Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees
Abstract
1. Introduction
2. Methods
2.1. Big Lysimeters
2.2. Tracking Phenology
2.3. Laboratory Analyses
2.4. Mass-Balance Computations
2.5. Data Processing and Statistics
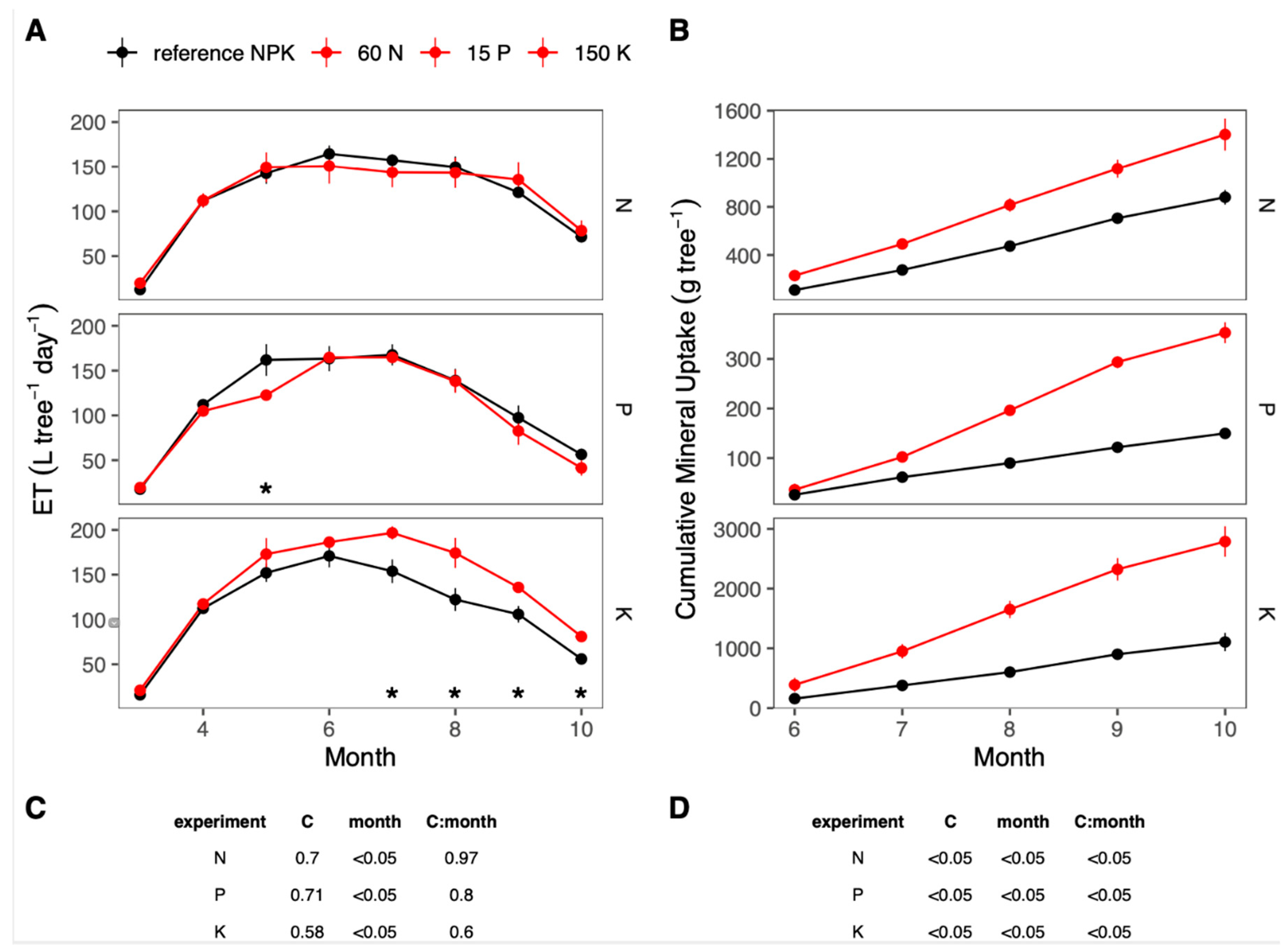
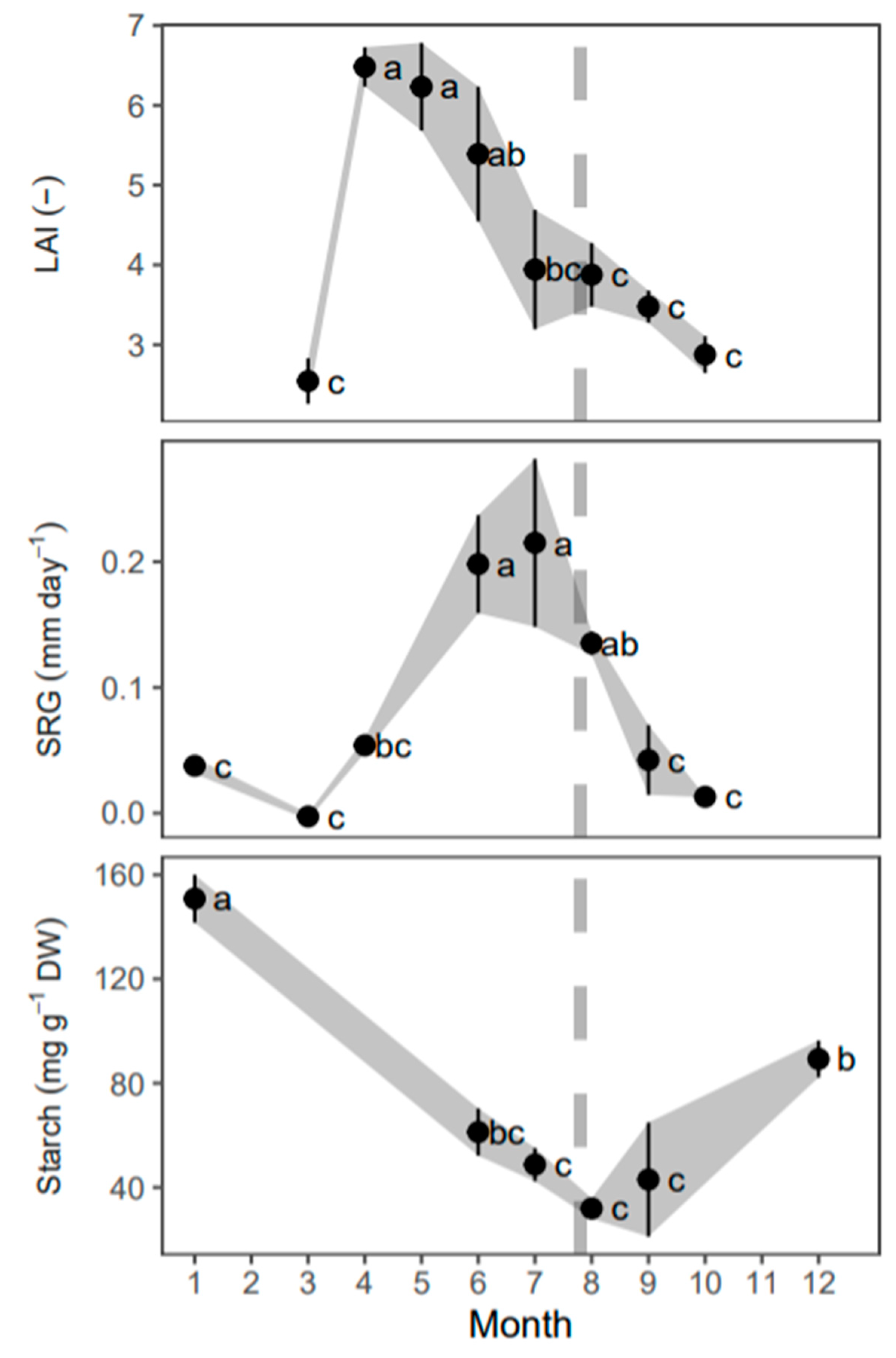
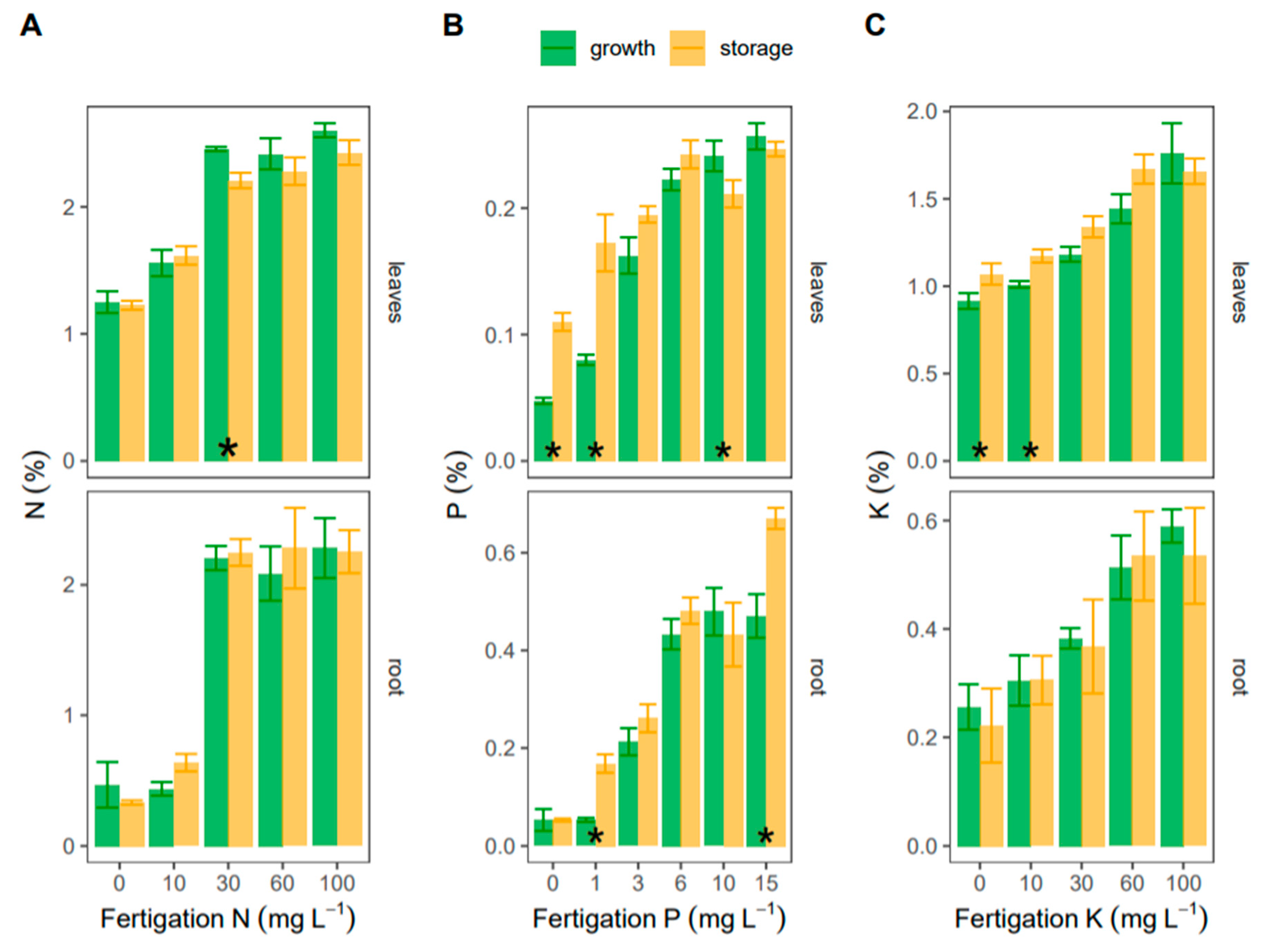
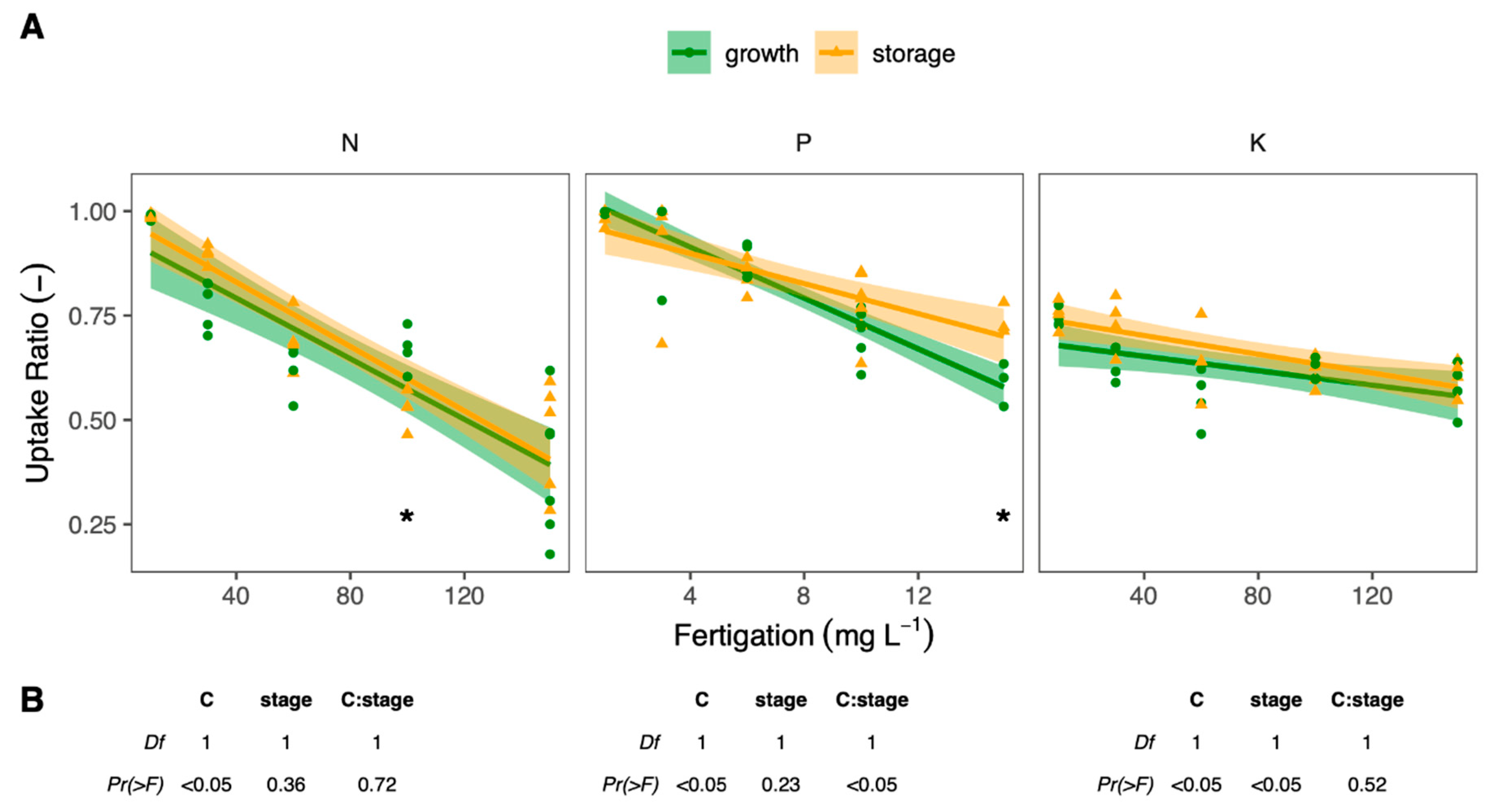
3. Results
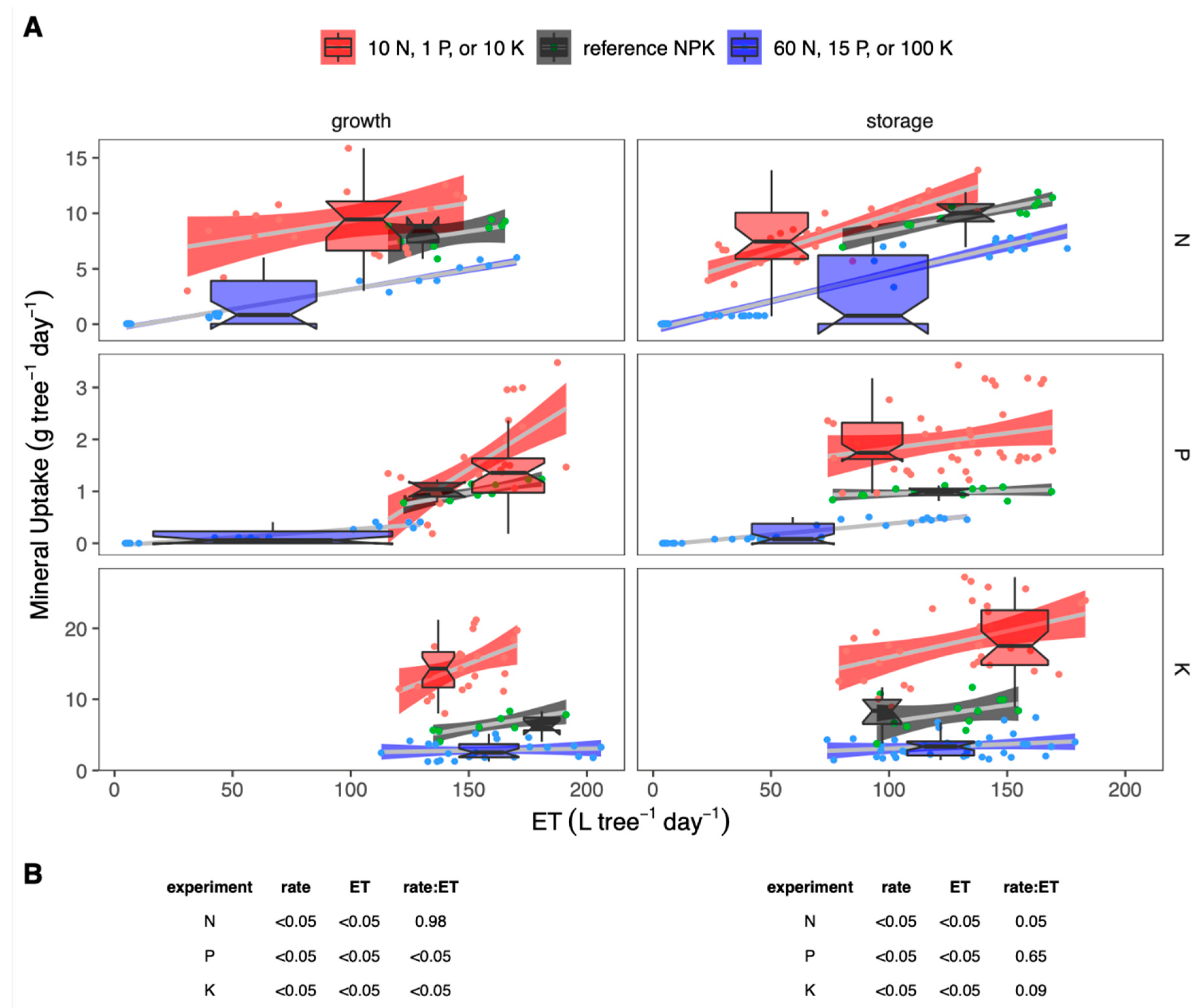
3.1. Seasonal Changes in Resource Uptake
3.2. Phenology and Fertigation Effects
3.3. Seasonal Changes in Fertigation Efficiency
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melillo, E.D. The First Green Revolution: Debt Peonage and the Making of the Nitrogen Fertilizer Trade, 1840–1930. Am. Hist. Rev. 2012, 117, 1028–1060. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Loop, T. The Facts about Nutrient Pollution; Environmental Protecting Agency (EPA): Washington, DC, USA, 2012. [Google Scholar]
- Sperling, O.; K, R.; Erel, R.; Yasuor, H.; Klipcan, L.; Yermiyahu, U.; Karuanakaran, R. Excessive nitrogen impairs hydraulics, limits photosynthesis, and alters the metabolic composition of almond trees. Plant Physiol. Biochem. 2019, 143, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Siebrecht, S.; Herdel, K.; Schurr, U.; Tischner, R. Nutrient translocation in the xylem of poplar—Diurnal variations and spatial distribution along the shoot axis. Planta 2003, 217, 783–793. [Google Scholar] [CrossRef]
