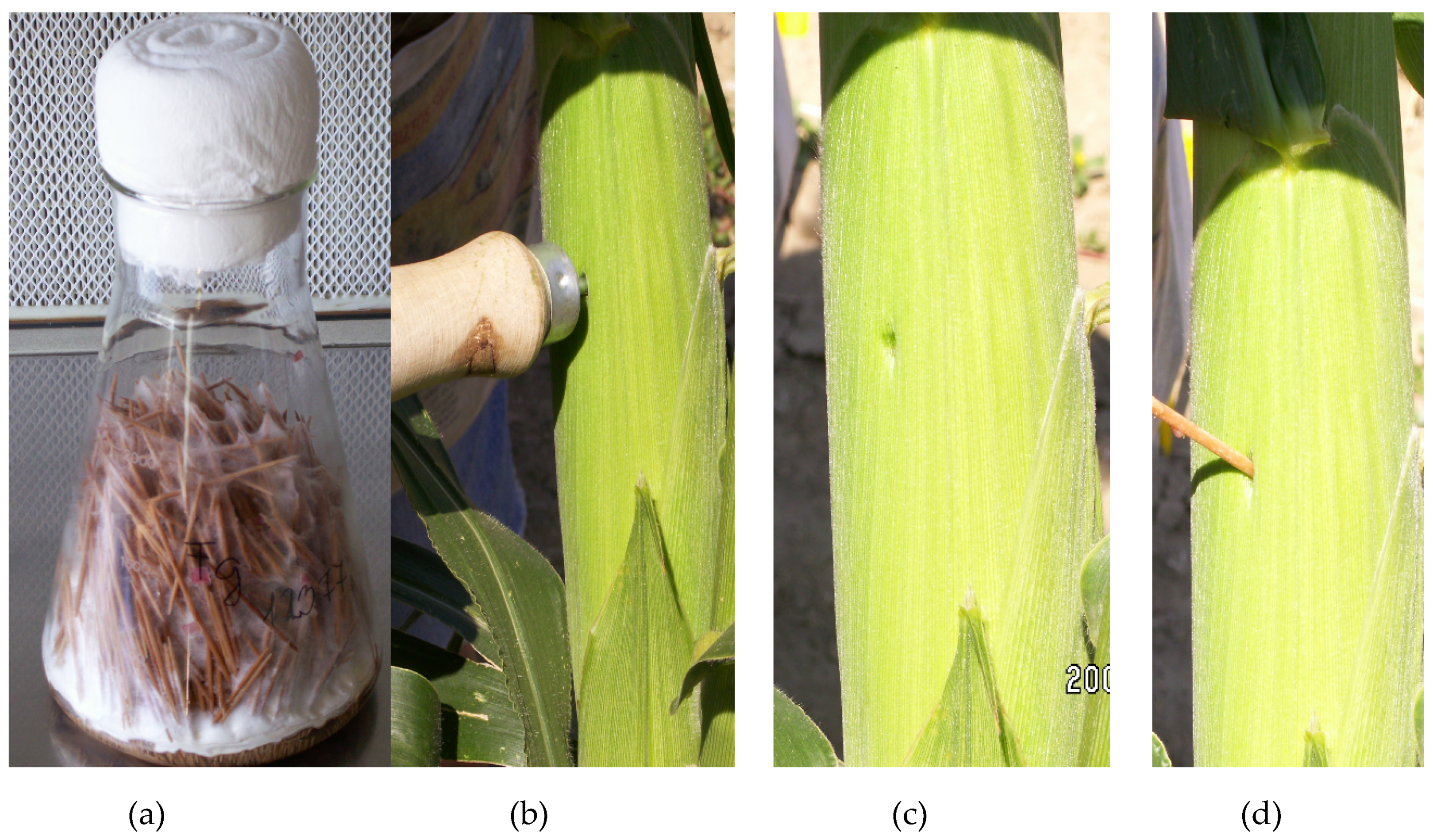
The toothpick method is very good at differentiating genotypes for KR by inserting infested toothpicks into the middle of the ear through husk leaves [
5,
12]. Clements et al. [
13] obtained the highest infection severity, with a side injection of the ears with 5 mL inoculum, representing a method for KR testing, but with suspension. For inoculation, Reid et al. [
10] used four stainless steel pins inserted into the suspension of the given fungal species before inoculation and four grains were inoculated at the same time. The possible infection severity was over 75% for susceptible hybrids in Gibberella ear rot GER. Robertson-Hoyt et al. [
14] found that carrying out inoculation by penetrating husk leaves with
F. verticillioides gave a much higher infection severity and toxin contamination than silk channel inoculation. It seems that KR testing has a higher stability and reproducibility. Stalk rot strongly reduces GER ear rot development [
14], so genetically valid ear data need healthy stalks (irrigation and a lower plant density).
