Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases
Abstract
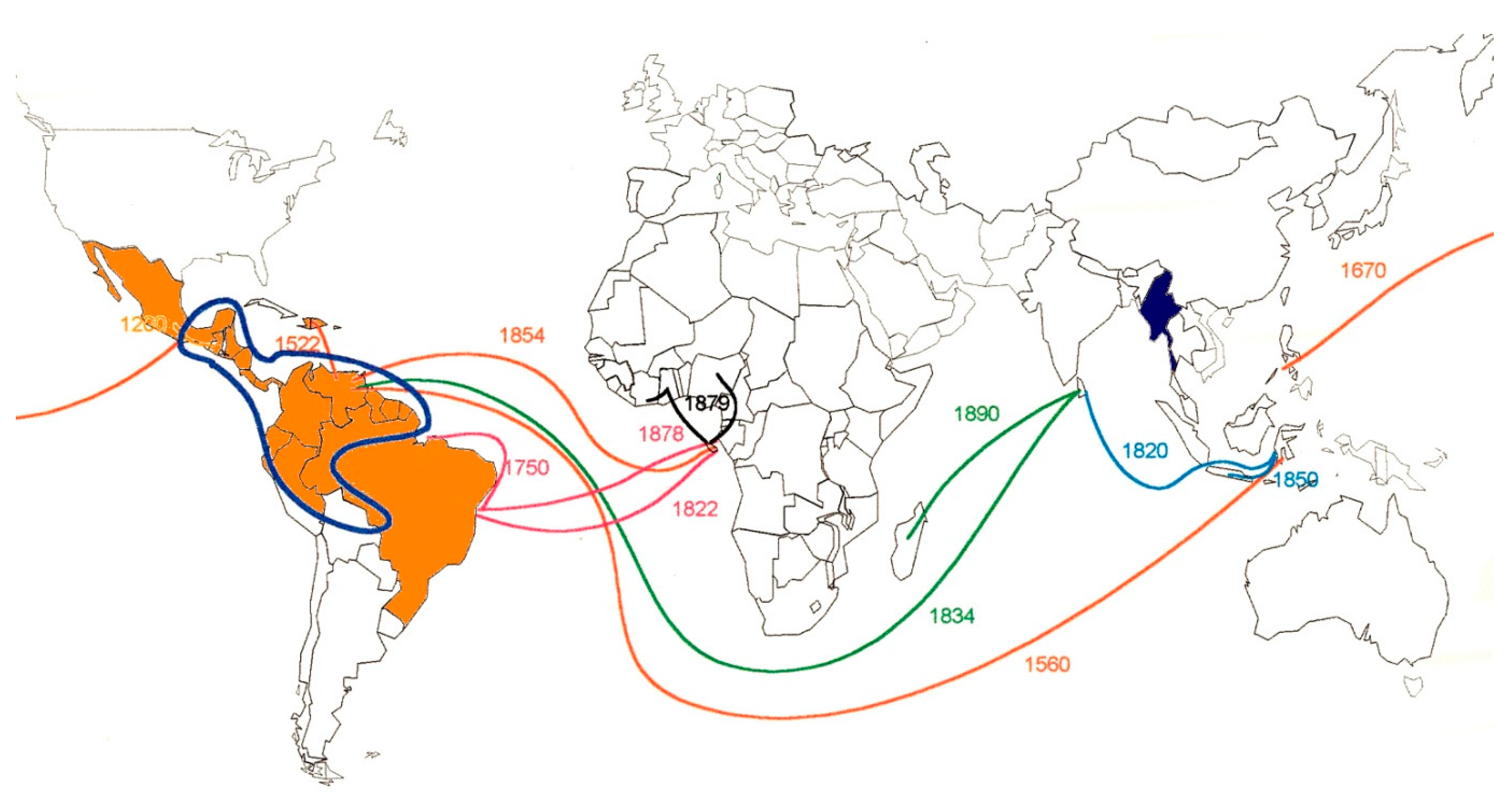
1. Historical Trajectory of Cocoa Production
2. Sanitary Constraints and Geographic Distribution of Main Cocoa Pests and Diseases
3. The Effects of Climate Change
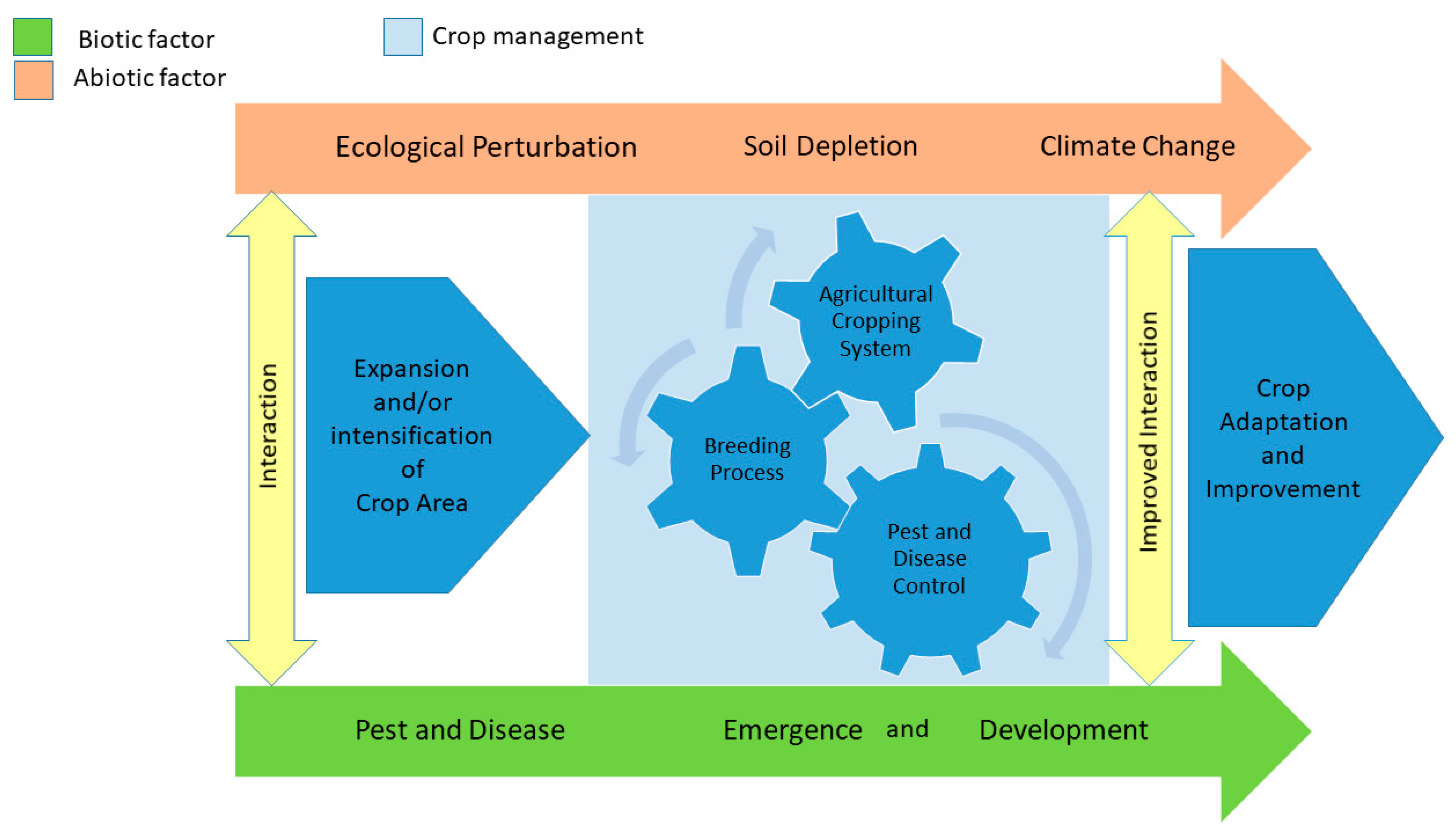
4. Climate Change, Global Change, and Plant Health Risks
5. Management in the Context of the Geographical Spread of Pests and Diseases and Climate Change
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Touzard, J.M. L’économie coloniale du Cacao en Amérique Centrale; Agris: Montpellier, France, 1993; p. 95. [Google Scholar]
- Powis, T.G.; Cyphers, A.; Gaikwad, N.W.; Grivetti, L.; Cheong, K. Cacao use and the San Lorenzo Olmec. Proc. Natl. Acad. Sci. USA 2011, 108, 8595–8600. [Google Scholar] [CrossRef] [PubMed]