- Le Bot, J.; Kirkby, E.A. Diurnal uptake of nitrate and potassium during the vegetative growth of tomato plants. J. Plant Nutr. 1992, 15, 247–264. [Google Scholar] [CrossRef]
- Muhammad, S.; Sanden, B.L.; Lampinen, B.D.; Saa, S.; Siddiqui, M.I.; Smart, D.R.; Olivos, A.; Shackel, K.A.; DeJong, T.; Brown, P.H. Seasonal changes in nutrient content and concentrations in a mature deciduous tree species: Studies in almond (Prunus dulcis (Mill.) D. A. Webb). Eur. J. Agron. 2015, 65, 52–68. [Google Scholar] [CrossRef]
- Geraldson, C.M.; Tyler, K.B. Plant Analysis as an Aid in Fertilizing Vegetable Crops. In Soil Testing and Plant Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 549–562. [Google Scholar] [CrossRef]
- Sabbe, W.E.; Zelinski, L.J.; Westerman, R. Plant Analysis as an Aid in Fertilizing Cotton. In Soil Testing and Plant Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 469–493. [Google Scholar] [CrossRef]
- Heerema, R.J.; Weinbaum, S.A.; Lampinen, B.D.; DeJong, T.M. Is nitrogen stress more apparent in shaded, fruiting almond spurs than in exposed, non-fruiting spurs? J. Hortic. Sci. Biotechnol. 2009, 84, 355–359. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Ben-Hayyim, G.; Kafkafi, U.; Ganmore-Neumann, R. Role of Internal Potassium in Maintaining Growth of Cultured Citrus Cells on Increasing NaCl and CaCl2 Concentrations. Plant Physiol. 1987, 85, 434–439. [Google Scholar] [CrossRef]
- Thompson, M.V.; Zwieniecki, M.A. The Role of Potassium in Long Distance Transport in Plants. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 221–240. [Google Scholar] [CrossRef]
- Cheng, L.; Fuchigami, L.H. 615 Growth Performance of Transplanted Young Apple Trees in Relation to Reserve Nitrogen and Carbohydrates. HortScience 1999, 34, 553. [Google Scholar] [CrossRef]
- Rosecrance, R.C.; Weinbaum, S.A.; Brown, P.H. Alternate Bearing Affects Nitrogen, Phosphorus, Potassium and Starch Storage Pools in Mature Pistachio Trees. Ann. Bot. 1998, 82, 463–470. [Google Scholar] [CrossRef]
- Alva, A.; Paramasivam, S.; Fares, A.; Obreza, T.; Schumann, A. Nitrogen best management practice for citrus trees. Sci. Hortic. 2006, 109, 223–233. [Google Scholar] [CrossRef]
- Collatz, G.; Ball, J.; Grivet, C.; A Berry, J. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- D’Ambrosio, N.; Arena, C.; De Santo, A.V. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Isidoro, D.; Grattan, S.R. Predicting soil salinity in response to different irrigation practices, soil types and rainfall scenarios. Irrig. Sci. 2010, 29, 197–211. [Google Scholar] [CrossRef]
- Halperin, O.; Gebremedhin, A.; Wallach, R.; Moshelion, M. High-throughput physiological phenotyping and screening system for the characterization of plant-environment interactions. Plant J. 2017, 89, 839–850. [Google Scholar] [CrossRef]
- Sperling, O.; Shapira, O.; Schwartz, A.; Lazarovitch, N. Direct in vivo evidence of immense stem water exploitation in irrigated date palms. J. Exp. Bot. 2014, 66, 333–338. [Google Scholar] [CrossRef]
- Mpelasoka, B.; Behboudian, M.; Green, S. Water use, yield and fruit quality of lysimeter-grown apple trees: Responses to deficit irrigation and to crop load. Irrig. Sci. 2001, 20, 107–113. [Google Scholar] [CrossRef]
- Fritschen, L.J.; Cox, L.; Kinerson, R. A 28-Meter Douglas-fir in a Weighing Lysimeter. For. Sci. 1973, 19, 256–261. [Google Scholar]
- Syvertsen, J.; Smith, M. Nitrogen Uptake Efficiency and Leaching Losses from Lysimeter-grown Citrus Trees Fertilized at Three Nitrogen Rates. J. Am. Soc. Hortic. Sci. 1996, 121, 57–62. [Google Scholar] [CrossRef]