Lanubile et al. [
12] reported that different techniques for silk inoculation are not equally effective. Clements et al. [
13] found that spraying the silks was ineffective, and the toothpicks inserted into the silk channel were also poor. The Szeged unpublished results support this view. Silk channel inoculation for SR is generally performed on the 6–7th day after midsilking [
15]. Miller et al. [
16] analyzed silk resistance to
F. graminearum, and broad differences were found. In susceptible genotypes, 7–9 days were required for the pathogen to reach the cob, and the resistant genotypes needed 12–15 days. For kernel inoculation, 10–15 days were suggested after midsilking [
17]. Morales et al. [
18] used toothpicks infested by
F. verticillioides, where the conidium concentration was irrelevant. Robertson-Hoyt et al. [
14] used an inoculation time of 10 days after midsilking, and another 7 days later, they applied 10 mL inoculum per primary ear. Therefore, the time required to reach the grain on the cob is about two weeks for both methods. However, the efficacy of the two methods significantly differs. The silk channel method has several setbacks. Husk leaves may be too short or too long, and they can remain closed or open early by influencing ear tip infection. At the injection of fungal suspension into a silk channel, the suspension may spread between rows. By using 2 mL of inoculum, in most cases, a part of the suspension dropped to the ground and could lead to different disease severities. Reid et al. [
10] reached GER by silk mediation with only a 5–7% GER severity. Reid et al. [
10] and Presello [
19] used 2 mL inoculum. Loeffler [
20] only applied 1 mL—there is no standard in this field.
Lemmens [
21,
22] found a low correlation (r = 0.12) between the two resistance types. In KR, the maximum value was 90%, and for silk channel inoculation, it was only 8%. However, Loeffler et al. [
20] reported a much closer correlation (r = 0.66). Chungu et al. [
23] also found a higher correlation between the two traits (r = 0.77–0.89). Reid et al. [
24] stressed that few genotypes have a good resistance to both resistance types. Loeffler et al. [
25] reported significant genotypic variance for both kernel and silk channel resistance. The correlation between silk and kernel resistance was moderate (r = 0.66), but there were genotypes with very diverging resistance levels with both methods. Therefore, the authors suggest the use of both inoculation methods. The rather close correlations between the results of the two inoculation methods seem to support the idea that most of the hybrids show overlapping results for the two resistance types. On the other hand, an inoculation method that reaches a higher disease severity is better. It should be added that stalk rot can significantly decrease the ear rot (pseudo resistance). Therefore, genetically valid ear rot results need plants with healthy stalks [
26]. This can be enhanced by irrigation and a lower plant density.
The conidium concentration only has a role in the silk channel method, as toothpicks do not need suspension. Very different concentrations were used from 5 × 10
5–10
6. For Fusarium ear rot (FER), the concentration is usually 10
6 conidia/mL [
13,
22,
26,
27,
28,
29], and for GER, 2.5–5 × 10
5 conidia concentrations were used [
21,
28,
29]. In another case [
30], no conidium concentration was given, and 10
6 conidia were also used [