- Soustelle, J. Les Olmèques. In La Plus Ancienne Civilisation du Mexique; Arthaud: Paris, France, 1979. [Google Scholar]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Muhs, D.R.; Kautz, R.R.; MacKinnon, J.J. Soils and the location of cacao orchards at a Maya site in western Belize. J. Archaeol. Sci. 1985, 12, 121–137. [Google Scholar] [CrossRef]
- Ponce, A. Relacion breve y verdadera del padre Fray Alonso Ponce. In Anales des Museo Guzman; Avri, Ed.; Museum Guzman: San Salvador, El Salvador, 1952; pp. 10, 24, 72. [Google Scholar]
- Ruf, F. Booms et Crises du Cacao: Les Vertiges de l’or Brun; Economie et développement; Karthala: Paris, France; CIRAD-SAR: Montpellier, France, 1995; p. 459. [Google Scholar]
- Ruf, F. Libéralisation, cycles politiques et cycles du cacao: Le décalage historique Côte-d’Ivoire-Ghana. Cah. Agric. 2009, 18, 343–349. [Google Scholar] [CrossRef]
- De Pineda, J. Descripcion de la provincia de Guatemala. In Anales del Museo Guzman; Museum Guzman: San Salvador, El Salvador, 1952; pp. 56–66. [Google Scholar]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases—Opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Morillo, F.; Sánchez, P.; Herrera, B.; Liendo-Barandiaran, C.; Muñoz, W.; Hernández, J.V. Pupal development, longevity and behavior of Carmenta theobromae (Lepidoptera: Sesiidae). Fla. Entomol. 2009, 92, 355–361. [Google Scholar] [CrossRef]
- Arshad, F.M.; Bala, B.K.; Alias, E.F.; Abdulla, I. Modelling boom and bust of cocoa production systems in Malaysia. Ecol. Model. 2015, 309, 22–32. [Google Scholar] [CrossRef]
- Malaysian Cocoa Board. Available online: https://www.koko.gov.my/lkm/industry/statistic/p_cocoabean.cfm (accessed on 4 May 2020).