- Peters, A.; Durner, W. Large zero-tension plate lysimeters for soil water and solute collection in undisturbed soils. Hydrol. Earth Syst. Sci. 2009, 13, 1671–1683. [Google Scholar] [CrossRef]
- Kislev, M.; Nadel, D.; Carmi, I. Epipalaeolithic (19,000 BP) cereal and fruit diet at Ohalo II, Sea of Galilee, Israel. Rev. Palaeobot. Palynol. 1992, 73, 161–166. [Google Scholar] [CrossRef]
- Doll, D. Almond Nutrients & Fertilization. 1996. Available online: http://fruitsandnuts.ucdavis.edu/almondpages/AlmondNutrientsFertilization/ (accessed on 25 March 2019).
- Saa, S.; Brown, P.H.; Muhammad, S.; Rio, A.O.-D.; Sanden, B.L.; Laca, E.A. Prediction of leaf nitrogen from early season samples and development of field sampling protocols for nitrogen management in Almond (Prunus dulcis [Mill.] DA Webb). Plant Soil 2014, 380, 153–163. [Google Scholar] [CrossRef]
- Neilsen, D.; Neilsen, G.; Forge, T. Building resilience: Future directions in mineral nutrition of woody perennial crops. Acta Hortic. 2018, 1–12. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Zipori, I.; Erel, R.; Ben-Gal, A.; Yermiyahu, U. Significance of proper nitrogen fertilization for olive productivity in T intensive cultivation. Sci. Hortic. 2019, 246, 710–717. [Google Scholar] [CrossRef]
