Target-Site Resistance to Glyphosate in Chloris Virgata Biotypes and Alternative Herbicide Options for its Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection and Storage
2.2. Experimental Approach and Design
2.3. Experiment 1. Glyphosate Dose Response
2.4. Experiment 2. EPSPS Gene Sequencing
2.5. Experiment 3. Alternative Herbicide Options
2.6. Experiment 4. Double Knock Approach
2.7. Response Variables Recorded
2.8. Statistical Analyses
3. Results and Discussion
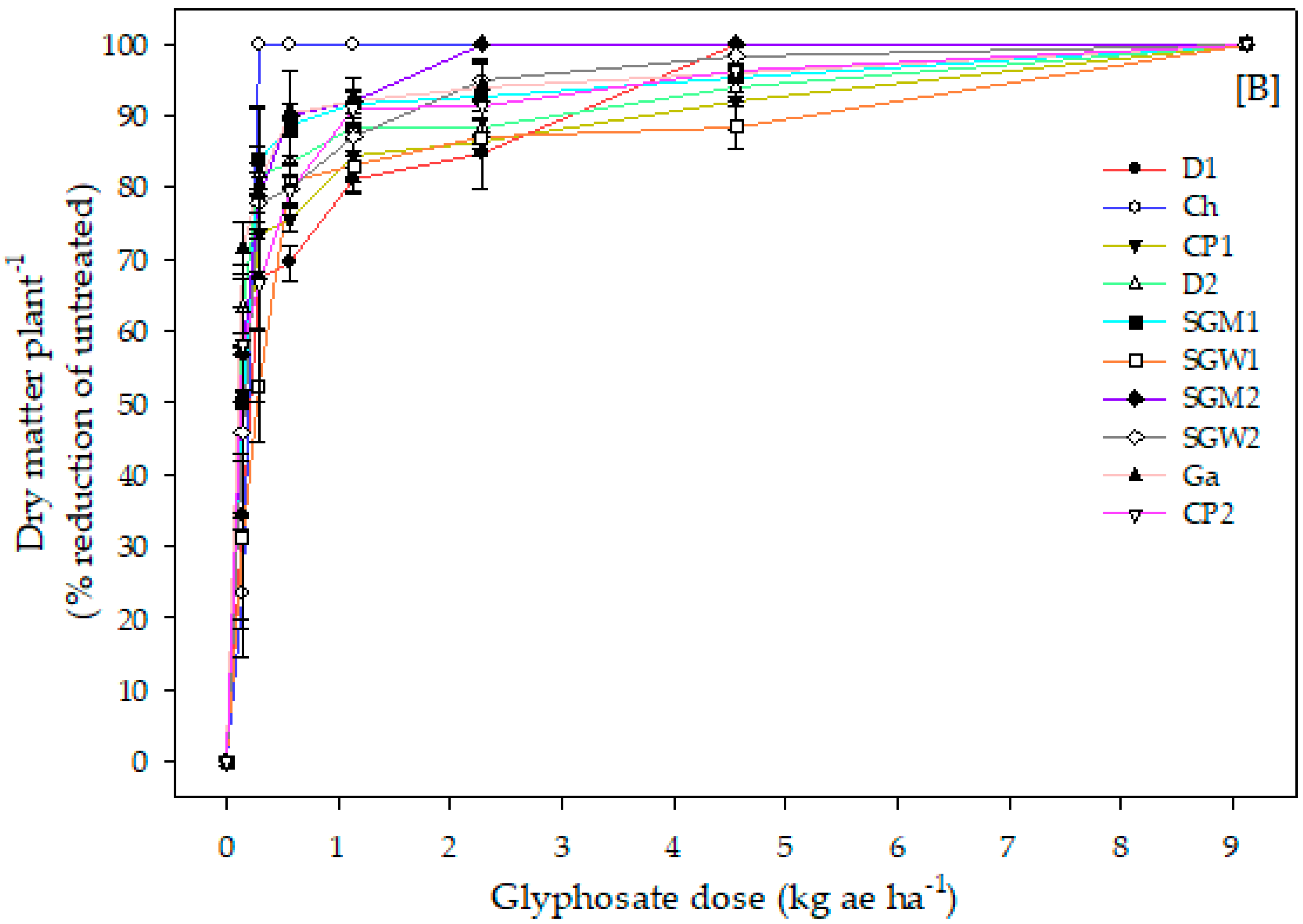
3.1. Experiment 1. Glyphosate Dose-Response
3.2. Experiment 2. EPSPS Gene Sequencing
3.3. Experiment 3. Alternative Options to Glyphosate
3.4. Experiment 4. Herbicide Treatments with Double Knock Approach
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osten, V. Feathertop Rhodes Grass: A Best Weed Management Guide; Department of Agriculture, Fisheries and Forestry, Queensland: Brisbane, Australia, 2012.