- Phillips-Mora, W.; Ortiz, C.F.; Aime, M.C. Fifty years of frosty pod rot in Central America: Chronology of its spread and impact from Panama to Mexico. In Proceedings of the 15th International Cocoa Research Conference, San José, Costa Rica, 9–14 October 2006; Cocoa Producers’ Alliance (COPAL)/CATIE: Ghana, Nigeria, 2006. [Google Scholar]
- Díaz-José, O.; Aguilar-Ávila, J.; Rendón-Medel, R.; Santoyo-Cortés, V.H. Current state of and perspectives on cocoa production in Mexico. Int. J. Agric. Nat. Resour. 2013, 40, 279–289. [Google Scholar] [CrossRef][Green Version]
- Evans, H.C.; Waller, J.M. Globalisation and the threat to biosecurity. In The Role of Plant Pathology in Food Safety and Food Security; Springer: Dordrecht, The Netherlands, 2009; pp. 53–71. [Google Scholar]
- Danquah, F.K. Sustaining a West African cocoa economy: Agricultural science and the swollen shoot contagion in Ghana, 1936–1965. Afr. Econ. Hist. 2003, 31, 43–74. [Google Scholar] [CrossRef]
- Turnbull, C.J.; Daymond, A.J.; Lake, H.; Main, B.E.; Radha, K.; Cryer, N.C.; End, M.J.; Hadley, P. The role of the international cocoa germplasm database and the international cocoa quarantine centre in information management and distribution of cocoa genetic resources. In Proceedings of the 16th International Cocoa Research Conference, Bali, Indonesia, 11–16 November 2009. [Google Scholar]
- Dufour, B.P.; Kerana, I.W.; Ribeyre, F. Effect of coffee tree pruning on berry production and coffee berry borer infestation in the Toba Highlands (North Sumatra). Crop Prot. 2019, 122, 151–158. [Google Scholar] [CrossRef]
- Sultan, B.; Defrance, D.; Iizumi, T. Evidence of crop production losses in West Africa due to historical global warming in two crop models. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V.P., Zhai, H.-O., Pörtner, D., Roberts, J., Skea, P.R., Shukla, A., Pirani, W., Moufouma-Okia, C., Péan, R., Pidcock, S., et al., Eds.; IPCC: Paris, France, 2018; Available online: https://www.ipcc.ch/sr15/download/ (accessed on 20 March 2018).
- Rosenzweig, C.; Iglesius, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate Change and Extreme Weather Events—Implications for Food Production, Plant Diseases, and Pests. Glob. Chang. 2001, 2, 90–104. [Google Scholar]
- Pan, X.; Zhu, Z.; Li, T. Forecasts of ENSO evolution using spatial–Temporal projection model. Int. J. Climatol. 2020. [Google Scholar] [CrossRef]
- Gateau-Rey, L.; Tanner, E.V.J.; Rapidel, B.; Marelli, J.-P.; Royaert, S. Climate Change could threaten cocoa production: Effects of 2015–2016 El Niño-related drought on cocoa agroforests in Bahia, Brazil. PLoS ONE 2018, 13, 17. [Google Scholar] [CrossRef]
- Carr, M.K.V.; Lockwood, G. The water relations and irrigation requirements of cocoa (Theobroma cacao L.): A review. Exp. Agric. 2011, 47, 653–676. [Google Scholar] [CrossRef]
- Almeida, A.-A.F.; de Valle, R.R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Lahive, F.; Hadley, P.; Daymond, A.J. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron. Sustain. Dev. 2019, 39, 22. [Google Scholar] [CrossRef]
- Läderach, P.; Martinez-Valle, A.; Schroth, G.; Castro, N. Predicting the future climatic suitability for cocoa farming of the world’s leading producer countries, Ghana and Côte d’Ivoire. Clim. Chang. 2013, 119, 841–854. [Google Scholar] [CrossRef]
- Gil Restrepo, J.P.; Leiva-Rojas, E.I.; Ramírez Pisco, R. Phenology of Cocoa Tree in a Tropical Moist Forest. Científica 2017, 45, 240–252. [Google Scholar] [CrossRef]
- Acheampong, K.; Daymond, A.J.; Adu-Yeboah, P.; Hadley, P. Improving Field Establishment of Cacao (Theobroma Cacao) through Mulching, Irrigation and Shading. Exp. Agric. 2009, 55, 898–912. [Google Scholar] [CrossRef]
- Schroth, G.; Läderach, P.; Martinez-Valle, A.I.; Christian Bunn, C.; Jassogne, L. Vulnerability to climate change of cocoa in West Africa: Patterns, opportunities and limits to adaptation. Sci. Total Environ. 2016, 556, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Garcia Lozano, J.; Moreno Fonseca, L.P. Respuestas fisiológicas de Theobroma cacao L. en etapa de vivero a la disponibilidad de agua en el suelo. Acta Agronómica 2015, 65, 44–50. [Google Scholar] [CrossRef]
- Luedeling, E.; Kindt, R.; Huth, N.; Koenig, K. Agroforestry systems in a changing climate—Challenges in projecting future performance. Curr. Opin. Environ. Sustain. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Kroeger, A.; Koenig, S.; Thomson, A.; Streck, C.; Weiner, P.-H.; Bakhtary, H. Forest- and Climate-Smart Cocoa in Côte d’Ivoire and Ghana, Aligning Stakeholders to Support Smallholders in Deforestation-Free Cocoa; World Bank: Washington, DC, USA, 2017; p. 57. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/29014/122086.pdf (accessed on 15 December 2017).