31]. It seems that no standard conidium concentration exists, and no aggressiveness test has been developed. As significant aggressiveness differences occur between isolates, a given concentration might not secure the aggressiveness that is desired. For aggressiveness tests, susceptible hybrids should be used, as the symptom severity depends also on resistance level.
Some breeders select for toxin resistance, whilst others select for disease resistance, and some even select for both. However, the data proving the effectiveness of such approaches do not have a solid basis. Robertson-Hoyt et al. [
14] found strong genetic correlations between ear rot and the fumonisin concentration. Eller et al. [
32] selected lines with a good resistance to FER and fumonisin. Hung et al. [
33] analyzed the general and specific combining ability in a genetic study on
F. verticillioides. Hybrids had 27% less ear rot and a 30% lower fumonisin content than their inbred parents, demonstrating the importance of hybrid vigor in disease resistance. Eller et al. [
17] identified sources for both disease resistance and toxin contamination in FER. Lanubile et al. [
12] concluded that screening for resistance to FER seems to be good enough, and toxin control is not regularly needed. Henry et al. [
34] identified a correlation between the FER severity and fumonisin content (r = 0.74) (
p = 0.0002), and between
Aspergillus flavus and aflatoxin production (r = 0.61) (
p = 0.004). They identified a genotype with a high resistance to both pathogens. Robertson-Hoyt et al. [
35] found a genotypic correlation between FER and the fumonisin content (r = 0.87), and phenotypic correlation between FER and the fumonisin content (r = 0.87 and r = 0.64, respectively). They concluded that ear rot should have priority over expensive toxin measurements. Szabó et al. [
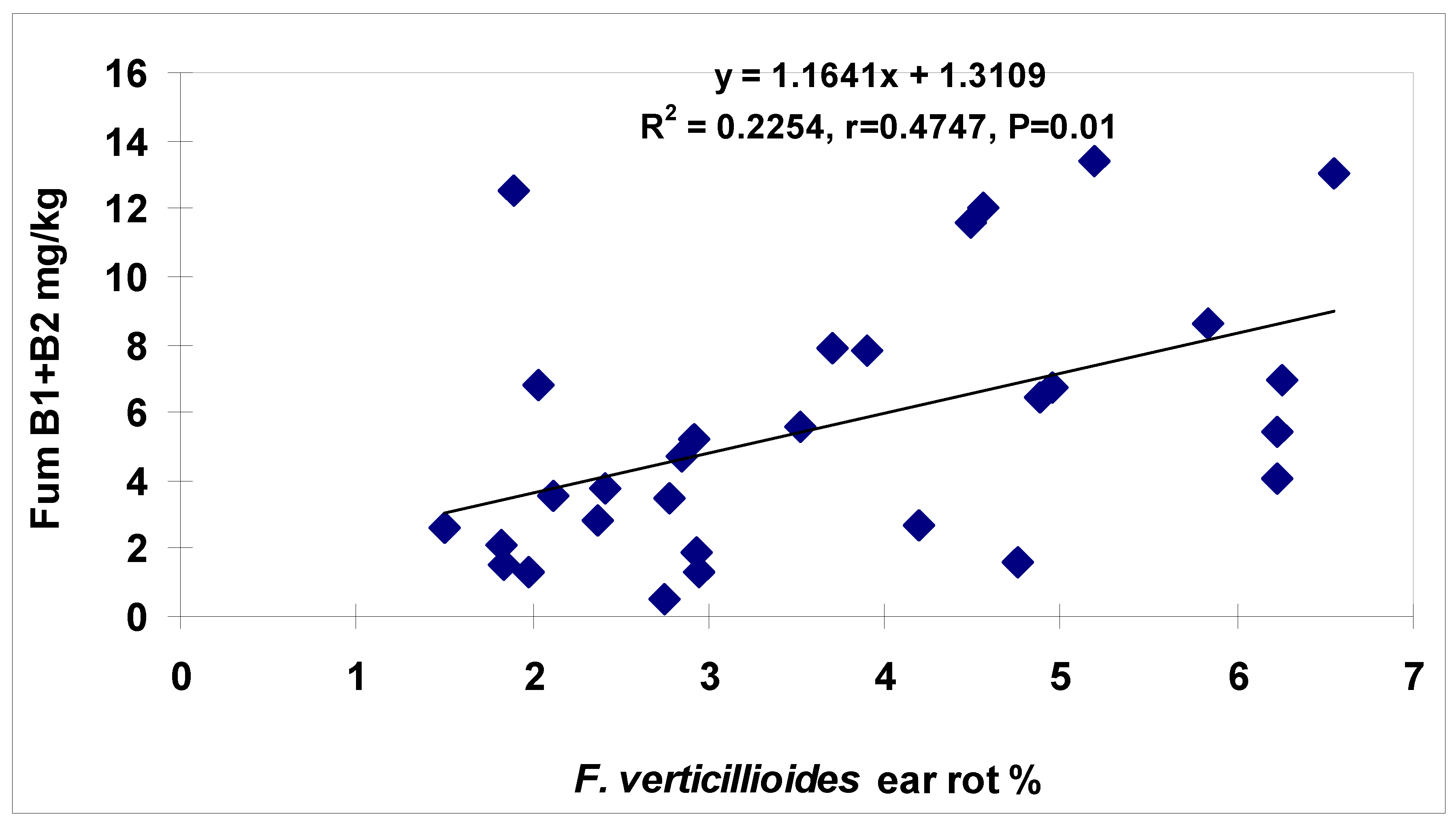
2] found a high correlation between GER and DON contamination (Fg r = 0.95 and Fc = 0.82, both
p = 0.001), but for FER and fumonisin, only r = 0.45 (
p = 0.05) could be demonstrated. The data support the hypothesis that, at a higher rate of the tested hybrids, the resistance to disease and toxin overlap and a part of the genotypes behave differently.
Loeffler [
25] found strong correlations between
F. graminearum and
F. verticillioides test results. Schaafsma et al. [
31] tested two hybrids against
F. graminearum; then, resistance tests on
F. verticillioides and
F. subglutinans were also conducted. Ear rot was the most severe for
F. graminearum, and the least severe for
F. subglutinans. The hybrid with a higher resistance to
F. graminearum also exhibited a higher resistance to the two other
Fusarium species. Other authors [
5,
7] found significant correlations between the resistance to
F. graminearum and
F. culmorum. Robertson-Hoyt et al. [
36] found a medium level of correlation between
F. verticillioides and
A. flavus resistance. Presello et al. [
37,
38] demonstrated resistance relationships between
F. graminearum and
F. verticillioides ear rots. Even though most papers have dealt with one pathogen, the possibility of multiple resistances is often mentioned. The genetic background is unknown, but in regions where more toxigenic species occur, this is a highly important breeding task that should be studied.
The objectives of the study are as follows: (1) Compare kernel and silk channel resistance data; (2) analyze resistance relations of maize hybrids to F. graminearum, F. culmorum, and F. verticillioides; (3) identify hybrids with a low toxin and ear rot severity for the three major pathogens; and (4) suggest better methodological approaches to identify hybrids with increased food and feed safety.