- Basile, B.; Reidel, E.; Weinbaum, S.; DeJong, T. Leaf potassium concentration, CO2 exchange and light interception in almond trees (Prunus dulcis (Mill) D.A. Webb). Sci. Hortic. 2003, 98, 185–194. [Google Scholar] [CrossRef]
- Wolff, M.W.; Hopmans, J.W.; Stockert, C.M.; Burger, M.; Sanden, B.L.; Smart, D.R. Effects of drip fertigation frequency and N-source on soil N2O production in almonds. Agric. Ecosyst. Environ. 2017, 238, 67–77. [Google Scholar] [CrossRef]
- Goyal, S.S.; Huffaker, R.C.; Hauck, R.D. Nitrogen Toxicity in Plants. In Nitrogen in Crop Production 97–118; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Woodward, F.I.; Lomas, M.R.; Kelly, C.K. Global climate and the distribution of plant biomes. Philos. Trans. R. Soc. B Boil. Sci. 2004, 359, 1465–1476. [Google Scholar] [CrossRef]
- Tixier, A.; Gambetta, G.A.; Godfrey, J.; Orozco, J.; Zwieniecki, M.A. Non-structural Carbohydrates in Dormant Woody Perennials; The Tale of Winter Survival and Spring Arrival. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- McKown, A.; Guy, R.D.; Quamme, L.K. Impacts of bud set and lammas phenology on root:shoot biomass partitioning and carbon gain physiology in poplar. Trees 2016, 30, 2131–2141. [Google Scholar] [CrossRef]
- Mason, A.C.; Whitfield, A.B. Seasonal Changes in the Uptake and Distribution of Mineral Elements in Apple Trees. J. Hortic. Sci. 1960, 35, 34–55. [Google Scholar] [CrossRef]