- Abdulai, I.; Vaast, P.; Hoffmann, M.P.; Asare, R.; Jassogne, L.; Van Asten, P.; Rötter, R.P.; Graefe, S. Cocoa agroforestry is less resilient to sub-optimal and extreme climate than cocoa in full sun. Glob. Chang. Biol. 2018, 24, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernández, M.; Wanger, T.C.; Tscharntke, T. Neglected pollinators: Can enhanced pollination services improve cocoa yields? A review. Agric. Ecosyst. Environ. 2017, 247, 137–148. [Google Scholar]
- Doare, F.; Ribeyre, F.; Cilas, C. Genetic and environmental links between traits of cocoa beans and pods clarify the phenotyping processes to be implemented. Sci. Rep. 2020, 10, 9888. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Cilas, C.; Goebel, F.R.; Babin, R.; Avelino, J. Tropical crop pests and diseases in a climate change setting—A few examples. In Climate Change and Agriculture Worldwide; Torquebiau, E., Manley, D., Cowan, P., Eds.; Springer: Heidelberg, Germany, 2016; pp. 73–82. [Google Scholar]
- Bandyopadhyay, R.; Frederiksen, R.A. Contemporary global movement of emerging plant diseases. Ann. N. Y. Acad. Sci. 1999, 894, 28–36. [Google Scholar] [CrossRef]
- Wilson, M.E. Travel and the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 39. [Google Scholar] [CrossRef]
- Oro, F.Z.; Bonnot, F.; Ngo-Bieng, M.A.; Delaitre, E.; Dufour, B.P.; Ametefe, K.E.; Mississo, E.; Wegbe, K.; Muller, E.; Cilas, C. Spatiotemporal pattern analysis of Cacao swollen shoot virus in experimental plots in Togo. Plant Pathol. 2012, 61, 1043–1051. [Google Scholar] [CrossRef]
- Schroth, G.; Krauss, U.; Gasparotto, L.; Duarte, J.A.; Vohland, K. Pests and Diseases in Agroforestry Systems of the Humid Tropics. Agrofor. Syst. 2000, 50, 199–241. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mills, N.J. Biological Control; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Deberdt, P.; Mfegue, C.V.; Tondje, P.R.; Bon, M.C.; Ducamp, M.; Hurard, C.; Begoude, D.; Ndoumbé Nkeng, M.; Hebbar, P.K.; Cilas, C. Impact of environmental factors, chemical fungicide and biological control on cacao pod production dynamics and black pod disease (Phytophthora megakarya) in Cameroon. Biol. Control 2008, 44, 149–159. [Google Scholar] [CrossRef]
- Rosmana, A.; Taufik, M.; Asman, A.; Jayanti, N.J.; Hakkar, A.A. Dynamic of Vascular Streak Dieback Disease Incidence on Susceptible Cacao Treated with Composted Plant Residues and Trichoderma asperellum in Field. Agronomy 2019, 9, 650. [Google Scholar] [CrossRef]
- Mbarga Manga, A.; Begoude, B.A.D.; Ambang, Z.; Meboma, M.; Kuaté, J.; Ewbank, W.; Ten Hoopen, G.M. Field testing an oil-based Trichoderma asperellum formulation for the biological control of cacao black pod disease, caused by Phytophthora megakarya. Crop Prot. 2020, 132, 105134. [Google Scholar] [CrossRef]