4. Discussion
Considering the role of resistance types, Munkvold and Desjardins [
11] argued that
F. verticillioides mostly infects through silks. Therefore, silk inoculation is the proper method for resistance testing, which explains its widespread use. However, several authors found kernel resistance to be more useful, with its higher stability [
13,
14,
15,
53,
54]. Schaafsma et al. [
31] found that inoculation conducted by penetrating husk leaves with
F. verticillioides gave a much higher infection severity and toxin contamination than silk channel inoculation. The fumonisin and FER results were more consequential when the kernel resistance was tested. Presello et al. [
55] found kernel resistance to be useful, but only a limited number of resistance sources were found. Reid et al. [
23] found only a few genotypes that were good for both resistance types. Despite the positive opinion on the role of silk in the infection process, spraying the silks (GER) was not successful [
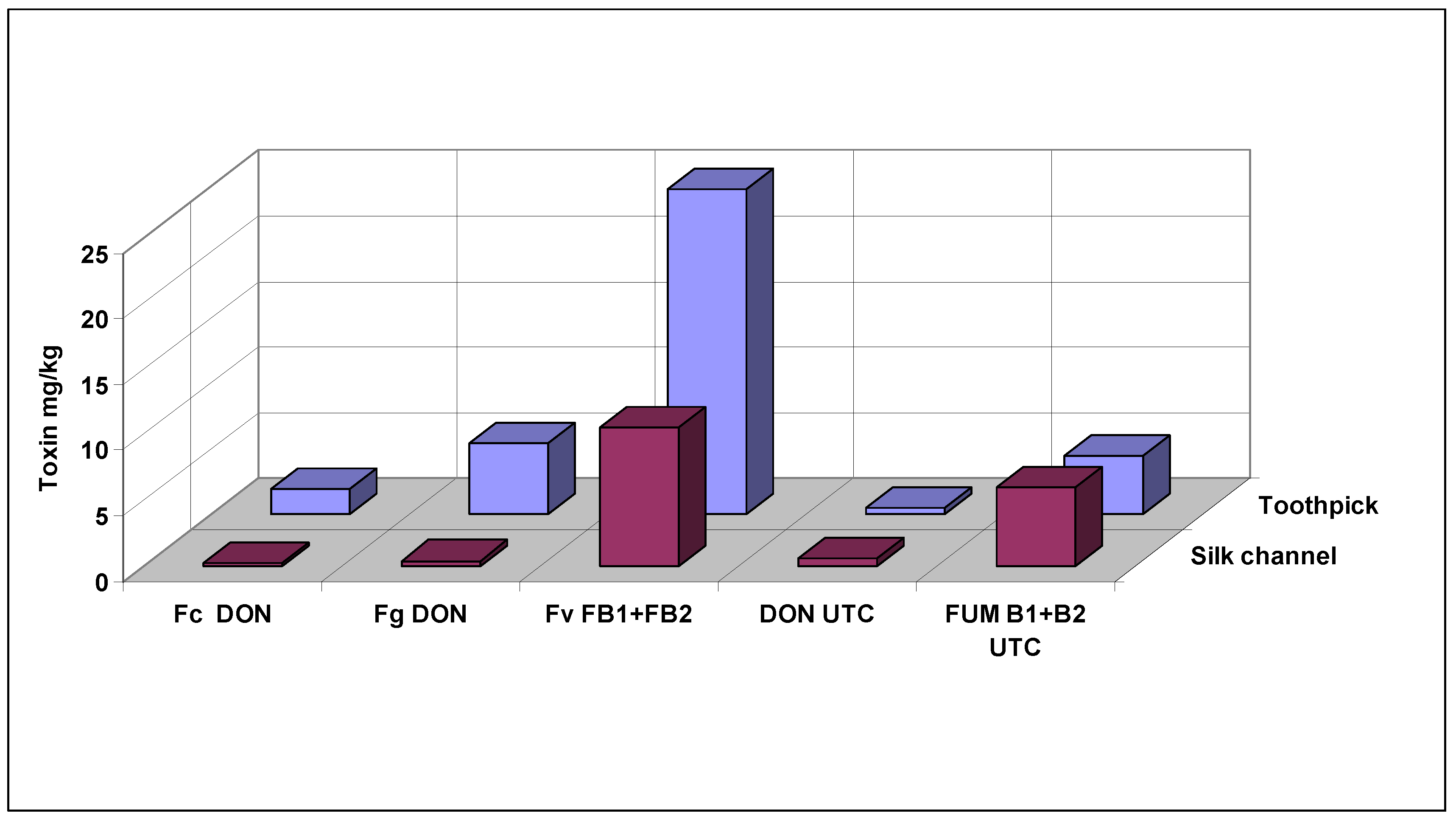
56]. The silk channel infection achieved better results, but exhibited a lower infection severity compared with the kernel resistance test. Our data support this latter view, as we received a much better differentiation of the genotypes.
Several authors have reported parallel tests and comparisons of relations between kernel and silk channel resistance, in which the correlations were moderate or higher [
20,
23,
25]. Löffler et al. [
25] suggested applying the two inoculation methods in parallel. In each case, genotypes were found to display large differences in response to the two methodologies. However, most of them provided similar reactions to both methodologies [
17]. Kebede et al. [
57] tested two lines with both methods, and the ear infection data were very similar. Most of the data confirmed the result that correlations between the two resistance components often overlap, and a large number of the hybrids react similarly [
12,
20,
23,
25]. Lemmens [
21,
22], with his low correlation, seems to be an exception, but this does not mean that such a case could not occur. The securing healthy stalks are also a precondition to lower the mistakes in determining resistance level (pseudoresistance) [
26].