- Millard, P.; Wendler, R.; Grassi, G.; Grelet, G.-A.; Tagliavini, M. Translocation of nitrogen in the xylem of field-grown cherry and poplar trees during remobilization. Tree Physiol. 2006, 26, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Shane, M.W.; McCully, M.E.; Lambers, H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J. Exp. Bot. 2004, 55, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Head, G.C. Effects of Seasonal Changes in Shoot Growth on the Amount of Unsuberized Root on Apple and Plum Trees. J. Hortic. Sci. 1967, 42, 169–180. [Google Scholar] [CrossRef]
- Rufat, J.; DeJong, T.M. Estimating seasonal nitrogen dynamics in peach trees in response to nitrogen availability. Tree Physiol. 2001, 21, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusià, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Falkiner, R.A.; Polglase, P.J. Transport of phosphorus through soil in an effluent-irrigated tree plantation. Soil Res. 1997, 35, 385. [Google Scholar] [CrossRef]
- Sharpley, A.; Moyer, B. Phosphorus Forms in Manure and Compost and Their Release during Simulated Rainfall. J. Environ. Qual. 2000, 29, 1462–1469. [Google Scholar] [CrossRef]
- Micke, W.C. Almond Production Manual; UCANR Publications: Davis, CA, USA, 1996. [Google Scholar]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Mallarino, A.P. Spatial Variability Patterns of Phosphorus and Potassium in No-Tilled Soils for Two Sampling Scales. Soil Sci. Soc. Am. J. 1996, 60, 1473–1481. [Google Scholar] [CrossRef]
- Mooshammer, M.; Hofhansl, F.; Frank, A.H.; Wanek, W.; Hämmerle, I.; Leitner, S.; Schnecker, J.; Wild, B.; Watzka, M.; Keiblinger, K.M.; et al. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci. Adv. 2017, 3, e1602781. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperling, O.; Karunakaran, R.; Yermiyahu, U. Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees. Agronomy 2020, 10, 1277. https://doi.org/10.3390/agronomy10091277
Sperling O, Karunakaran R, Yermiyahu U. Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees. Agronomy. 2020; 10(9):1277. https://doi.org/10.3390/agronomy10091277
Chicago/Turabian StyleSperling, Or, Ranjith Karunakaran, and Uri Yermiyahu. 2020. "Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees" Agronomy 10, no. 9: 1277. https://doi.org/10.3390/agronomy10091277
APA StyleSperling, O., Karunakaran, R., & Yermiyahu, U. (2020). Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees. Agronomy, 10(9), 1277. https://doi.org/10.3390/agronomy10091277