- Andres, C.; Blaser, W.J.; Dzahini-Obiatey, H.K.; Ameyaw, G.A.; Domfeh, O.K.; Awiagah, M.A.; Gattinger, A.; Schneider, M.; Offei, S.K.; Six, J. Agroforestry systems can mitigate the severity of cocoa swollen shoot virus disease. Agric. Ecosyst. Environ. 2018, 252, 83–92. [Google Scholar] [CrossRef]
- Babin, R.; Ten Hoopen, G.M.; Cilas, C.; Enjalric, F.; Yede Gendre, P.; Lumaret, J.P. Impact of shade on the spatial distribution of Sahlbergella singularis in traditional cocoa agroforests. Agric. For. Entomol. 2010, 12, 69–79. [Google Scholar] [CrossRef]
- Gidoin, C.; Babin, R.; Bagny-Beilhe, L.; Cilas, C.; Ten Hoopen, G.M.; Ngo Bieng, M.A. Tree spatial structure, host composition and resource availability influence mirid density or Black pod prevalence in cacao agroforests in Cameroon. PLoS ONE 2014, 9, e109405. [Google Scholar] [CrossRef]
- Nyassé, S.; Efombagn, M.I.B.; Kébé, B.I.; Tahi, M.; Despréaux, D.; Cilas, C. Integrated management of Phytophthora diseases on cocoa (Theobroma cacao L.): Impact of plant breeding on pod rot incidence. Crop Prot. 2007, 26, 40–45. [Google Scholar] [CrossRef]
- Phillips-Mora, W.; Castillo, J.; Krauss, U.; Rodríguez, E.; Wilkinson, M.J. Evaluation of cacao (Theobroma cacao) clones against seven Colombian isolates of Moniliophthora roreri from four pathogen genetic groups. Plant Pathol. 2005, 54, 483–490. [Google Scholar] [CrossRef]
- Teh, C.L.; Pang, J.T.Y.; Ho, C.T. Variation of the response of clonal cocoa to attack by cocoa pod borer Conopomorpha cramerella (Lepidoptera: Gracillariidae) in Sabah. Crop Prot. 2006, 25, 712–717. [Google Scholar] [CrossRef]
- Ndoumbé, M.; Bieysse, D.; Cilas, C. Multi-trait selection in a diallel crossing scheme of cocoa. Plant Breed. 2001, 120, 365–367. [Google Scholar] [CrossRef]
- Jaimez, R.E.; Vera, D.I.; Mora, A.; Loor, R.G.; Bailey, B.A. A disease and production index (DPI) for selection of cacao (Theobroma cacao) clones highly productive and tolerant to pod rot diseases. Plant Pathol. 2020, 69, 698–712. [Google Scholar] [CrossRef]
- Domfeh, O.; Ameyaw, G.A.; Dzahini-Obiatey, H.K.; Ollennu, L.A.A.; Osei-Bonsu, K.; Acheampong, K.; Aneani, F.; Owusu-Ansah, F. Use of immune crops as barrier in the management of cacao swollen shoot virus disease (CSSVD)—Long-term assessment. Plant Dis. 2016, 100, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilas, C.; Bastide, P. Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases. Agronomy 2020, 10, 1232. https://doi.org/10.3390/agronomy10091232
Cilas C, Bastide P. Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases. Agronomy. 2020; 10(9):1232. https://doi.org/10.3390/agronomy10091232
Chicago/Turabian StyleCilas, Christian, and Philippe Bastide. 2020. "Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases" Agronomy 10, no. 9: 1232. https://doi.org/10.3390/agronomy10091232
APA StyleCilas, C., & Bastide, P. (2020). Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases. Agronomy, 10(9), 1232. https://doi.org/10.3390/agronomy10091232