The general conclusion is that the kernel resistance for breeding might be more important, especially in dry regions, where silk channel inoculation seems to be much less effective. As two different methods cannot be used in a breeding program, the KR model can be used as the main method and additional control for possible silk channel resistance. The higher consistency of data is understandable, as, in 4–5 weeks; the infection can spread under closed husk leaves in a “humid chamber”, which is not always the case for the silk channel method. We suppose that the application of KR allows less false positive resistance identification. It should be stressed that a lot of information is absent, and future research should answer the open questions, but practical screening can present an improved methodology.
Resistance to different
Fusarium spp. has not been a central problem in the past. The
F. graminearum isolate was shown to be the most pathogenic, the
F. culmorum isolate was seen to be less pathogenic, and the least coverage was recorded for
F. verticillioides. This agrees well with earlier findings [
31]. In other tests [
2],
F. culmorum had a higher aggressiveness than
F. graminearum. This is not an accident, as one inoculum cannot represent the mean pathogenicity of the species, so any difference is possible. As the two leading
Fusarium species (
F. graminearum and
F. verticillioides) need strongly different conditions, medium or higher correlations were found between two pathogens [
5,
7,
20,
31,
37,
38], and our data support the fact that a smaller part of the genotype responses is similar to different pathogens. Butron et al. [
58] also found very large toxin differences for different hybrids with very similar visual ear rot data. This is in agreement with what was found by others [
2,
12,
20,
23,
25]. This should have a genetic basis. However, nobody can currently say that similar responses regulated by quantitative trait loci (QTLs) with a pleiotropic effect on more pathogens or QTLs with different functions are responsible. As the