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag. Sci. 2018, 74, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, R.; Ronning, D.; Ouzman, J.; Walker, S.; Mayfield, A.; Clarke, M. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for Grains Research & Development Corporation: Canberra, Australia, 2016. [Google Scholar]
- Mahajan, G.; Walsh, M.; Chauhan, B.S. Junglerice (Echinochloa colona) and feather fingergrass (Chloris virgata) seed production and retention at sorghum maturity. Weed Technol. 2020, 34, 272–276. [Google Scholar] [CrossRef]
- Ngo, T.; Boutsalis, P.; Preston, C.; Gill, G. In Seed biology of Chloris truncata (windmill grass) and Chloris virgata (feathertop Rhodes grass). In Proceedings of the 19th Australasian Weeds Conference, “Science, Community and Food Security: The Weed Challenge”, Hobart, Tasmania, Australia, 1–4 September 2014; pp. 75–78. [Google Scholar]
- Fernando, N.; Humphries, T.; Florentine, S.K.; Chauhan, B.S. Factors affecting seed germination of feather fingergrass (Chloris virgata). Weed Sci. 2016, 64, 605–612. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Manalil, S.; Florentine, S.; Jha, P. Germination ecology of Chloris truncata and its implication for weed management. PloS ONE 2018, 13, e0199949. [Google Scholar]
- Davidson, B.; Cook, T.; Chauhan, B.S. Alternative options to glyphosate for control of large Echinochloa colona and Chloris virgata plants in cropping fallows. Plants 2019, 8, 245. [Google Scholar] [CrossRef]
- Herbicide Resistant Weeds. Available online: https://www.croplife.org.au/resources/programs/resistance-management/herbicide-resistant-weeds-list-draft-3/ (accessed on 6 June 2020).
- Délye, C.; Duhoux, A.; Pernin, F.; Riggins, C.W.; Tranel, P.J. Molecular mechanisms of herbicide resistance. Weed Sci. 2015, 63, 91–115. [Google Scholar] [CrossRef]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N., Jr. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef]
- McGillion, T.; Storrie, A. Integrated Weed Management in Australian Cropping Systems—A Training Resource for Farm Advisors; CRC for Australian Weed Management: Adelaide, Australia, 2006. [Google Scholar]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from plant tissues. In Plant Molecular Biology Manual; Springer: Cham, Switzerland, 1989; pp. 73–83. [Google Scholar]
- Minati, M.H.; Preston, C.; Malone, J. Resistance of flaxleaf fleabane (Conyza bonariensis (L.) Cronquist) to glyphosate. Bull. Natl. Res. Cent. 2020, 44, 1–6. [Google Scholar] [CrossRef]
- Preston, C.; Wakelin, A.M.; Dolman, F.C.; Bostamam, Y.; Boutsalis, P. A decade of glyphosate-resistant Lolium around the world: Mechanisms, genes, fitness, and agronomic management. Weed Sci. 2009, 57, 435–441. [Google Scholar] [CrossRef]
- Powles, S.B.; Lorraine-Colwill, D.F.; Dellow, J.J.; Preston, C. Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Sci. 1998, 46, 604–607. [Google Scholar] [CrossRef]
- Lorraine-Colwill, D.; Powles, S.; Hawkes, T.; Preston, C. Inheritance of evolved glyphosate resistance in Lolium rigidum (Gaud.). Theor. Appl. Genet. 2001, 102, 545–550. [Google Scholar] [CrossRef]
- Widderick, M.; McLean, A. Optimal intervals differ for double knock application of paraquat after glyphosate or haloxyfop for improved control of Echinochloa colona, Chloris virgata and Chloris truncata. Crop Prot. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Walsh, M.J.; Powles, S.B. High seed retention at maturity of annual weeds infesting crop fields highlights the potential for harvest weed seed control. Weed Technol. 2014, 28, 486–493. [Google Scholar] [CrossRef]