Fusarium species spectrum can change sharply from year to year [
59], the natural infection cannot be the base of the selection. As no paper on QTL analysis simultaneously analyzing the resistance to two or three different
Fusarium spp. was found, research on this objective is a task for the future [
60]. Zila et al. [
61] found that the genome-wide association study for
Fusarium ear rot resistance resulted in SNP variants explaining 1–3% of the variation found. Butron et al. [
58] found two genotypes with a good resistance of ten (20%). In this study, we demonstrated that about 10–15% of the hybrids had a good phenotypic resistance to more toxigenic species. We can say that around 20% of hybrids can have a good or higher resistance to the different
Fusarium spp., and in about 80%, the resistance to different species varies. Additionally, several are highly susceptible to all. What is behind this rather complicated picture? Considering the genetics, several papers have reported different functions of QTL. Lanubile [
12] and Lanubile et al. [
62] reported QTLs where, in addition to the ear rot value, toxin production was mapped. In another case, maize inbreds showed a high variability in resistance that was very differently expressed [
63]. Silks were found with differing resistances to
F. verticillioides [
62,
63]. Other differing functions have also been reported [
18,
24,
60,
64,
65], which is similar to what has been found in wheat [
66]. For this reason, we should test the resistance to the different toxigenic species separately, so that a better evaluation of the resistance evaluation and risk analysis is possible. As we tested large amounts of hybrids, two facts are important. The rate is different in each breeding firm, up to 20–30% of hybrids can be identified with similarly good resistance for the three pathogens, and the vast majority of hybrids vary in their resistance. For this reason, it is necessary to screen hybrids (and inbreds) against all
Fusarium species that play a role in the given region. Generally, it seems that
F. graminearum and
F. verticillioides will be the focus, but other
Fusarium species may also have significance. As
F. verticillioides dominates under dryer and warmer conditions,
F. graminearum prefers warm and humid conditions; both resistances are necessary, and this also means an adaptation to climatic changes.
Disease and toxin resistance are often considered to be synonyms. The toxin data from the kernel resistance tests differed, but both were significantly higher than the silk channel data. Schaafsma et al. [
67] found the kernel resistance to be better, and other data seem to support the better preciosity of kernel resistance [
10,
15,
54]. The three year toxin test with 10 selected hybrids produced similar results, supporting the conclusions of the larger hybrid set. The extremely high toxin production difference in several genotypes at very similar ear rot values indicates extra toxin production ability that can be called toxigenic hybrid. In the majority, however, the symptoms and toxin data correlate, well. Butron et al. [
58] also reported such cases. The term ‘toxigenic’ fungus is a commonly used term. In this case, we employ the term
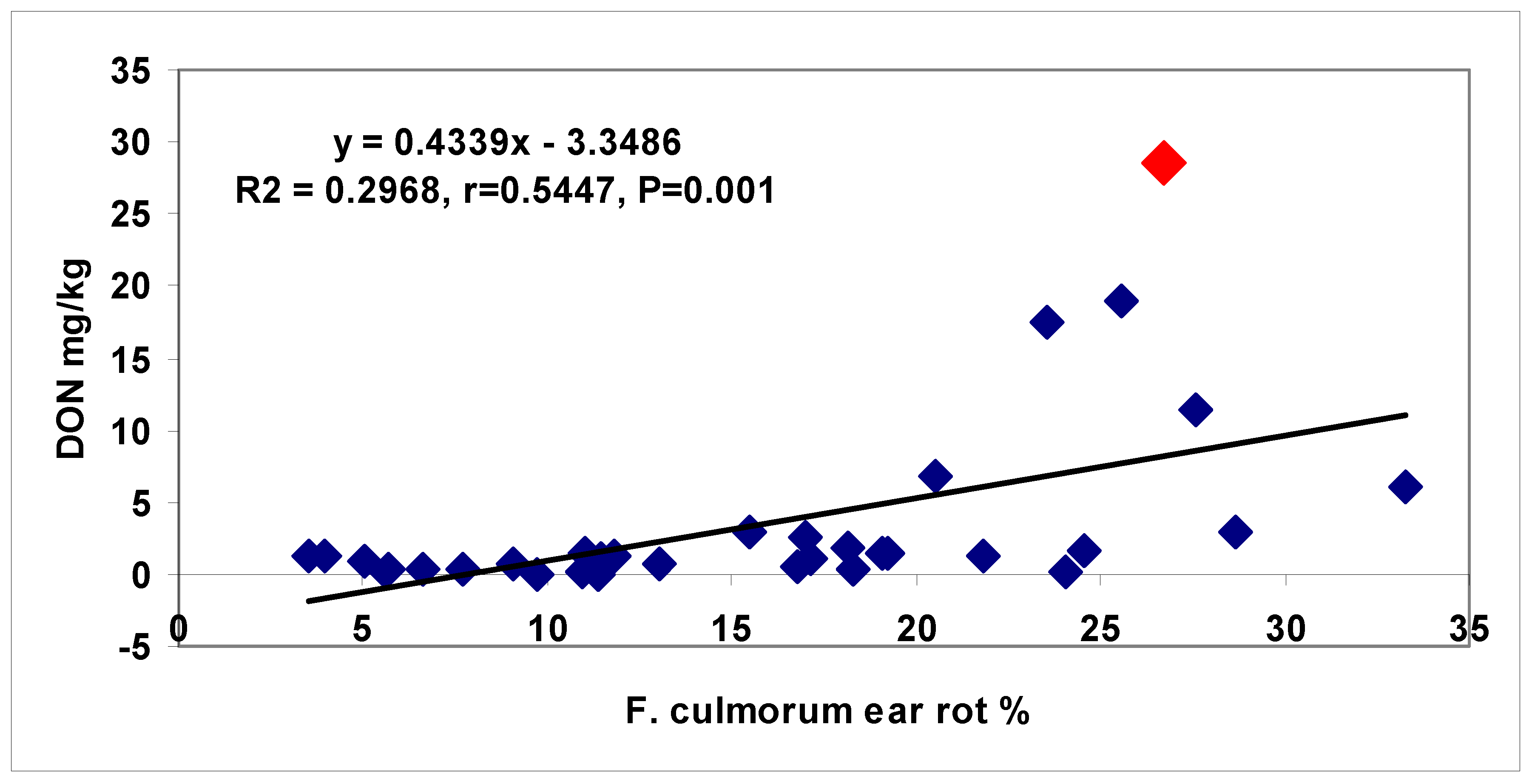
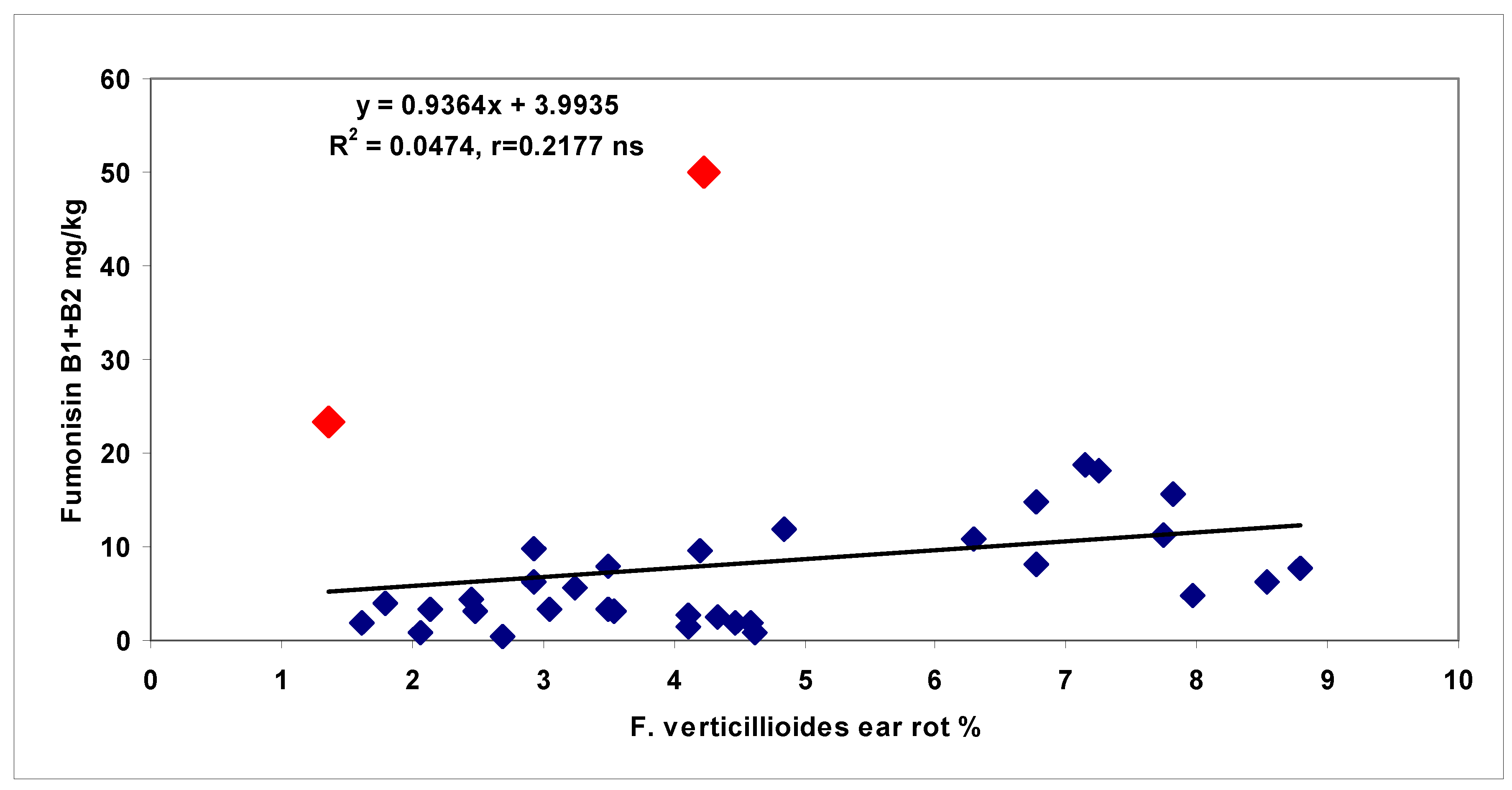
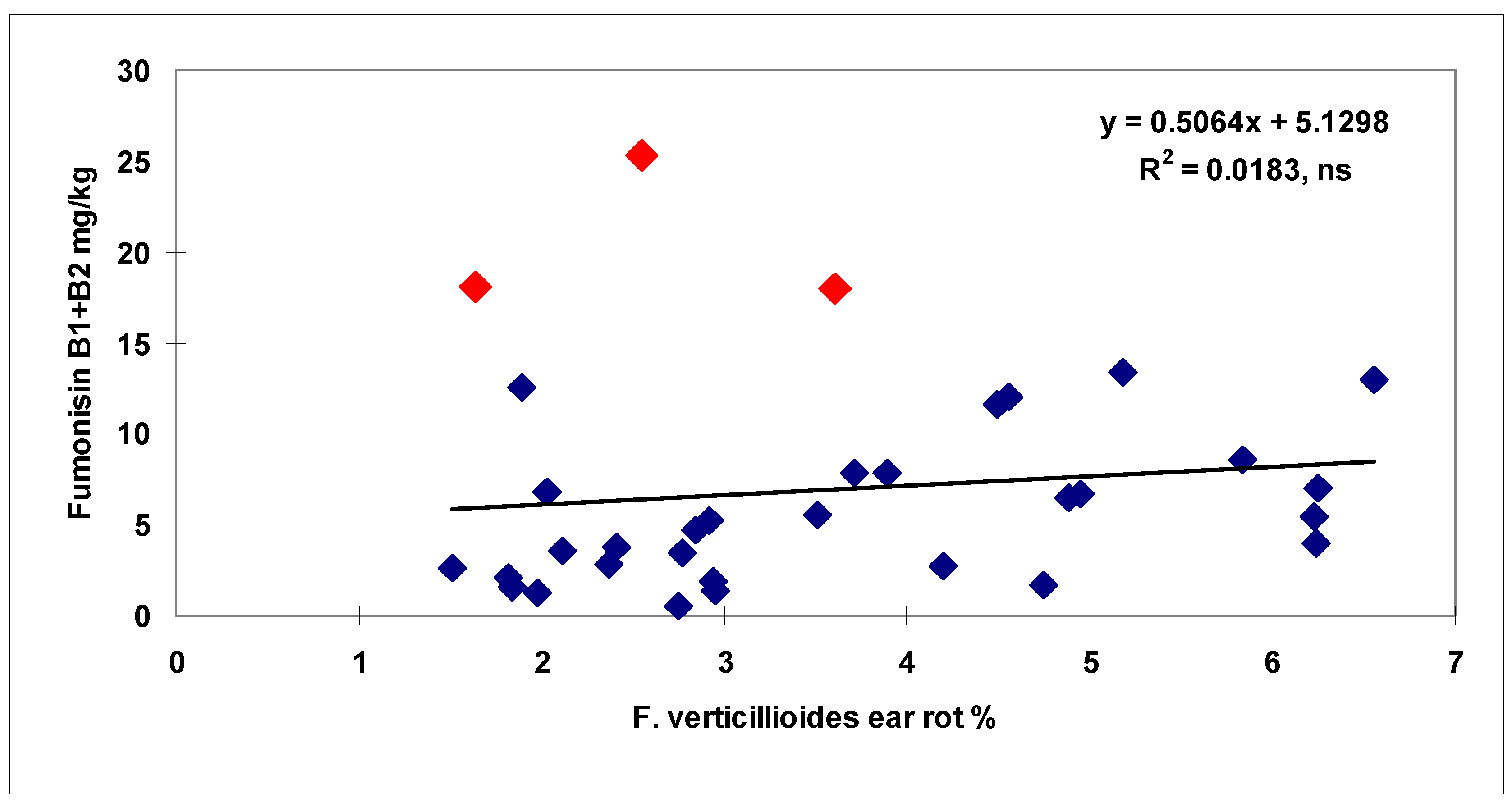
toxigenic genotype, and referring to maize toxigenic hybrid, which is very dangerous from the point of view of food safety. One correlation breaking hybrid with very high DON production could be identified from the 34 genotypes for
F. graminearum, one for
F. culmorum, and two for
F. verticillioides. Whilst, for ear rot symptoms, most of the correlations were significant, in the case of toxins, this was not the case mostly by these correlation breaking hybrids.