- Werth, J.; Walker, S.; Boucher, L.; Robinson, G. Applying the double knock technique to control Conyza bonariensis. Weed Biol. Manag. 2010, 10, 1–8. [Google Scholar] [CrossRef]
- Borger, C.P.; Hashem, A. Evaluating the double knockdown technique: Sequence, application interval, and annual ryegrass growth stage. Aust. J. Agric. Res. 2007, 58, 265–271. [Google Scholar] [CrossRef]
- Shaw, D.R.; Arnold, J.C. Weed control from herbicide combinations with glyphosate. Weed Technol. 2002, 16, 1–6. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Ngo, T.D. Biology and Glyphosate Resistance in Chloris truncata (Windmill Grass) and Chloris virgata (Feathertop Rhodes Grass) in Southern Australia. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, April 2017. [Google Scholar]
- Preston, C.; Stone, L.M.; Rieger, M.A.; Baker, J. Multiple effects of a naturally occurring proline to threonine substitution within acetolactate synthase in two herbicide-resistant populations of Lactuca serriola. Pestic. Biochem. Physiol. 2006, 84, 227–235. [Google Scholar] [CrossRef]
- Brunharo, C.A.D.C.G.; Morran, S.; Martin, K.; Moretti, M.L.; Hanson, B.D. EPSPS duplication and mutation involved in glyphosate resistance in the allotetraploid weed species Poa annua L. Pest Manag. Sci. 2019, 75, 1663–1670. [Google Scholar] [CrossRef]
- Kaundun, S.S.; Dale, R.P.; Zelaya, I.A.; Dinelli, G.; Marotti, I.; McIndoe, E.; Cairns, A. A novel P106L mutation in EPSPS and an unknown mechanism (s) act additively to confer resistance to glyphosate in a South African Lolium rigidum population. J. Agric. Food Chem. 2011, 59, 3227–3233. [Google Scholar] [CrossRef]
- Baerson, S.R.; Rodriguez, D.J.; Biest, N.A.; Tran, M.; You, J.; Kreuger, R.W.; Dill, G.M.; Pratley, J.E.; Gruys, K.J. Investigating the mechanism of glyphosate resistance in rigid ryegrass (Lolium ridigum). Weed Sci. 2002, 50, 721–730. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Abugho, S.B. Effect of growth stage on the efficacy of postemergence herbicides on four weed species of direct-seeded rice. Sci. World J. 2012. [Google Scholar] [CrossRef] [PubMed]
- Geier, P.W.; Stahlman, P.W.; White, A.D.; Miller, S.D.; Alford, C.M.; Lyon, D.J. Imazamox for winter annual grass control in imidazolinone-tolerant winter wheat. Weed Technol. 2004, 18, 924–930. [Google Scholar] [CrossRef]
- Peppers, J.M.; Gonçalves, C.G.; McElroy, J.S. Rate response of select grass weeds to pinoxaden. Weed Technol. 2020. [Google Scholar] [CrossRef]
- Chhokar, R.; Sharma, R.; Verma, R. Pinoxaden for controlling grass weeds in wheat and barley. Indian J. Weed Sci. 2008, 40, 41–46. [Google Scholar]
- Sasaki, Y.; Konishi, T.; Nagano, Y. The compartmentation of acetyl-coenzyme A carboxylase in plants. Plant Physiol. 1995, 108, 445. [Google Scholar] [CrossRef]
- Ke, J.; Behal, R.H.; Back, S.L.; Nikolau, B.J.; Wurtele, E.S.; Oliver, D.J. The role of pyruvate dehydrogenase and acetyl-coenzyme A synthetase in fatty acid synthesis in developing Arabidopsis seeds. Plant Physiol. 2000, 123, 497–508. [Google Scholar] [CrossRef]
- Hawke, J.; Leech, R. Acetyl-CoA-carboxylase activity in normally developing wheat leaves. Planta 1987, 171, 489–495. [Google Scholar] [CrossRef]
- Ellis, A.T.; Steckel, L.E.; Main, C.L.; De Melo, M.S.; West, D.R.; Mueller, T.C. A survey for diclofop-methyl resistance in Italian ryegrass from Tennessee and how to manage resistance in wheat. Weed Technol. 2010, 24, 303–309. [Google Scholar] [CrossRef]