As both significantly higher toxin production and underproduction (the latter could not be proved in this paper) can be the case, the disease resistance data alone are not suitable for predicting toxin behavior and also not for variety registration. For this reason, a hybrid without toxin control cannot be allowed for commercial production, and highly susceptible hybrids should be withdrawn from commercial production.
The silk channel data from 2010 and 2010–2012 tell another story. Compared to the natural infection, no significant increase was seen in ear rot severity in the artificially induced inoculation regime. In DON producers, no increase was measured compared to the control. However, in
F. verticillioides, a significant toxin increase was recorded, even if the visual data did not show any difference. This means that
F. verticillioides can increase fumonisin contamination so that the visual symptom severity does not change. Of course, there are initial infections which are not visible, but toxin production can be identified. There may have been an infection between rows or on the surface of the cob, where grains can be infected from the germ side, but during the evaluation of the surface of ears, this will not be recognized (Mesterhazy unpublished). This is also an argument for the decisive factor being the toxin contamination. For us, the conclusion is that the toxin contamination should be measured, as trade and self-use are determined by toxin contamination, and not by ear rot severity in the field. It seems that toxin contamination and disease spread may need different ecological conditions. As many papers have reported successful SR tests [
10,
54,
68], even though the ear rot severity is mostly moderate, our very dry and hot climatic conditions may be responsible for it.
This would mean that artificial inoculation can really increase the fumonisin level. From the contradictious data on natural and artificial toxin levels, further research is needed to explain the results.
Visual rating and toxin contamination represent an old problem. Henry et al. [
34] found a correlation for FER (r = 0.74)
, and Robertson–Hoyt et al. [
14] and Robertson–Hoyt et al. [
35] found r = 0.29, and in another study, r = 0.69 [
36]. This is lower than the value found by Bolduan [
28] (r = 0.94,
p = 0.001). Szabo et al. [
2] also found acceptable correlation values between them. Santiago et al. [
69] found enough variability for FER in terms of resistance to select more resistant genotypes. Balconi et al. [
27] also found very high resistance differences for FER; in 2010, 0.56 and 240 mg/kg, and in 2009, 0.09 and 190 mg/kg, fumonisins were the maximum and minimum values, respectively. The data presented here fit well with the general experience. Therefore, the ear rot is the most powerful toxin regulating factor. In the cited papers, genotypes with unusually high toxin contamination were presented, but we realized that the toxin overproduction is an important feature when significant differences can be shown. The vast majority of hybrids have proportional toxin contamination to ear infection severity. Therefore, resistance is the most powerful regulation agent. As there are toxin overproducers for each ear rot pathogen, without a toxin control, we cannot secure food or feed safety. In wheat, we could identify genotypes with relative toxin resistance, meaning that they produced significantly less toxin than would have been supposed on the symptom severity. In this hybrid set, we could not identify such hybrids. For this, a significantly improved testing methodology will be needed.
Several studies have found agreements between resistances to two toxigenic fungi. For
F. graminearum and
F. verticilliodes, Presello [
37,
38] found similar results. Löffler et al. [
20] found a good agreement for flint and dent groups. Presello et al. [
70] selected inbreds from F
2 populations for
F. proliferatum resistance, and their hybrids were also tested with
F. graminearum and
F. verticillioides. The selection was also effective for these two species. Rose et al. [
71] identified, in FER and
Aspergillus, ear rot resistant genotypes, showing that breeding is an effective way to reduce toxin contamination.
It seems that we should adapt to the fact that, based on a visual evaluation of resistance, the forecast of toxin data is problematic. It also seems that resistance to a Fusarium species does not automatically mean resistance to another one and resistance to toxins. By comparing the data with a much larger population, it is clear that a larger data basis allows more profound conclusions to be drawn. The correlations between traits are seldom significant and there are surprising data.
In this study, the resistance to three toxigenic species was tested. It is clear that a smaller proportion of the hybrids have a similar resistance, whilst the others show a variable resistance structure. As for KR, reasonable correlations were found for symptom severity; this was not true for toxin production. For this reason, an automatic correspondence between ear rot severity and toxin contamination is not granted. As for relatively low ear rot severity, very high toxin amounts can be yielded, and without toxin control, successful breeding is not possible. For this reason, we need to identify hybrids that have low ear rot values with low toxin contamination against the most important toxigenic species in the region. By using more isolates and locations, the reliability of the data can be higher, and smaller toxin differences are enough to present clear proof for correlation breaking genotypes, as we have seen in wheat [
72]. We also need many cheaper toxin analytic methods besides the improving of phenotyping. We agree with the remark of Gaikpa and Miedaner [
59], that an improved methodology on a wider experimental basis would be needed to improve the usefulness of experimental results.