- Baldwin, J.L.; Coats, G.E.; Street, J.E.; Langston, V.B. Effect of growth stage and application site on tolerance of rice (Oryza sativa) to haloxyfop. Weed Technol. 1996, 10, 268–272. [Google Scholar] [CrossRef]
- Bridges, D.C.; Smith, A.E.; Falb, L.N. Effect of adjuvant on foliar absorption and activity of clethodim and polar degradation products of clethodim. Weed Sci. 1991, 39, 543–547. [Google Scholar] [CrossRef]
- Werth, J.; Thornby, D.; Widderick, M. “Double knock” as a tactic for problematic weeds. In Proceedings of the Australian Cotton Conference, Gold Coast, Australia, 10 August 2008. [Google Scholar]
- Daniel, R.; Alliance, N.G. Fallow Management of Grass Weeds. In GRDC Grains Research Update; GRDC: Sydney, Australia, 2016. [Google Scholar]
- Diggle, A.; Neve, P.; Smith, F. Herbicides used in combination can reduce the probability of herbicide resistance in finite weed populations. Weed Res. 2003, 43, 371–382. [Google Scholar] [CrossRef]
- Neve, P.; Diggle, A.; Smith, F.; Powles, S. Simulating evolution of glyphosate resistance in Lolium rigidum I: Population biology of a rare resistance trait. Weed Res. 2003, 43, 404–417. [Google Scholar] [CrossRef]

| Biotype | Location | Situation | Coordinates | Collection Date |
|---|---|---|---|---|
| D1 | Dalby | Sorghum | −27.1844, 151.2668 | March 2017 |
| Ch | Chinchilla | Wheat fallow | −26.8264, 150.5802 | March 2017 |
| CP1 | Cecil Plains | Sorghum | −27.1799, 151.2554 | April 2017 |
| D2 | Dalby | Mungbean | −27.2866, 151.3228 | March 2017 |
| SGM1 | St. George | Mungbean boundary | −28.0041, 148.4100 | April 2017 |
| SGW1 | St. George | Wheat | −28.0775, 148.4100 | April 2017 |
| SGM2 | St. George | Mungbean | −28.0916, 148.4271 | April 2017 |
| SGW2 | St. George | Wheat fallow | −28.0454, 148.3158 | April 2017 |
| Ga | Gatton | Mungbean | −27.3318, 150.2011 | April 2017 |
| CP2 | Cecil Plains | Sorghum | −27.2935, 151.1283 | April 2017 |
| Active Constituent | Chemical Family | Mode of Action | Rates |
|---|---|---|---|
| Clethodim 240 g/L | Cyclohexanediones | Inhibits acetyl co-enzyme A carboxylase | 90 g ai ha−1 + 1% Supercharge |
| Haloxyfop 520 g/L | Aryloxyphenoxypropionates | Inhibits acetyl co-enzyme A carboxylase | 78 g ai ha−1 + 1% Hasten |
| Imazamox 33 g/L plus Imazapyr 15 g/L | Imidazolinones | Inhibits acetolactate synthesis | 25 g ai ha−1 + 1% Hasten |
| Paraquat 360 g/L | Bipyridils | Inhibits photo synthesis 1 | 600 g ai ha−1 + 1% BS 1000 |
| Pinoxaden 50 g/L | Phenylpyrazoles | Inhibits acetyl co-enzyme A carboxylase | 20 g ai ha−1 + 0.5% Adigor |
| Active Constituent | Application Type | Chemical Family | Mode of Action | Rate |
|---|---|---|---|---|
| Glyphosate 570 g/L | Single | Glycines | Inhibits EPSP synthase | 1.14 kg ae ha−1 |
| Glufosinate-ammonium 200 g/L | Single | Phosphinic acids | Inhibits glutamine synthetase | 0.75 kg ai ha−1 |
| Glyphosate 570 g/L fb Paraquat 360 g/L | Sequential | Glycines fb Bipyridils | Inhibits EPSP synthase fb Inhibits photo synthesis 1 | 1.14 kg ae ha−1 fb 0.6 kg ai ha−1 |
| Glyphosate 570 g/L fb Paraquat 360 g/L | Sequential | Glycines fb Bipyridils | Inhibits EPSP synthase fb Inhibits photo synthesis 1 | 1.14 kg ae ha−1 fb 1.2 kg ai ha−1 |
| Glufosinate-ammonium 200 g/L fb Paraquat 360 g/L | Sequential | Phosphinic acids fb Bipyridils | Inhibits glutamine synthetase fb Inhibits photo synthesis 1 | 0.75 kg ai ha−1 fb 0.6 kg ai ha−1 |
| Glufosinate-ammonium 200 g/L fb Paraquat 360 g/L | Sequential | Phosphinic acids fb Bipyridils | Inhibits glutamine synthetase fb Inhibits photo synthesis 1 | 0.75 kg ai ha−1 fb 1.2 kg ai ha−1 |
| Biotypes | LD50 (kg ae ha−1) | R/S Ratio | Resistance Status |
|---|---|---|---|
| Ch | 0.21 | 1 | Susceptible |
| SGM2 | 0.85 | 4.04 | |
| D1 | 2.64 | 12.53 | Moderately Resistant |
| CP1 | 2.75 | 13.03 | |
| SGW1 | 2.89 | 13.67 | |
| SGM1 | 3.88 | 18.38 | |
| Ga | 3.96 | 18.78 | |
| D2 | 4.24 | 20.00 | |
| CP2 | 5.72 | 27.09 | Highly Resistant |
| SGW2 | 5.76 | 27.28 |
| Amino Acid Number: | 96 | 97 | 98 | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acids: | Phe | Leu | Gly | Asn | Ala | Gly | Thr | Ala | Met | Arg | Pro | Leu | Thr |
| Consensus sequence: | TTC | TTG | GGG | AAT | GCT | GGA | ACT | GCA | ATG | CGG | CCA | TTG | ACA |
| Ch | - | - | - | - | - | - | - | - | - | - | CCX (3) | - | - |
| SGM2 | - | - | - | - | - | - | - | - | - | - | ACX (2)(3) | - | - |
| SGW2 | - | - | - | - | - | - | - | - | - | - | ACX (2)(3) | - | - |
| CP2 | - | - | - | - | GCY (1)(4) | - | - | - | - | CCZ (1)(5) | TCX (2)(3) | - | - |
| Treatments | Survival (%) ± SE | Dry Matter Per Plant (g) ± SE | ||||||
|---|---|---|---|---|---|---|---|---|
| Ch | SGM2 | SGW2 | CP2 | Ch | SGM2 | SGW2 | CP2 | |
| Untreated | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 0.964 ± 0.15 | 0.533 ± 0.10 | 0.720 ± 0.13 | 0.930 ± 0.14 |
| Clethodim | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Haloxyfop | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Imazamox + imazapyr | 83.3 ± 8.3 | 100 ± 0 | 87.5 ± 8.5 | 94.4 ± 5.6 | 0.148 ± 0.060 (85) | 0.141 ± 0.040 (74) | 0.215 ± 0.050 (70) | 0.219 ± 0.040 (77) |
| Paraquat | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Pinoxaden | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 0.518 ± 0.020 (46) | 0.521 ± 0.100 (2) | 0.609 ± 0.100 (15) | 0.775 ± 0.080 (17) |
| Treatments | Survival % ± SE | Dry Matter Per Plant (g) ± SE | ||||||
|---|---|---|---|---|---|---|---|---|
| Ch | SGM2 | SGW2 | CP2 | Ch | SGM2 | SGW2 | CP2 | |
| Untreated | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 1.152 ± 0.1 | 0.957 ± 0.06 | 0.870 ± 0.12 | 1.168 ± 0.25 |
| Glyphosate | 0 ± 0 | 81.66 ± 9.1 | 100 ± 0 | 100 ± 0 | 0 ± 0 (100) | 0.479 ± 0.12 (51.83) | 0.406 ± 0.05 (53.33) | 0.475 ± 0.14 (62.33) |
| Glufosinate-ammonium | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Glyphosate fb paraquat | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Glyphosate fb paraquat | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Glufosinate-ammonium fb paraquat | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
| Glufosinate-ammonium fb paraquat | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) | 0 ± 0 (100) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, H.S.; Thompson, M.; Chauhan, B.S. Target-Site Resistance to Glyphosate in Chloris Virgata Biotypes and Alternative Herbicide Options for its Control. Agronomy 2020, 10, 1266. https://doi.org/10.3390/agronomy10091266
Desai HS, Thompson M, Chauhan BS. Target-Site Resistance to Glyphosate in Chloris Virgata Biotypes and Alternative Herbicide Options for its Control. Agronomy. 2020; 10(9):1266. https://doi.org/10.3390/agronomy10091266
Chicago/Turabian StyleDesai, Het Samir, Michael Thompson, and Bhagirath Singh Chauhan. 2020. "Target-Site Resistance to Glyphosate in Chloris Virgata Biotypes and Alternative Herbicide Options for its Control" Agronomy 10, no. 9: 1266. https://doi.org/10.3390/agronomy10091266
APA StyleDesai, H. S., Thompson, M., & Chauhan, B. S. (2020). Target-Site Resistance to Glyphosate in Chloris Virgata Biotypes and Alternative Herbicide Options for its Control. Agronomy, 10(9), 1266. https://doi.org/10.3390/agronomy10091266






